DNA Rearrangement: Recombination, Transposons, Insertion Sequences
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
DNA Rearrangement: Recombination, Transposons, Insertion Sequences
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
Describe, and differentiate among, the different types of recombination.
Recognize the structure and function of the different mobile genetic elements.
Describe the function of RecA in homologous recombination and in the SOS response.
Mention the function of other important recombination proteins.
Discuss examples where recombination plays a biomedical relevant role.
all of these have been done individually.
need to go to textbook for clinical correlations.
what is a recombination?
rearrangement of DNA
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
ATP-Dependent Chromatin Remodeling
Histone Modifications (The "Histone Code")
Histone Variant Incorporation
DNA Methylation: PRIMARILY represses gene expression, but CAN activate it in certain contexts.
Chromatin Looping and 3D Genome Architecture (CTF and Cohesin)
Phase Separation and Biomolecular Condensates
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
1. ATP-Dependent Chromatin Remodeling
1. ATP-Dependent Chromatin Remodeling
Large multi-protein complexes use the energy from ATP hydrolysis to physically slide, evict, restructure, or reposition nucleosomes.
How ATP-Dependent Chromatin Remodeling Creates Dynamism: These complexes act as molecular motors, constantly reshaping the nucleosomal landscape to make specific DNA regions accessible or inaccessible on a timescale of seconds to minutes.
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
2. Histone Modifications (The "Histone Code")
2. Histone Modifications (The "Histone Code")
Molecular Basis: Enzymes covalently add or remove chemical groups (e.g., acetyl, methyl, phosphate) to the tails of histone proteins. This is a reversible process.
Writers: Add marks (e.g., Histone Acetyltransferases - HATs).
Erasers: Remove marks (e.g., Histone Deacetylases - HDACs).
Readers: Proteins that bind to these marks and initiate downstream effects.
How it Creates Dynamism: This creates a rapidly changing, combinatorial code that dictates chromatin state. Acetylation, for example, can quickly loosen histone-DNA interactions, while certain methylations can just as quickly recruit proteins that promote condensation.
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
3. Histone Variant Incorporation
Histone Variant Incorporation
Molecular Basis: Standard core histones can be replaced with non-allelic variants (e.g., H3.3, H2A.Z, CenH3) outside of DNA replication.
How it Creates Dynamism: These variants alter the biophysical properties of the nucleosome. For example, H2A.Z-containing nucleosomes are less stable and often mark nucleosomes that are poised for activation, making regions more dynamically responsive to signals.
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
4. DNA Methylation
4. DNA Methylation: PRIMARILY represses gene expression, but CAN activate it in certain contexts.
Molecular Basis: The addition of methyl groups to cytosine bases in CpG islands. This process is also reversible, though generally more stable than histone modifications.
How it Creates Dynamism: While often associated with long-term silencing, active methylation and demethylation processes allow the genome to adapt to developmental and environmental cues, dynamically shutting down or priming gene regions.
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
5. Chromatin Looping and 3D Genome Architecture (CTF and Cohesin)
the end goal is gene regulation.
5. Chromatin Looping and 3D Genome Architecture
Molecular Basis: Protein complexes like CTCF and Cohesin mediate long-range interactions by extruding DNA loops, bringing distant regulatory elements (enhancers) into close physical proximity with gene promoters.
extrude: making a DNA loop stand out.
How CTCF and Cohesin Work Together: The Collaborative Dance
CCCTC: This is the specific DNA sequence that the protein was first discovered to bind to. It's a five-nucleotide sequence (Cytosine-Cytosine-Cytosine-Thymine-Cytosine
Cohesin: multi-subunit protein complex that forms a large, ring shaped structure, physically holding two strands of DNA together.
Think of it as a molecular handcuff or a carabiner that can encircle and tether DNA segments. This simple yet powerful ability allows it to perform two critical, but distinct, roles in the cell:
In Cell Division: Holding sister chromatids together.
In Interphase (between divisions): Organizing the 3D genome by extruding DNA loops.
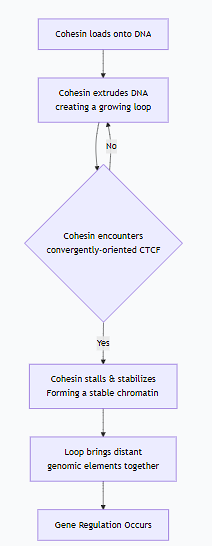
The true magic happens in the collaboration between these two players. This is often called the "Loop Extrusion Model."
Here is a step-by-step sequence of how they create a chromatin loop:
The cohesin complex is loaded onto the chromatin fiber.
Cohesin begins its motor activity, extruding DNA to form a growing loop. During this phase, the loop is dynamic and transient.
Encounter and Stall: The extruding cohesin complex encounters a pair of CTCF proteins bound to their specific DNA sites. Crucially, these CTCF sites must be in a convergent orientation (facing towards each other) to form a stable "roadblock."
SThe collision with the correctly oriented CTCF proteins stalls the cohesin complex, stabilizing the loop.
Functional Outcome: This stabilized loop now brings two distant genomic elements (e.g., an enhancer and a gene promoter) into close physical proximity, enabling gene regulation.
Summary Table: Roles of CTCF and Cohesin
Feature | CTCF (The Architect) | Cohesin (The Motor) |
|---|---|---|
Primary Role | Defines loop anchors and boundaries | Actively extrudes DNA to form loops |
Molecular Function | DNA sequence-specific binder | ATP-dependent motor complex |
Structure | Zinc-finger protein | Multi-subunit ring |
Analogy | Stanchions / Anchor Points | Motorized Winch |
Key Action | Creates a stable roadblock | Performs active loop extrusion |
In conclusion, CTCF provides the static, address-specific landmarks, while Cohesin provides the dynamic, motorized force. Together, they orchestrate the complex 3D folding of the genome, which is essential for proper gene expression, DNA replication, and cellular identity. Disruptions in either protein can lead to severe developmental disorders and cancers by misregulating gene expression.
How it Creates Dynamism: These loops are not permanent. They can form, break, and reform, creating and dissolving regulatory hubs in the nucleus, allowing a single enhancer to dynamically interact with different promoters in different cell states.
Explain the molecular basis for the dynamic nature of the chromosome and its genetic consequences.
6. Phase Separation and Biomolecular Condensates (hubs of active transcription)
6. Phase Separation and Biomolecular Condensates
Molecular Basis: Regions of chromatin with high concentrations of specific transcription factors and activating marks can undergo liquid-liquid phase separation, forming membrane-less condensates (hubs of active transcription).
liquid-liquid phase separation→ membrane-less condensates→ hubs of activate transcription.
How it Creates Dynamism: This allows for the rapid, reversible assembly and disassembly of transcriptionally active compartments, concentrating the necessary machinery for efficient gene expression and releasing it when no longer needed.
Describe, and differentiate among, the different types of recombination.
Describe, and differentiate among, the different types of recombination.
what is genetic recombination?
genetic recombination is the process by which genetic material is broken and rejoined to produce new combinations of alleles.
how many types of recombinations are there?
There are three primary types of recombination, each with distinct mechanisms, functions, and biological roles.
1. Homologous Recombination (HR)
2. Site-Specific Recombination
3. Transposition (Transpositional Recombination)
what is homologous recombination (general recombination)?
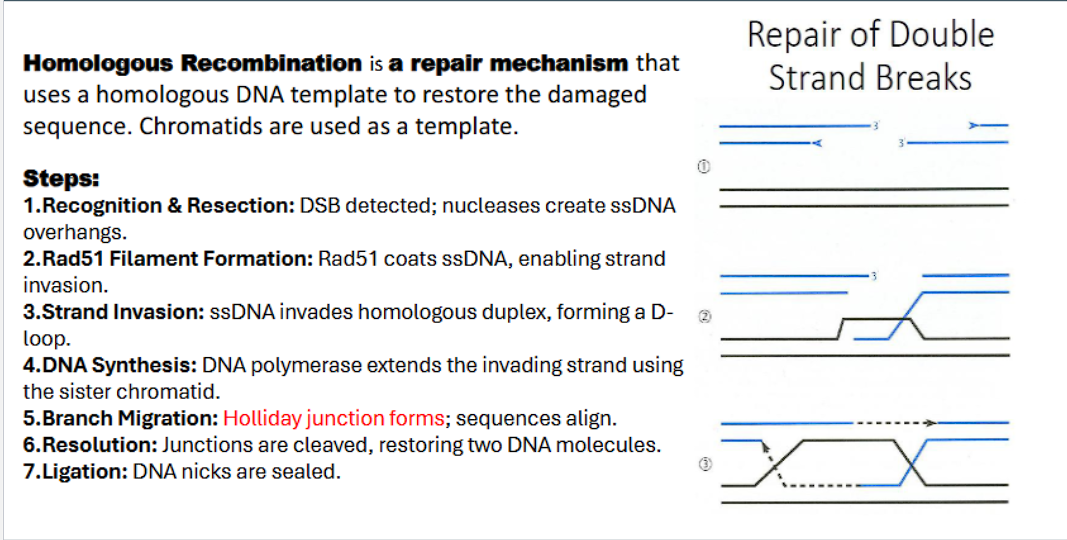
Homologous Recombination is a repair mechanism that uses a homologous DNA template to restore the damaged sequence. Chromatids are used as a template.
“homologous recombination” uses homologous DNA TEMPLATE to restore the damaged sequence.
end goal: fix the damaged sequence
homologous recombination steps:
double stranded break detected by MRE complex
nucleases act as molecular scissors to chew back the 5’ ends on either side of break to create single strand 3’ DNA overhangs.
the 3’ DNA overhangs are sticky, and the protein RPA immediate binds to these overhangs to prevent them from forming secondary structures or being degraded.
Rad51 binds to the 3’ overhang
Rad51 coats ssDNA with a presynaptic filament, forming the enabling strand invasion.
The Rad51-ssDNA filament scans the genome and locates a homologous sequence (on the sister chromatid).
“strand invasion”: ssDNA catalyzes invasion, the invading strand base pairs with complementary bases on homologous duplex, the base pairing displaces the homologous strand, forming a Displacement-loop (D-loop).
Why D-loop is Important: This step physically connects the damaged DNA to the undamaged template. The invading 3' end is now perfectly positioned to act as a primer for new DNA synthesis.
DNA polymerase extends the invading strand using the sister chromatid.
Branch migration: holiday junction forms: sequences align.
A Holliday junction is a cross-shaped DNA structure central to homologous recombination, where two double-stranded DNA molecules are joined, allowing for the exchange of genetic information.
resolution: resolvases recognize and cut the holliday junctions, restoring two DNA molecules
The Key Action: Cleavage in Two Planes. Each Holliday junction can be cleaved in one of two ways:
Crossover: Cleavage results in the reciprocal exchange of DNA arms, creating new combinations of alleles on the repaired chromosomes.
Non-crossover: Cleavage results in the restoration of the original chromosomes without exchange of flanking sequences.
Why It's Important: This step separates the two interconnected DNA molecules. The cell has a bias toward the non-crossover pathway during DSB repair to prevent potentially harmful rearrangements.
Ligation: DNA ligase is the enzyme that rejoins the broken DNA ends by catalyzing the formation of a phosphodiester bond to seal the sugar-phosphate backbone.
DNA nick: a break in the phosphodiester bonds, therefore DNA ligase rejoins broken DNA by catalyzing the formation of phosphodiester bonds.
which enzyme determines crossing over or non-crossing over?
resolvase! (recognizes and cuts holliday junctions)
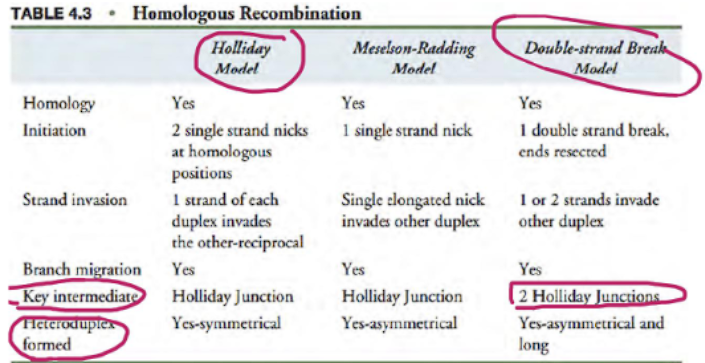
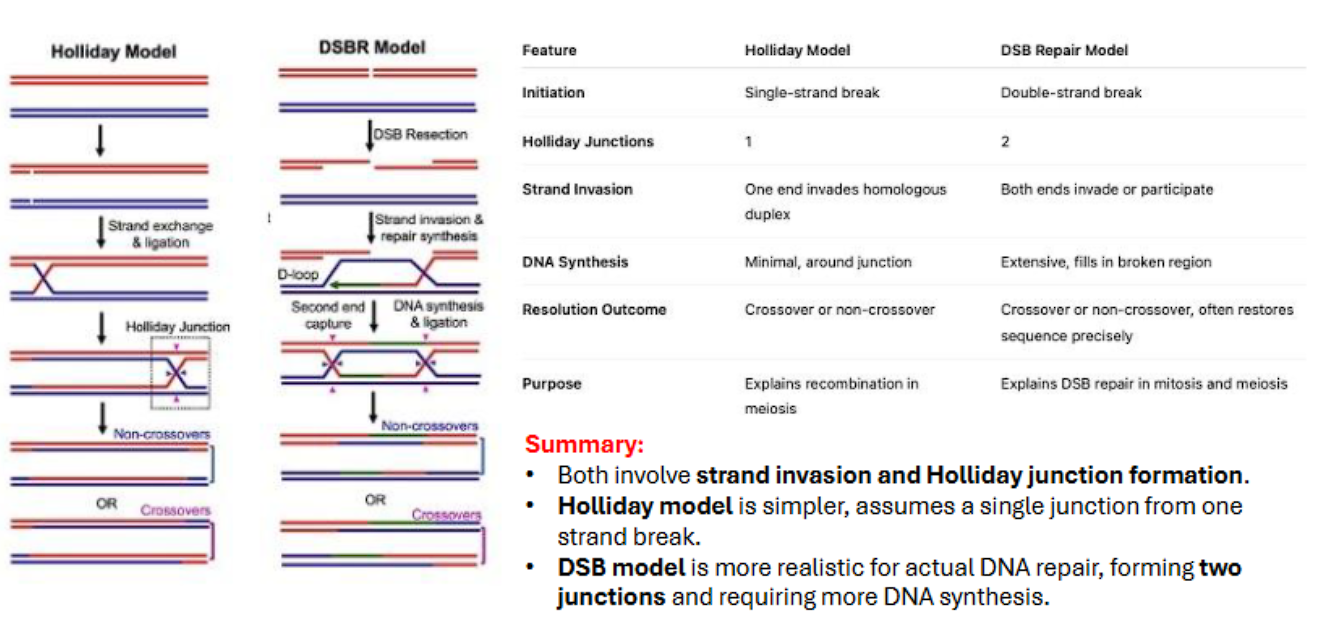
what are two models of homologous recombination?
holliday model
double-strand rebreak model
when is homologous recombination mostly seen?
what does is another function of homologous recombination?
during meiosis (eukaryotes) and conjugation (prokaryotes)
linking newly made chromatids together for proper segragation

what are the key difference between the holliday model and double stranded break model?
key intermediate (holliday junction): the holliday model has 1 holliday junction because only one strand breaks, the double stranded break model (DSBM) has 2 holliday junctions because both strands break.
heteroduplex formed (symmetrical vs assymetrical): the holliday model has a symmetrical duplex, the double stranded break model has a assymetrical duplex.
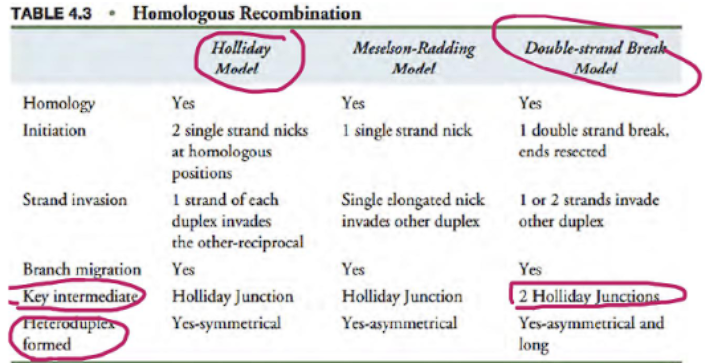
what are the differences between holliday model and double stranded break repair?
initiation: holliday model: single stranded break, double stranded break repair has double stranded break
holliday junctions: holliday model has 1, double stranded break repair has 2
invasions: holliday model has 1 invasion, dsbr has 2 invasions
dna synthesis: holliday model has minimal, dsbr has extensive
resolution or outcome is the same (cross over or non crossover)
MITOSIS VS MEIOSIS: holliday model occurs in meiosis, dsbr occurs in BOTH mitosis and meiosis
crossing over requires…
crossing over requires a double stranded break with resolvase enzyme (this can occur in holliday model or dsb model)
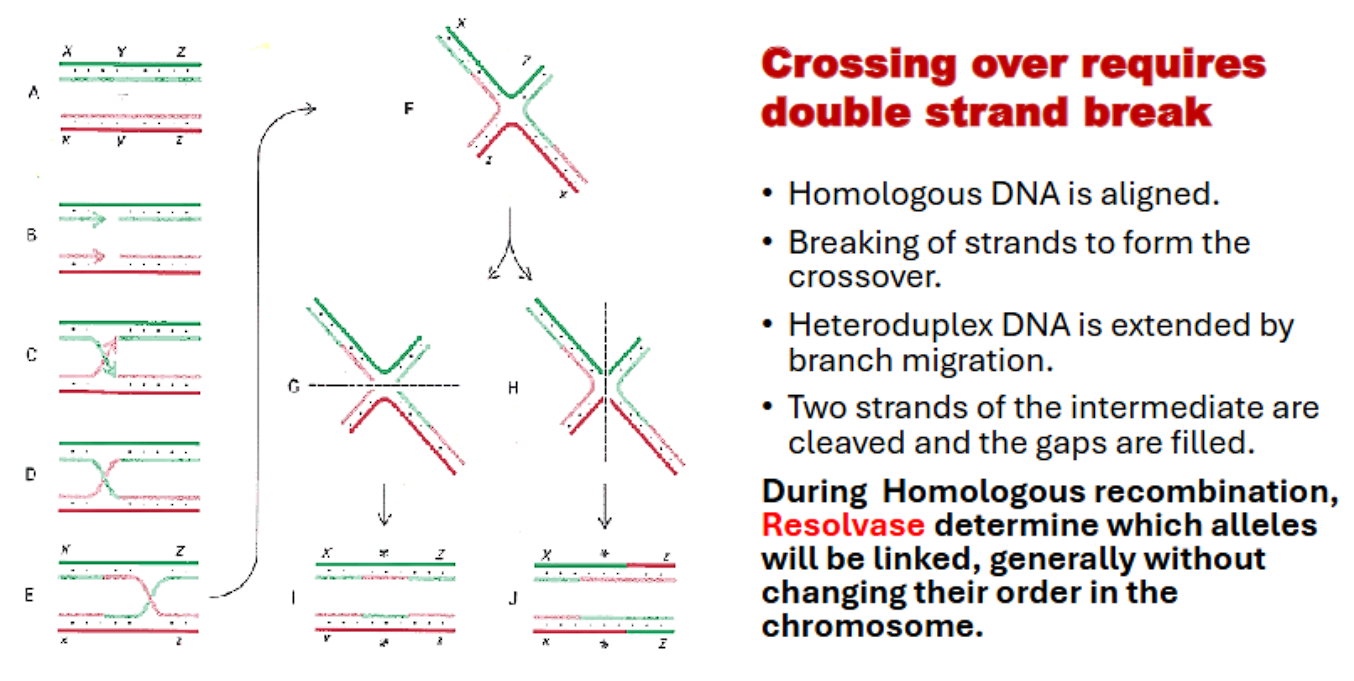
which enzyme determines crossing over?
resolvase, because it recognizes and cuts holliday junctions
The Holliday junction is a "tangled" or "cross-linked" intermediate that represents a problem. The resolvase is the enzyme that solves (resolves) this problem by cutting the DNA at the crossover point, allowing the two DNA duplexes to separate.
what is a holliday junction?
A Holliday junction is a cross-shaped structure that forms during homologous recombination between two molecules. It is named after Robin Holliday, who proposed it.
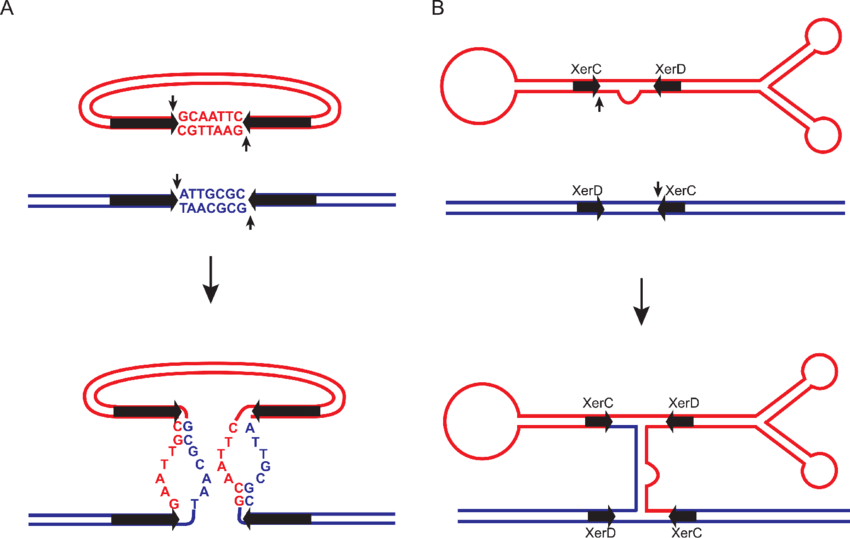
what is site specific recombination (overview)?
2. Site-Specific Recombination
“Site-specific recombination” involves the exchange of DNA segments (recombination) at specific, short DNA sequences (recognition sites)
-mediated by specialized enzymes called recombinases (e.g., integrases, resolvases). It does not require extensive homology.
The recombinase enzyme recognizes DNA sequence
The recombinase enzyme binds to the specific DNA sequence on both molecules.
The two recognition sites are brought together in a complex.
The recombinase cleaves the DNA at precise points within the recognition sites
recombinase catalyzes the reciprocal exchange of DNA strands.
DNA ligase is the enzyme that rejoins the broken DNA ends by catalyzing the formation of a phosphodiester bond to seal the sugar-phosphate backbone.
Primary Function:
Viral Integration: The insertion of a bacteriophage (like lambda phage) genome into a specific site in the bacterial chromosome.
Resolution of Co-integrates: Separating intertwined DNA molecules that result from transposition or replication.
Gene Expression Regulation: Controlling gene expression by inverting or excising DNA segments (e.g., phase variation in Salmonella to switch flagellar proteins).
Assembly of Immune System Genes: In vertebrates, V(D)J recombination uses a site-specific mechanism to generate antibody diversity.
Key Organisms: Common in viruses and bacteria; also occurs in eukaryotes for specific functions like immune system development
what is site specific recombination?
“Site-specific recombination” involves the exchange of DNA segments (recombination) at specific, short DNA sequences (recognition sites)
which enzymes mediate site-specific recombination?
recombinases (basically does the whole of site-specific recombination except in the end, where DNA ligase seals the strands)
The name perfectly captures its role:
It takes existing DNA sequences.
It breaks them at specific sites.
It rejoins them in a new combination.
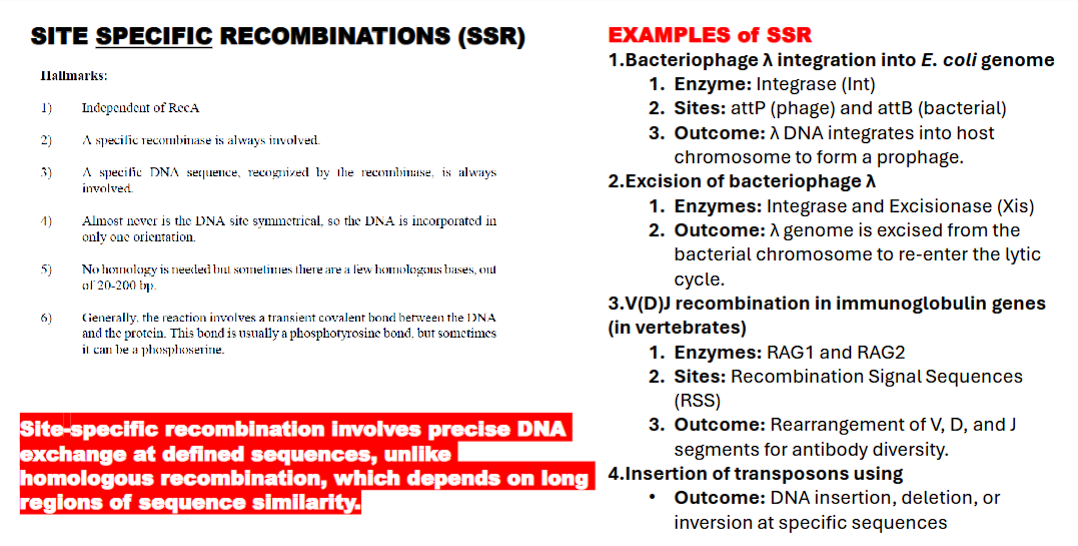
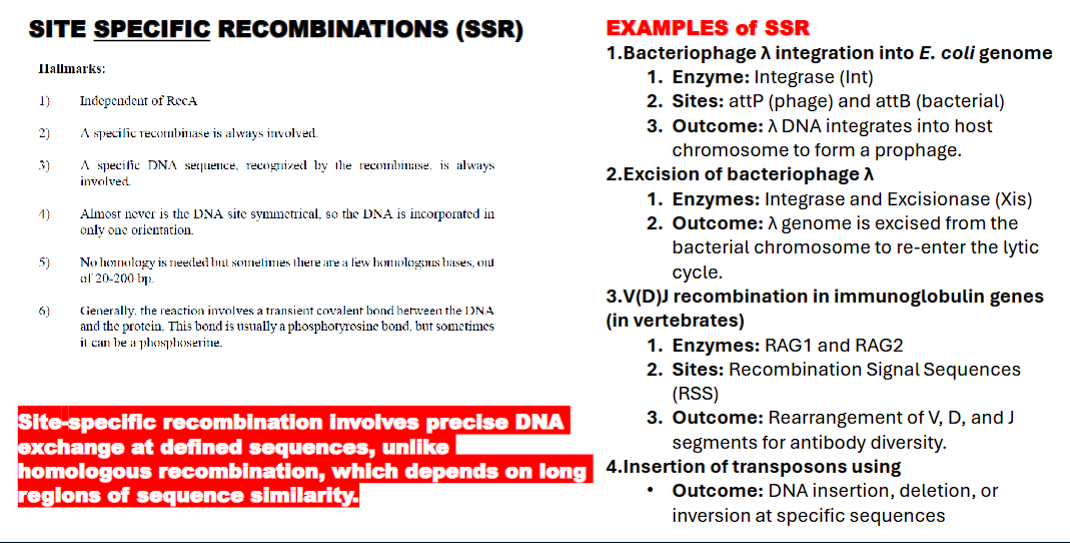
hallmarks of site specific recombination (SSR)
independent of RecA
recombinase always involved
a specific dna sequence recognized by recombinase is always involved
the dna is NEVER symmetrical
no homologous strand is needed
reaction involves transient covalent bond between DNA and the protein. usually a phototyrosinic bond or a photoserine.
what is the difference between site specific recombination and SSR?
site specific recombination involves precise DNA exchange at defined sequences, UNLIKE HOMOLOGOUS recombination
homologous recombination depends on long regions of sequence similarity.
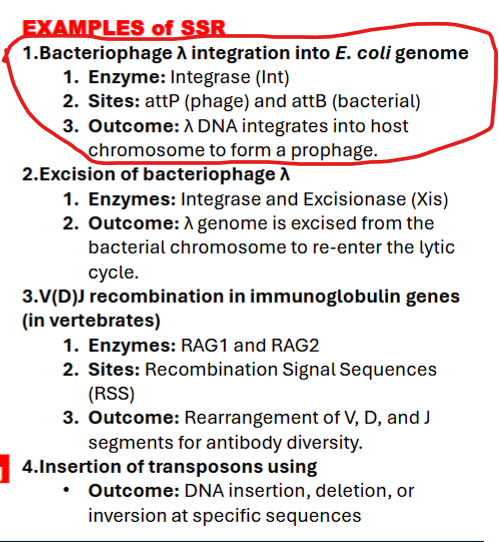
what are examples of SSR?
bacteriophage lambda integration into e.coli
exicision of bacteriophase lambda
V(D)J recombination in immunoglobulin genes
(in vertebrates)
Insertion of transposons using
what are examples of SSR (bacteriophase lambda integration into e.coli mechanism)?
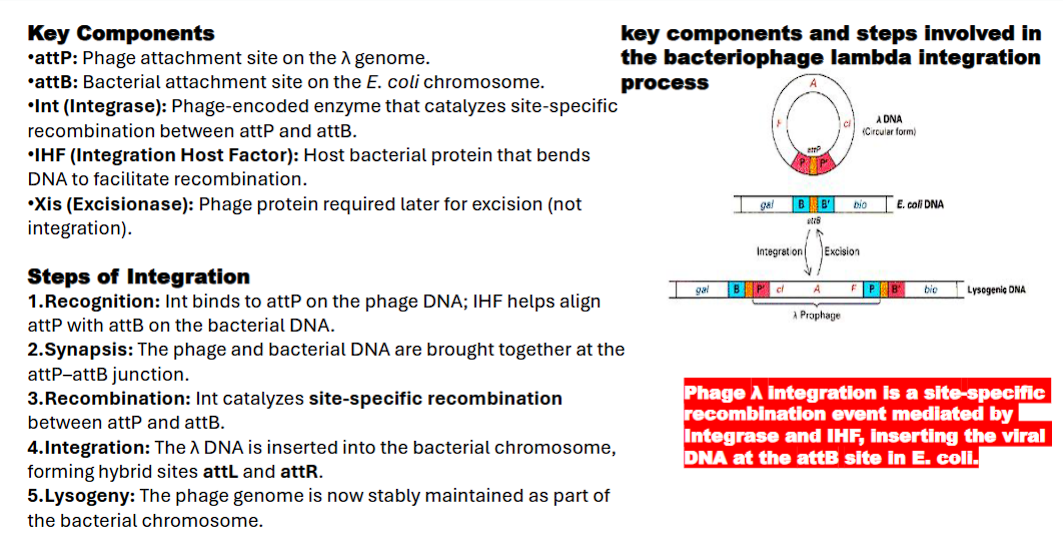
1. Bacteriophage λ Integration into E. coli Genome
Goal: The lambda virus, after infecting the bacterium, needs to become a permanent, passive part of the bacterial chromosome (a "prophage") to lie dormant.
attP: Phage attachment site on the λ genome
attB: Bacterial attachment site on the E. coli chromosome.
Int (Integrase): Phage-encoded enzyme that catalyzes site-specific recombination between attP and attB.
IHF (Integration Host Factor): Host bacterial protein that bends DNA to facilitate recombination.
Xis (Excisionase): Phage protein required later for excision (not integration)
Step 1: Recognition
1. Integrase and Integration host factor proteins bind to the attP site on the phage DNA. IHF bends the DNA, forming a precise nucleoprotein structure called the intasome. This complex is now "primed" and ready to find the target.
Step 2: Synapsis
The intasome complex searches for and captures the bacterial attB site. The two DNA molecules are brought together in a specific alignment at the attP–attB junction.
Step 3: Recombination
The Integrase enzyme catalyzes the recombination:
It makes staggered cuts (like an offset pair of scissors) in the
attPandattBsequences.It exchanges the DNA strands between the phage and bacterial chromosomes.
It reseals the breaks by forming new phosphodiester bonds.
Step 4: Integration
The exchange is complete. The phage DNA is now physically integrated into the bacterial chromosome. The original attP and attB sequences are gone. In their place, two new "hybrid" sites are created:
attL(Left hybrid site)attR(Right hybrid site)
These new sites are crucial because they are the ones recognized by the Int/Xis complex for excision later.
Step 5: Lysogeny
The phage genome, now called a prophage, is stably maintained as part of the bacterial chromosome. It is replicated passively every time the host cell divides. A single phage repressor protein is produced that keeps the viral genes silent, maintaining this dormant state.
what are examples of SSR (Excision of bacteriophage λ)
2. Excision of Bacteriophage λ
Goal: Later, under stress (e.g., UV light), the dormant virus decides to wake up, replicate, and burst out of the cell. To do this, it must first cut itself out of the chromosome.
A dormant virus refers to a virus that remains inactive or hidden within the body of a host without causing any active symptoms or disease
Mechanism:
A second viral protein, excisionase (Xis), is produced. Xis teams up with the integrase (Int) already present.
Together, the Int-Xis complex recognizes the new hybrid sites (
attLandattR) that were created during integration and reverses the process.
Outcome: The viral DNA is precisely excised from the host chromosome, restoring the original
attPandattBsites and freeing the phage to begin its lytic cycle.
what are examples of SSR (V(D)J recombination in immunoglobulin genes)
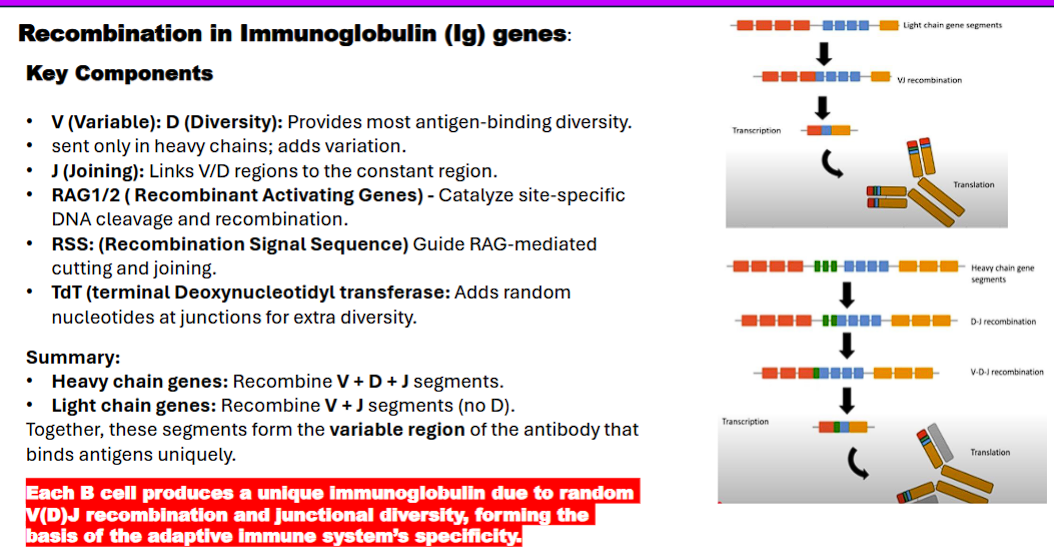
3. V(D)J Recombination in Immunoglobulin Genes
Goal: Each B cell produces a unique immunoglobulin due to random V(D)J recombination and junctional diversity, forming the basis of the adaptive immune system’s specificity
Detailed Explanation of Key Components
V (Variable), D (Diversity), J (Joining) Segments: These are the "parts" in your genetic toolbox.
Your genome has multiple copies of each type (e.g., ~65 V segments, ~27 D segments, ~6 J segments for the human heavy chain).
Heavy Chain: The process combines one V, one D, and one J segment.
Light Chain: The process combines one V and one J segment (no D segments). This two-chain structure (heavy + light) creates the unique antigen-binding site.
RAG1/RAG2 (Recombinase): These are the "molecular scissors and glue" that perform the cut-and-paste operation. They are only active in developing B and T cells.
They search the DNA for specific signals to know where to cut.
RSS (Recombination Signal Sequence): This is the "instruction manual" for the RAG scissors. Each V, D, and J segment is flanked by an RSS.
The RSS ensures that a V segment only joins to a D or J segment, and not randomly to another part of the genome.
TdT (Terminal Deoxynucleotidyl Transferase): This is the "editing tool" that introduces randomness.
After RAG makes the cut, the DNA ends are messy. TdT randomly adds and removes nucleotides at these junctions before they are sealed.
This process, called Junctional Diversity, is the single biggest source of antibody variation. It can completely change the amino acid sequence at the critical center of the binding site.
The Step-by-Step Process (for a Heavy Chain)
Commitment: A developing B cell decides to rearrange its immunoglobulin genes.
D-J Joining: First, one D segment and one J segment on the heavy chain locus are chosen at random
D segment and J segment are joined together by the RAG complex.
V-DJ Joining: Next, one V segment is chosen and joined to the pre-formed DJ segment.
Junctional Diversity: During the joining process, TdT adds and removes random nucleotides (N-nucleotides) at the junctions between V, D, and J.
Testing: If the rearrangement is successful and produces a functional protein, the cell moves on. If not, it attempts to rearrange the gene on the other chromosome.
Light Chain Rearrangement: The same process happens for the light chain (kappa first, then lambda if kappa fails), combining a V and J segment.
Outcome: The deletion of intervening DNA and the inversion/joining of specific gene segments to create a unique, functional antibody gene. This is a programmed, developmentally controlled use of SSR.
what is the difference between heavy chain genes and light chain genes?
Summary:
• Heavy chain genes: Recombine V + D + J segments.
• Light chain genes: Recombine V + J segments (no D).
Together, these segments form the variable region of the antibody that
binds antigens uniquely.
what are examples of SSR
4. Insertion of Transposons
Important Clarification: This example is a bit of a gray area. "Classic" transposition (like "cut-and-paste") is often categorized separately from SSR because the target site is often random, not specific.
The Link to SSR: However, the integration step for some elements (particularly retrotransposons and some complex transposons) is carried out by an integrase enzyme that acts in a site-specific manner. Furthermore, the transposase enzyme itself acts on specific sequences at the ends of the transposon.
Outcome: As you stated, this can lead to DNA insertion, deletion, or inversion at or near specific sequences.
what is transposition (jumping genes)?
3. Transposition (Transpositional Recombination)
Transposition, often called "jumping genes," is the movement of a discrete DNA segment (a transposon or transposable element) from one location in the genome to another. It is a form of recombination because it integrates into a new site, but it is fundamentally different from HR and site-specific recombination.
Mechanism: Mediated by an enzyme called transposase. The key feature is that the transposase acts only on the ends of its own transposon.
"Cut-and-Paste" Transposition: The transposon is excised from its original location and inserted into a new, often random, target site.
"Copy-and-Paste" Transposition (Retrotransposition): The transposon is transcribed into RNA, which is then reverse-transcribed back into DNA by a reverse transcriptase, and the new DNA copy is inserted into a new genomic location. This is how retrotransposons (like LINEs and SINEs in humans) proliferate.
Primary Function:
Selfish DNA Propagation: The primary "function" is the propagation and survival of the transposable element itself within the genome.
Genome Evolution: A major driver of mutation, gene duplication, and genome expansion and rearrangement. It is a significant source of genetic diversity and disease.
Key Organisms: Found in all organisms. A huge portion of the human genome (~45%) is comprised of transposable elements or their remnants.
what is transposition (jumping genes)?
Transposition, often called "jumping genes," is the movement of a discrete DNA segment (a transposon or transposable element) from one location in the genome to another. It is a form of recombination because it integrates into a new site, but it is fundamentally different from HR and site-specific recombination.
what enzymes mediates transposition?
transposase: cut the transposon out of its original location and paste it into a new target site (CUT and PASTE transposition)
integrase: The enzyme that inserts a DNA copy of a retroelement (like a retrotransposon or a retrovirus) into a host genome. (for COPY and PASTE/ retrotransposition)
cut and paste transposition (class I)
"Cut-and-Paste" Transposition:
transposase exices the transposon from its original location.
transposase inserts the transposon into a new, often random, target site.
copy and paste transposition (retrotransposition) (class II)
integrase, not transposase
"Copy-and-Paste" Transposition (Retrotransposition):
The transposon (DNA) is transcribed into RNA
reverse transcriptase reverse-transcribes the RNA back into DNA
integrase inserts the new DNA copy is inserted into a new genomic location.
This is how retrotransposons (like LINEs and SINEs in humans) proliferate.
Integrase: The enzyme that inserts a DNA copy of a retroelement (like a retrotransposon or a retrovirus) into a host genome.
what is a similarity between all of these types of dna recombination?
complex structure intermediate
what are consequences of the dynamic nature of the chromosome (recombination)
1) a source of genetic diversity is created.
2) DNA can be repaired
3) gene expression is modified
4) some programmed gene arrangements can occur during development.
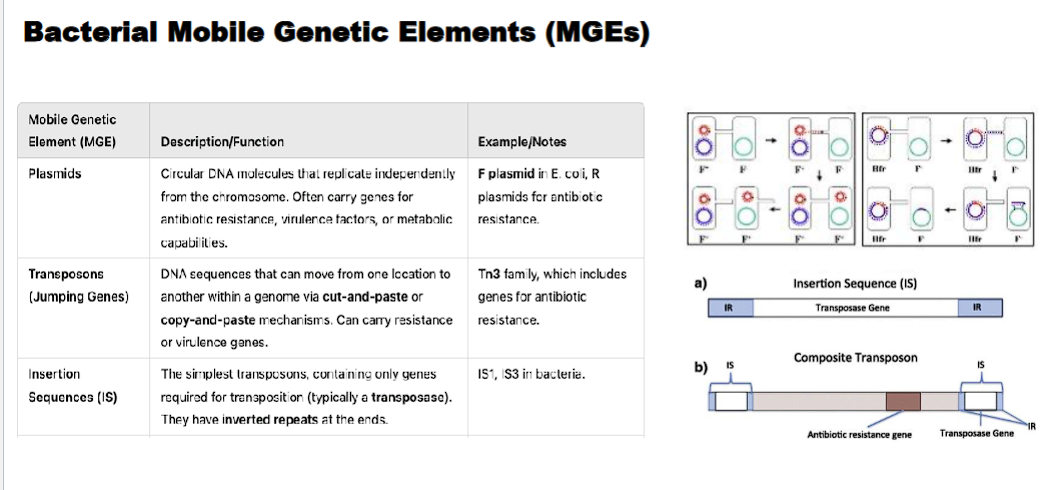
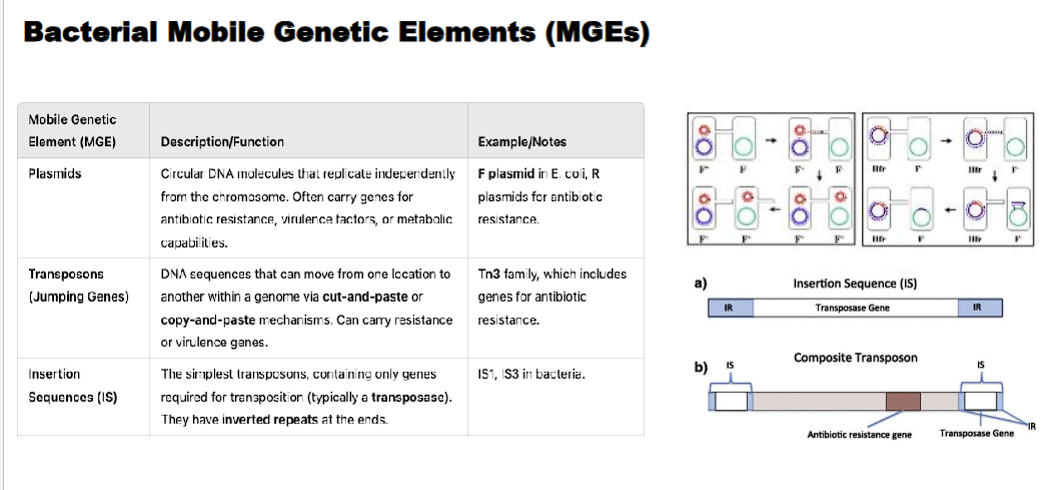
Recognize the structure and function of the different mobile genetic elements.
Mobile genetic elements (MGEs), often called "jumping genes," are DNA sequences that can move from one location to another within a genome. They are fundamental drivers of genome evolution and plasticity.
Here is a breakdown of the major classes, their structure, and their functions.
Overview: Major Classes of Mobile Genetic Elements
MGEs can be broadly categorized by their mechanism of movement:
Class II: Transposons - Move via a "cut-and-paste" or "copy-and-paste" mechanism directly as DNA.
Class I: Retrotransposons - Move via a "copy-and-paste" mechanism using an RNA intermediate.
Other Elements: Including Insertion Sequences (the simplest MGEs) and Integrons (gene capture systems).
1. Transposons (Class II)
These are often called "DNA transposons" and move without an RNA intermediate.
Structure:
Core Component: A gene encoding the enzyme Transposase.
Recognition Sequences: Short, inverted repeat (IR) sequences flanking the transposase gene. The transposase recognizes and binds to these IRs to initiate excision.
Composite Transposons: Two insertion sequences (IS elements) flanking one or more accessory genes (e.g., for antibiotic resistance).
Function & Mechanism:
Mechanism: "Cut-and-Paste" Transposition.
The transposase enzyme binds to the inverted repeats at the ends of the transposon.
It cuts the transposon out of its original donor site in the DNA.
It inserts it into a new target site in the genome.
Genetic Consequences:
Mutation: Insertion into a gene can disrupt its function.
Genome Rearrangements: The excision and insertion process can cause deletions, inversions, or duplications of adjacent DNA.
2. Retrotransposons (Class I)
These are the most abundant MGEs in many eukaryotic genomes (e.g., over 40% of the human genome). They move via an RNA intermediate that is reverse-transcribed into DNA.
There are two main types:
A. LTR Retrotransposons (Long Terminal Repeat)
Structure:
Long Terminal Repeats (LTRs): Direct repeat sequences at both ends that contain promoters and regulatory signals.
Internal Genes: Typically include:
gag: Codes for structural proteins of the virus-like particle.
pol: Codes for a polyprotein containing Reverse Transcriptase (makes DNA from RNA) and Integrase (inserts the DNA copy into the genome).
Function & Mechanism: They resemble retroviruses but lack an env gene for leaving the cell. Their mechanism is "Copy-and-Paste":
The element is transcribed into RNA.
Reverse Transcriptase uses the RNA as a template to synthesize a double-stranded DNA copy.
Integrase inserts this new DNA copy into a random site in the genome.
B. Non-LTR Retrotransposons
Structure:
No Long Terminal Repeats.
Internal Genes: Often contain genes for proteins like ORF1p (RNA-binding protein) and ORF2p (which has both Reverse Transcriptase and Endonuclease activity).
Poly-A Tail: The DNA copy ends with a poly-A tail, just like the mRNA it was copied from.
The most common type in humans is the LINE (Long Interspersed Nuclear Element).
Function & Mechanism:
LINEs are transcribed and translated.
The ORF2p protein nicks the target DNA.
It uses the nicked DNA to prime reverse transcription of the LINE RNA, directly synthesizing DNA at the insertion site ("target-primed reverse transcription").
A Special Case: SINEs (Short Interspersed Nuclear Elements)
Structure: Short sequences (e.g., Alu elements in humans) that do not encode their own reverse transcriptase or integrase.
Function & Mechanism: SINEs are parasites of the LINE machinery. They are transcribed by RNA Polymerase III, and they hijack the enzymes encoded by LINEs to reverse transcribe and integrate themselves back into the genome.
3. Insertion Sequences (IS Elements)
These are the simplest autonomous MGEs, found primarily in bacteria.
Structure:
Only two components:
A gene encoding a Transposase.
Short Inverted Repeat (IR) sequences flanking the transposase gene.
Function:
They are the basic unit of transposition in bacteria.
They are the building blocks for composite transposons (where two IS elements flank a gene).
4. Integrons
These are sophisticated "gene capture and expression systems" found in bacteria, often located on plasmids and transposons. They are a major driver of the spread of antibiotic resistance.
Structure:
intI gene: Encodes an Integrase enzyme.
attI site: A specific attachment site where new gene cassettes are inserted.
Promoter (Pc): A promoter that drives expression of the captured genes.
Function & Mechanism:
Integrons can capture promoter-less gene cassettes (often antibiotic resistance genes) from the environment.
The integrase enzyme catalyzes the site-specific recombination of the cassette into the attI site.
The promoter (Pc) then allows for the expression of the newly captured gene.
Summary Table: Structure and Function
Mobile Element | Key Structural Features | Mechanism of Movement | Primary Genetic Consequence |
|---|---|---|---|
Transposon | Transposase gene, Flanking Inverted Repeats (IR) | Cut-and-Paste (DNA intermediate) | Gene disruption, small deletions/insertions. |
LTR Retrotransposon | Long Terminal Repeats (LTRs), gag, pol (Reverse Transcriptase, Integrase) | Copy-and-Paste (RNA intermediate) | Genome expansion, new regulatory influences. |
Non-LTR Retrotransposon (LINE) | No LTRs, ORF1p, ORF2p (Reverse Transcriptase/Endonuclease) | Copy-and-Paste (RNA intermediate, target-primed) | Genome expansion, provides machinery for SINEs. |
SINE | Short, no coding capacity, internal promoter, Poly-A tail | Copy-and-Paste (hijacks LINE machinery) | Genome expansion, gene disruption. |
Insertion Sequence (IS) | Transposase gene, Flanking Inverted Repeats (IR) | Cut-and-Paste | Simple insertion mutagenesis, forms composite transposons. |
Integron | intI (integrase), attI site, Promoter (Pc) | Site-Specific Recombination | Capture and spread of antibiotic resistance genes. |
In summary, mobile genetic elements are powerful evolutionary forces. They can disrupt genes and cause disease, but they are also a major source of genetic diversity, new regulatory sequences, and genome evolution, shaping the genomes of all living organisms.
MGE (mobile genetic elements)/ Jumping Genes
Mobile genetic elements (MGEs), often called "jumping genes," are DNA sequences that can move from one location to another within a genome. They are fundamental drivers of genome evolution and plasticity.
-plasmids
-transposons
-insertion sequences
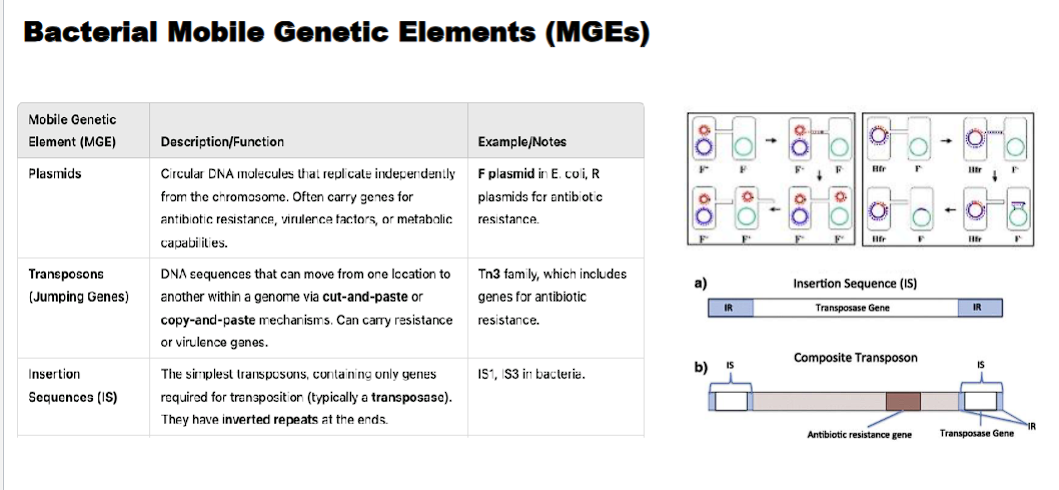
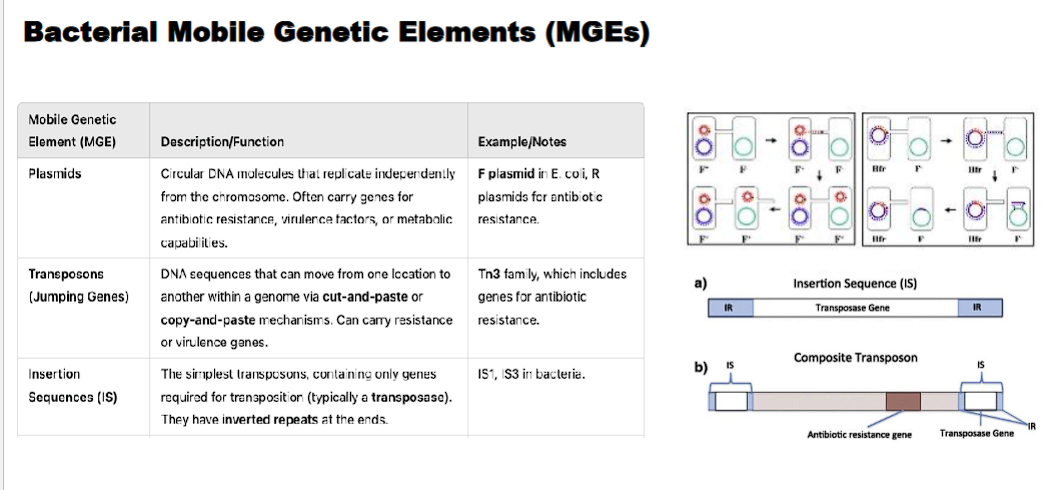
plasmid
circular dna molecules that replicate independently from the chromosome.
often carry genes for antibiotic resistance, virulence factors, or metabolic capabilities.
transposon (jumping genes)
DNA sequences that can move from one location to another within a genome via cut-and-paste (class II) OR copy and paste (class I) mechanisms. can carry resistance or virulence genes.
insertion sequences
An Insertion Sequence (IS) is a short segment of DNA that acts as a simple transposable element. It contains only the genetic information necessary for its own movement (transposition) within a genome.
Minimalist Structure: An IS element consists of two essential components:
A Transposase Gene: A single gene that codes for the transposase enzyme. This enzyme recognizes the ends of the IS and catalyzes the "cut-and-paste" movement of the entire element.
Inverted Repeats (IRs): Short, repeated sequences (15-25 bp) at both ends of the element. These sequences are perfect or near-perfect reverses of each other. They are the recognition sites for the transposase enzyme.
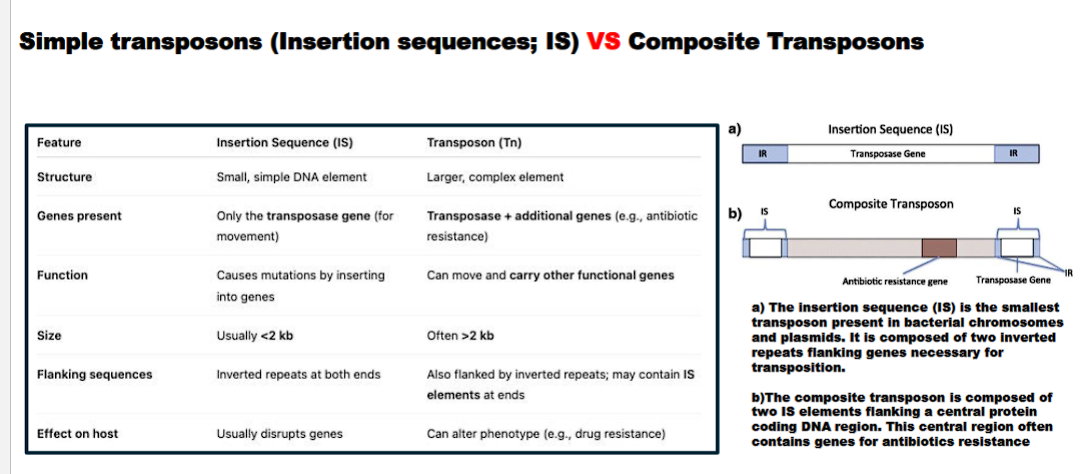
what is the difference between an insertion sequence and composite transposon
Feature | Insertion Sequence (IS) | Composite Transposon (Tn) |
|---|---|---|
Structure | Minimal: Only Transposase + Inverted Repeats | Two IS elements flanking additional genes (e.g., for antibiotic resistance). |
Carries Extra Genes? | No. | Yes. (e.g., antibiotic resistance, toxin production). |
Function | Self-propagation; "selfish DNA." | Self-propagation + conferring new traits to the host bacterium. |
insertion sequences usually disrupt genes
composite transposon can alter the phenotype
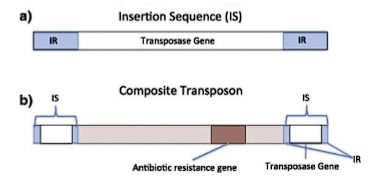
type of transposable element
simple transposon
complex transposon
retrotransposon
jumping genes in humans
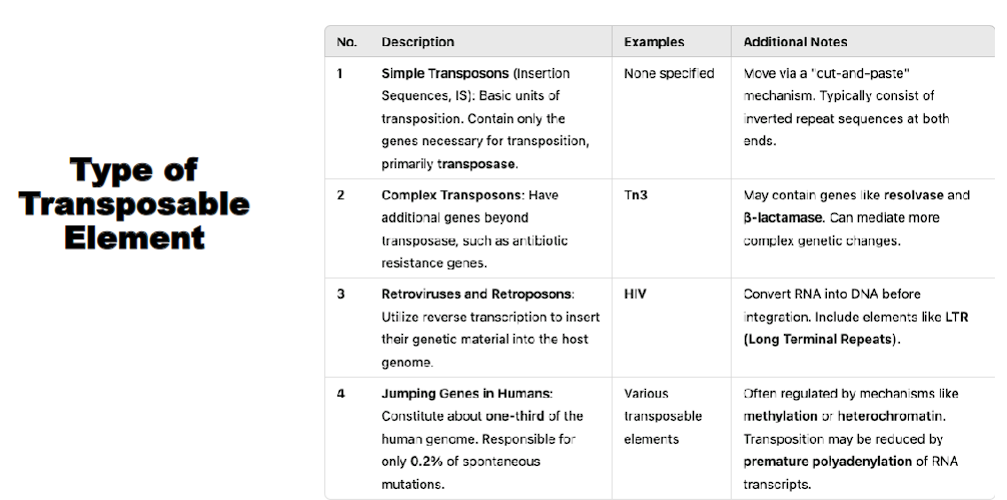
simple transposon
“simple” contain only the genes necessary for transposition
ex: insertion sequence
complex transposon
“complex” have the genes necessary for transposition AND ADDITIONAL genes beyond transposase, such as antibiotic resistance genes
retrotransposon
utilize reverse transcription to insert their genetic material into the host genome
jumping genes
MGE categorization (Class II, Class I, other elements)
Overview: Major Classes of Mobile Genetic Elements
MGEs can be broadly categorized by their mechanism of movement:
Class II: Transposons - Move via a "cut-and-paste" or "copy-and-paste" mechanism directly as DNA.
trans: cross over to the other side
poson: to place
Class I: Retrotransposons - Move via a "copy-and-paste" mechanism using an RNA intermediate.
Literal Meaning: "To place across, via a backwards step."
This etymology reveals the key mechanistic difference. A retrotransposon does not move directly. Its movement is "backwards" in terms of the Central Dogma of molecular biology (DNA → RNA → Protein).
Other Elements: Including Insertion Sequences (the simplest MGEs) and Integrons (gene capture systems).
Describe the function of RecA in homologous recombination and in the SOS response.
RecA is a quintessential multifunctional protein in bacteria, playing two critical and interrelated roles
1. RecA is the central catalyst of homologous recombination and
RecA is thekey regulator of the SOS response to DNA damage.
Its function can be summarized as a DNA-dependent ATPase that forms a nucleoprotein filament on single-stranded DNA (ssDNA), and this filament is the active structure for both processes.
1. Function of RecA in Homologous Recombination
Homologous recombination is a fundamental process for repairing double-stranded DNA breaks and exchanging genetic material. RecA's role is to facilitate the central step: strand invasion.
The Process:
Initiation and Processing:
A double-strand break occurs or a replication fork stalls.
Nucleases (like RecBCD in E. coli) resect the ends, generating 3' single-stranded DNA (ssDNA) tails.
Formation of the RecA Filament (The Key Step):
RecA protein, in its active form (bound to ATP), polymerizes cooperatively onto the ssDNA, forming a right-handed helical filament. This filament is often called the presynaptic filament.
This filament stretches the DNA, making it about 1.5 times longer than standard B-DNA. This is crucial for the next step.
Homology Search and Strand Invasion:
The RecA-ssDNA filament scans the intact, double-stranded donor DNA for a homologous sequence (a matching sequence).
Upon finding a homologous region, the filament catalyzes strand invasion: the ssDNA displaces one strand of the donor duplex and pairs with its complementary strand.
This forms a critical intermediate called the D-loop (Displacement loop).
Branch Migration and Resolution:
The RecA filament continues to promote the exchange of DNA strands, a process called branch migration, extending the heteroduplex region (where one strand is from each original DNA molecule).
Finally, other enzymes (e.g., RuvAB, RecG) resolve the resulting cross-shaped structure (Holliday junction) into two separate, repaired DNA molecules.
In summary for recombination: RecA is the matchmaker and catalyst. It finds the matching sequence in a sister chromosome or homologous DNA and drives the strand exchange that is the heart of homologous recombination.
2. Function of RecA in the SOS Response
The SOS response is a global, inducible DNA damage repair system in bacteria. RecA's role here is not enzymatic in the traditional sense, but allosteric—it acts as a cellular alarm signal and co-protease.
The Process:
Activation by DNA Damage:
The same signal that initiates recombination—ssDNA gaps caused by replication forks encountering DNA damage—also activates RecA for its SOS role.
RecA forms its active filament on this ssDNA, but in the context of the SOS response, this filament is now called the RecA filament* ("RecA-star"). The * signifies that it is activated by its association with ssDNA and ATP.
LexA Autocleavage (The "Switch"):
In an undamaged cell, the LexA repressor protein binds to the promoter regions (SOS boxes) of about 40 genes, repressing their transcription. These genes include DNA repair proteins (e.g., UvrA, UvrB for nucleotide excision repair), error-prone translesion polymerases (e.g., UmuC/D, also called DNA Pol V), and RecA itself.
The activated RecA* filament does not cleave LexA itself. Instead, it facilitates the self-cleavage (autoproteolysis) of LexA.
RecA* acts as a catalytic cofactor, inducing a conformational change in LexA that stimulates its latent protease activity to cut itself.
Derepression of SOS Genes:
Cleaved LexA can no longer dimerize or bind DNA.
This de-represses all the SOS genes, leading to a massive surge in the production of DNA repair enzymes.
This includes the error-prone polymerases, which can replicate past damaged DNA bases but at the cost of increased mutations—a last-resort "translesion synthesis" strategy for cell survival.
Switching Off the Response:
Once the DNA damage is repaired, ssDNA gaps are no longer present.
RecA* filaments disassemble, as they lack their ssDNA scaffold.
Without RecA*, LexA no longer undergoes autocleavage. Newly synthesized LexA repressor accumulates and re-binds the SOS boxes, shutting down the response.
In summary for the SOS response: RecA acts as a damage sensor and signal transducer. Its filament form signals the severity of DNA damage and directly triggers the dismantling of the LexA repressor, turning on the cell's emergency repair systems.
Summary Table: The Dual Roles of RecA
Feature | Role in Homologous Recombination | Role in SOS Response |
|---|---|---|
Active Form | RecA-ATP filament polymerized on ssDNA. | RecA-ATP filament polymerized on ssDNA (RecA). |
Primary Action | Catalytic: Actively performs homology search and strand exchange. | Allosteric: Acts as a co-protease to facilitate LexA autocleavage. |
Key Partner | Double-stranded DNA with a homologous sequence. | The LexA repressor protein. |
Biological Outcome | High-fidelity repair of DNA breaks, restoration of replication forks, genetic exchange. | Global induction of diverse DNA repair genes, including error-prone repair (increased mutagenesis). |
Connection | Both roles are triggered by the same signal: the presence of ssDNA, a common intermediate after DNA damage or replication fork stalling. |
In essence, RecA is the master conductor of the bacterial DNA damage response. Its ability to form a specific nucleoprotein filament allows it to perform two distinct but vital functions: precisely mending broken DNA through recombination and sounding the alarm to activate a global, emergency repair network.
what is RecA?
RecA is a multifunctional protein in bacteria, playing two critical and interrelated roles
1. RecA is the central catalyst of homologous recombination
RecA regulator of the SOS response to DNA damage.
Its function can be summarized as a DNA-dependent ATPase that forms a nucleoprotein filament on single-stranded DNA (ssDNA), and this filament is the active structure for both processes.
RecA etymology
RecA is an acronym where:
Rec = Recombination
A = Gene A
1. RecA is the central catalyst of homologous recombination
RecA regulator of the SOS response to DNA damage.
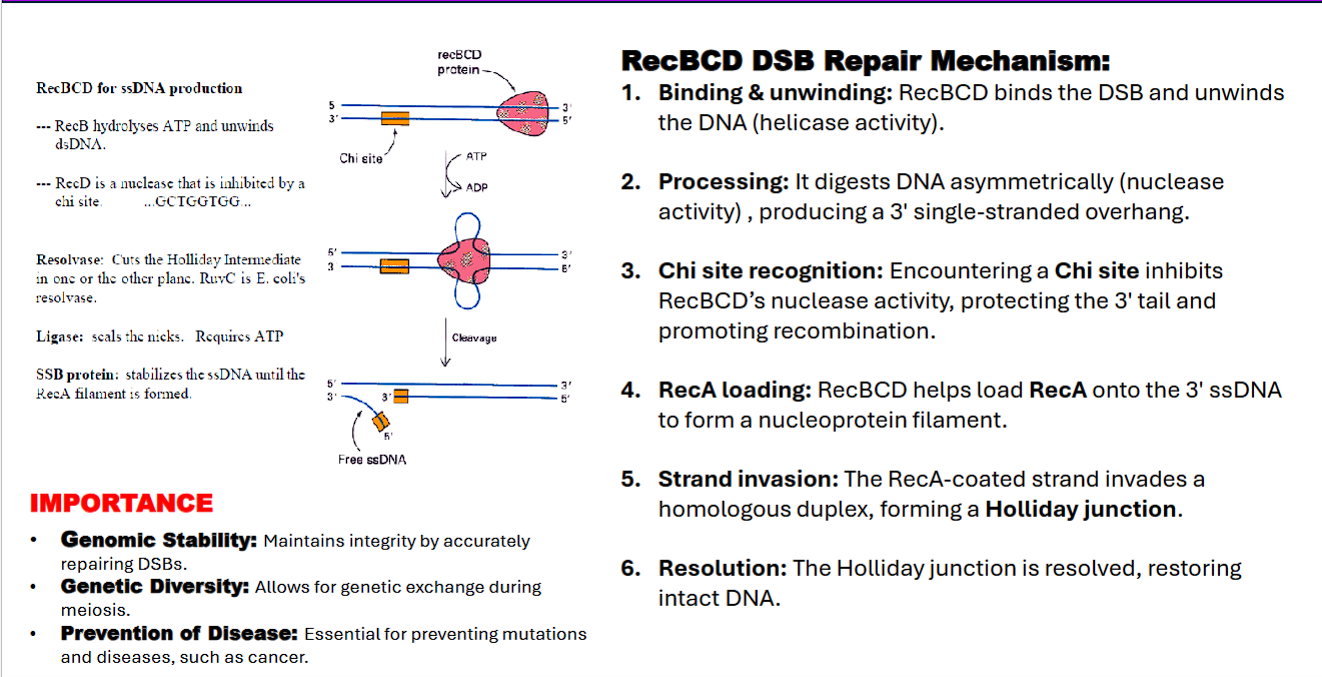
what is the RecBCD and RecA repair mechanism for a double stranded break?
binding
unwind DNA
chi site
Rec A
Holliday Junction
RuvC resolvase
DNA ligase
RecBCD DSB Repair Mechanism:
1. RecBCD binds the DSB
RecBCD hydrolyzes ATP and unwinds DNA (showing helicase activity)
RecBCD digests DNA asymmetrically (nuclease activity) , producing a 3′ single-stranded overhang.
Encountering a Chi site inhibits RecBCD’s nuclease activity, protecting the 3′ tail and promoting recombination.
Chi site: Chi site (Crossover hotspot instigator) is a specific 8-base-pair sequence (5'-GCTGGTGG-3' in E. coli)
RecBCD undergoes a conformational change upon the Chi site, leading to inhibition.
RecBCD helps load RecA onto the 3′ ssDNA to form a nucleoprotein filament.
The RecA-coated ssDNA strand performs strand invasion by invading a homologous duplex.
forming a Holliday junction.
RuvA binds to holiday junction to stabilize it
Ruv B drives branch migration through ATP hydrolysis to facilitate resolution
The Holliday junction is cleaved ("resolved") by enzymes like RuvC resolvase. Depending on how the junction is cut, this results in either crossover or non-crossover products, restoring two intact, double-stranded DNA molecules.
DNA ligase seals the nicks.
RecBCD complex
what does RecB do?
what does RecD do?
waht does RecC do?
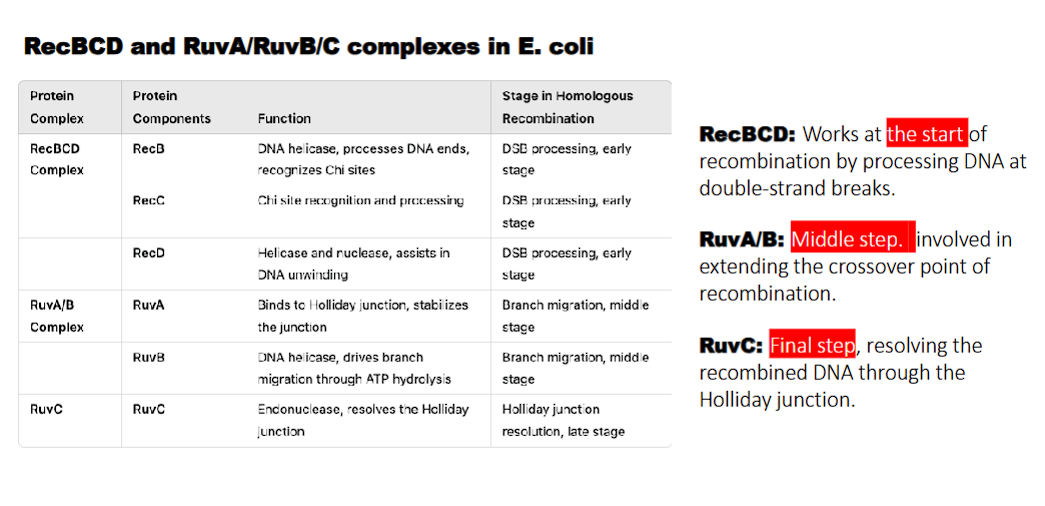
RecB: DNA helicase, processes DNA ends, recognizes Chi sites
RecD: Helicase and nuclease, assists in DNA unwinding
RecC: Chi site recognition
RecBCD: Works at the start of recombination by processing DNA at double-strand breaks.
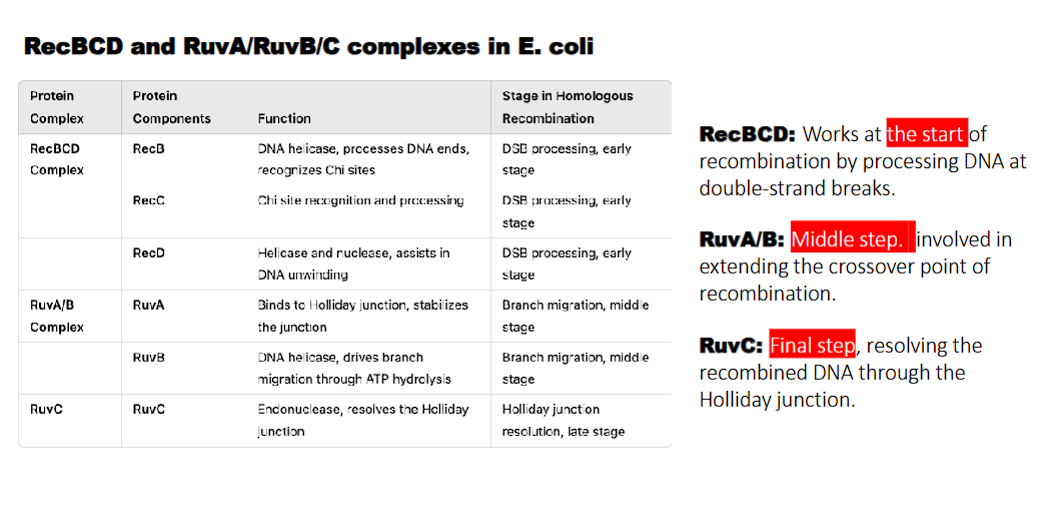
what does RuvA/B do?
RuvA: binds to holliday junction and stabilizes the junction
RuvB: DNA helicase, drives branch migration through ATP hydrolysis
RuvA/B: Middle step. involved in extending the crossover point of recombination.
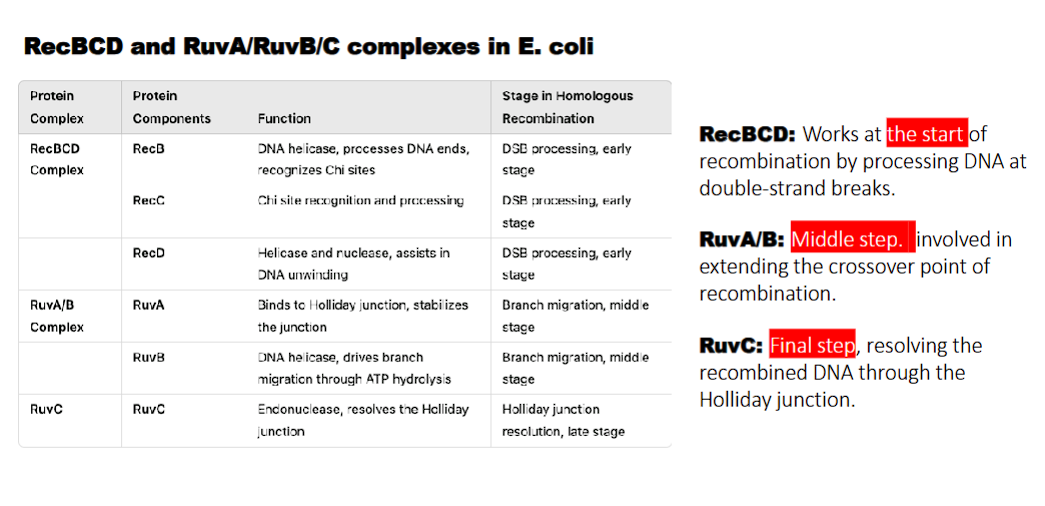
what does RuvC do?
holliday junction resolution
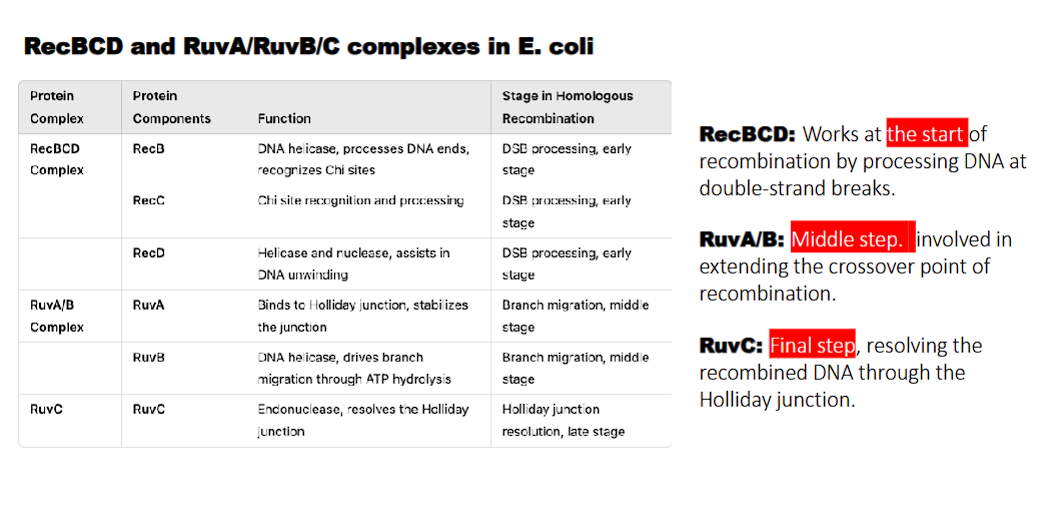
what do the RecBCD and RuvA/RuvB/C complexes in E. coli perform?
homologous recombination
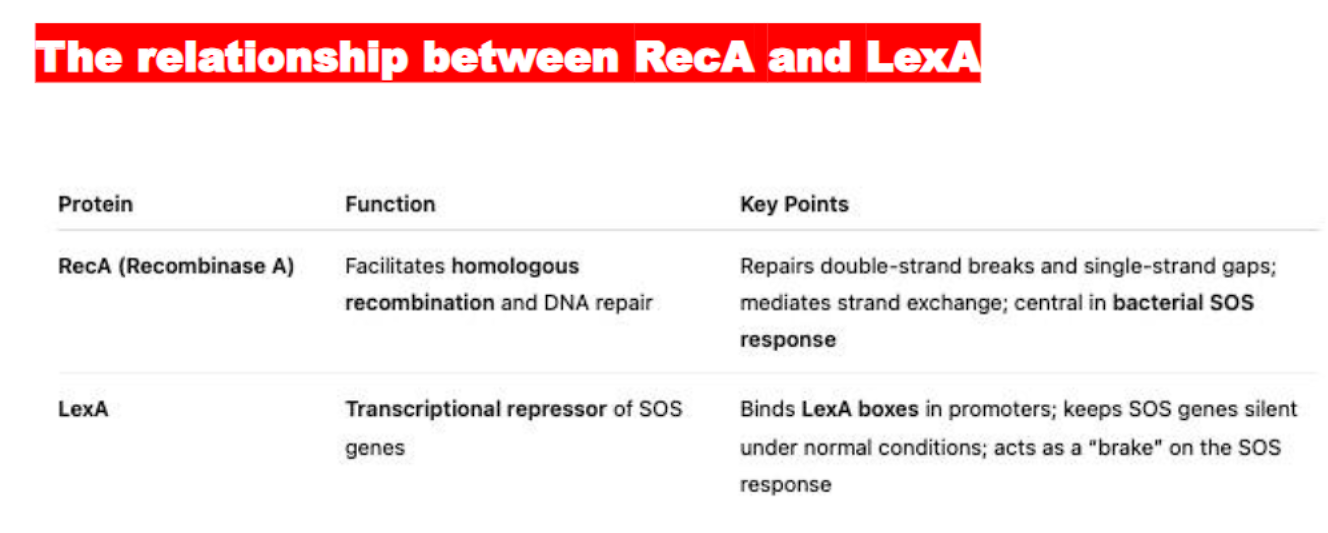
what is the difference and similarity between RecX and LecA?
RecX represses the activity of the RecA protein
LecA represses the EXPRESSION of the Rec A gene, acting as a brake, keeping the SOS response genes silent when DNA is undamaged.

what is the importance of the RecBCD DSB Repair Mechanism?
genomic stability
genomic diversity
prevention of disease

the point: both eukaryotes and prokaryotes have different mechanisms for fixing double stranded breaks for homologous recombination.
what are the differences?
Key Distinction from Eukaryotic HR
It's useful to note how this bacterial pathway parallels but uses different proteins than the eukaryotic pathway you learned about earlier:
Function | Bacteria (RecBCD Pathway) | Eukaryotes (Homologous Recombination) |
|---|---|---|
DSB Recognition/Resection | RecBCD (helicase/nuclease) | MRN Complex + Other Nucleases (e.g., Exo1, Dna2) |
ssDNA Protection | SSB protein | RPA |
Strand Invasion Filament | RecA | Rad51 |
Mediator for Loading | RecBCD itself (post-Chi) | BRCA2 and others |
Holliday Junction Resolution | RuvC | GEN1, MUS81-EME1 etc. |
1. Function of RecA in Homologous Recombination (only found in prokaryotes)
Binds single-stranded DNA (ssDNA) generated at a double-strand break
Forms a nucleoprotein filament on ssDNA
Mediates strand invasion into a homologous DNA duplex to initiate homologous recombination. (formation of D-loop)
Facilitates the formation of a Holliday junction, which is later resolved to repair the break
RecA also plays a critical role in the bacterial SOS response
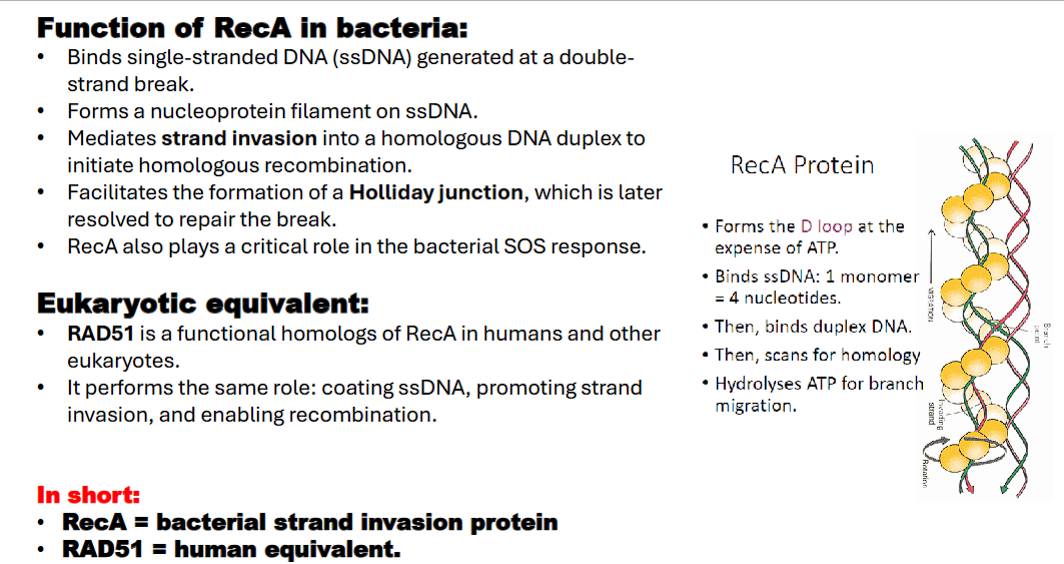
what is the eukaryotic equivalent of RecA?
*remember, RecA is found in prokaryotes, so the eukaryotic equivalent is….
Rad51 (go back to flashcard 13)
Rad51 coats ssDNA, enabling strand invasion
RecA Mediates strand invasion into a homologous DNA duplex to initiate homologous recombination. (formation of D-loop)
RecBCD complex
RecB: DNA helicase, processes DNA ends, recognizes Chi sites
RecC: Chi recognition and processing
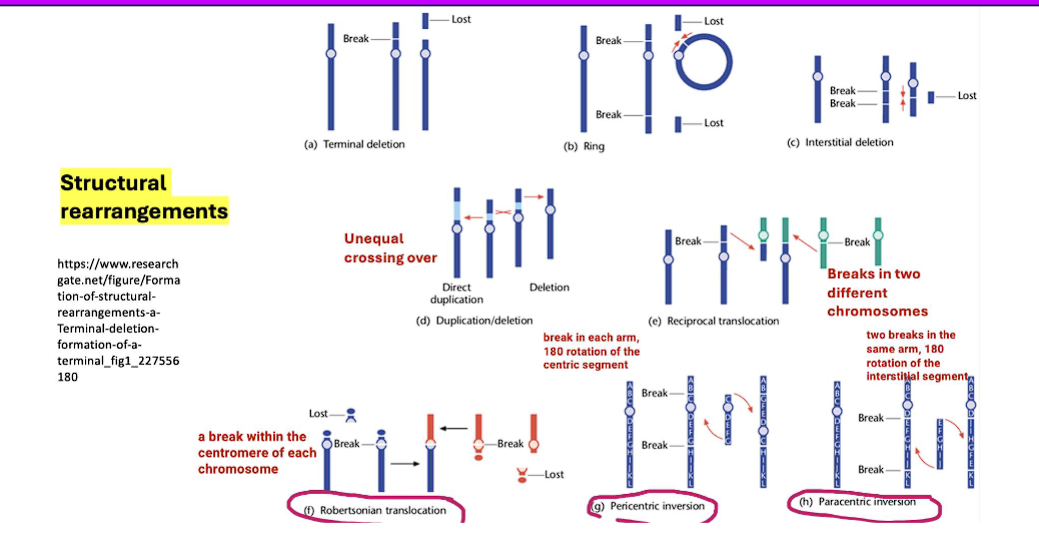
what are the different types of rearrangements?
1) Translocations
2) Inversions
3) Deletions
4) Duplications
what are the different types of rearrangements?
1) Translocations: Robertsonian translocation is a specific and common type of translocation
2) Inversions: Pericentric inversion and c) Paracentric inversion are the two types of inversions.
3) Deletions
4) Duplications
translocation
1. Translocations
A translocation occurs when a segment of one chromosome is transferred to a non-homologous chromosome (a different chromosome).
Reciprocal Translocation: Two non-homologous chromosomes swap segments. This is the most common type.
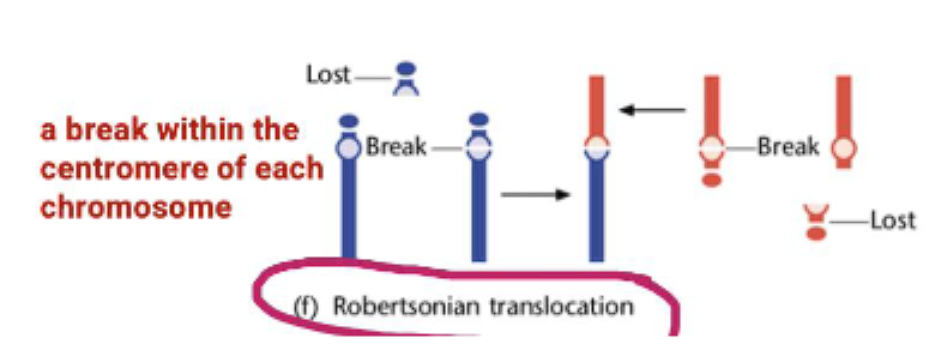
Robertsonian Translocation: A special type where two acrocentric chromosomes (chromosomes with the centromere near the end) break at the centromere and their long arms fuse. This results in a single, large chromosome and the loss of the two short arms (which typically contain non-essential rRNA genes).
robertsonian translocation
Robertsonian Translocation: A special type where two acrocentric chromosomes (chromosomes with the centromere near the end) break at the centromere and their long arms fuse. This results in a single, large chromosome and the loss of the two short arms (which typically contain non-essential rRNA genes).
inversion
paracentric inversion
pericentric inversion
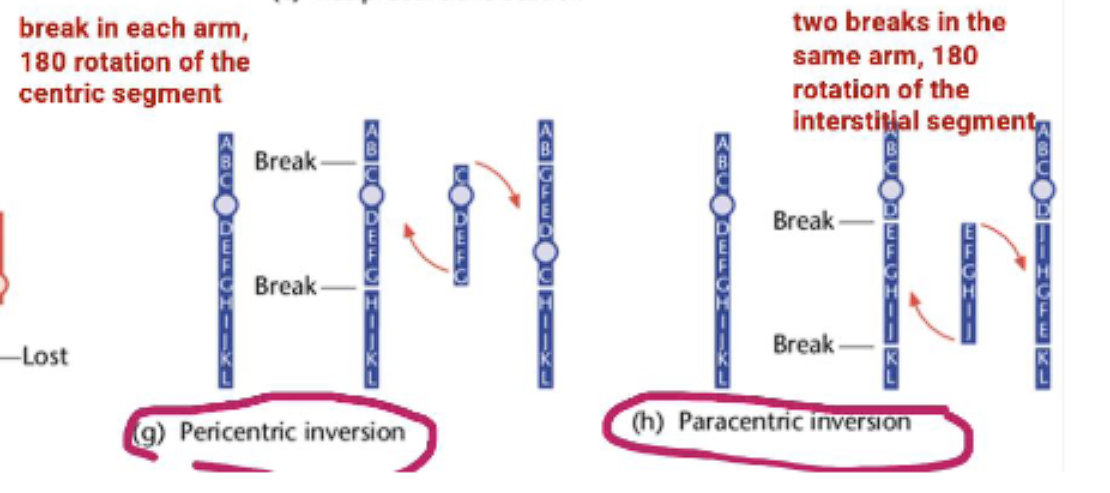
2. Inversions
An inversion occurs when a segment of a chromosome is reversed end-to-end. The genetic material is present, but the order is flipped.
Paracentric Inversion: The inverted segment does NOT include the centromere. (Remember: Paracentric = Parallel to the centromere, but not including it).
para= beside the centromere, not including it
Pericentric Inversion: The inverted segment DOES include the centromere. (Remember: Pericentric = around the Perimeter, including the centromere).
peri= including the centromere.
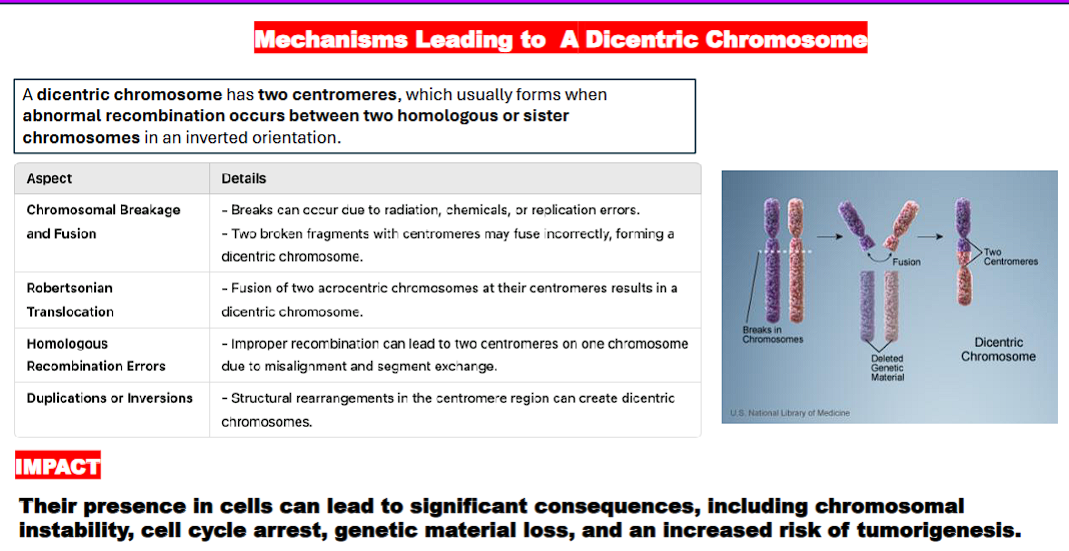
mechanisms leading to a dicentric chromosome (bad)
dicentric chromosome= a chromosome with two centromeres
2 chromosomes
a break in each chromosome caused by radiation, chemicals, or replication errors
2 chromosome ends with centromeres
2 chromosome ends with centromeres fuse together to create a dicentric chromosome
a dicentric chromosome can come about through different ways such as:
chromosomal breakage and fusion
Robertsonian translocation
homologous recombination errors
duplications or inversions.
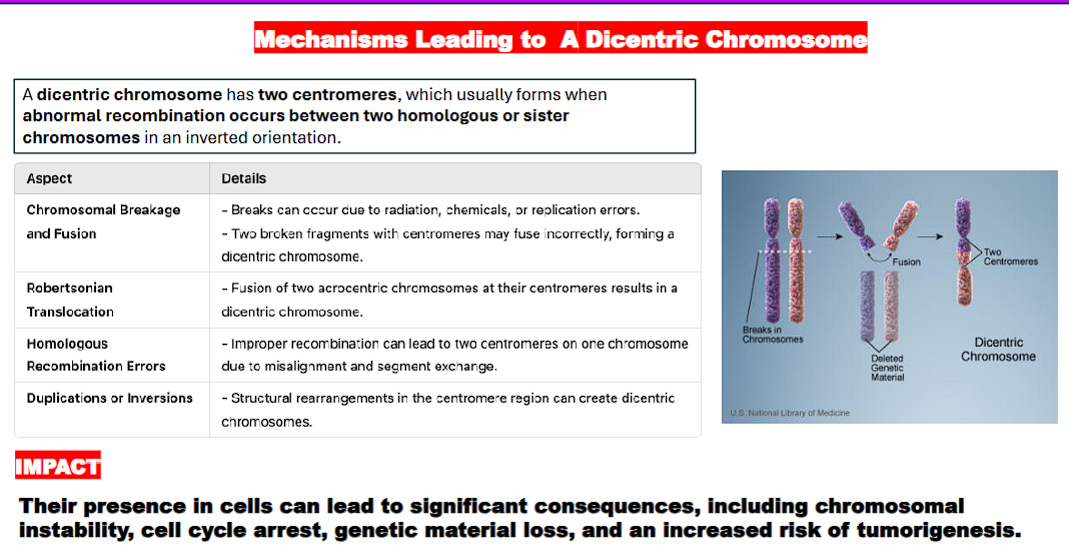
what are the consequences of dicentric chromosomes?
chromosomal instability,
cell cycle arrest,
genetic material loss, and an
increased risk of tumorigenesis (the process by which normal cells transform into cancer cells, leading to the formation of tumors).
mechanisms of DNA structural rearrangements
non-homologous end joining (NHEJ)
homologous recombination (HR)
transposition
replication slippage
unequal crossing over
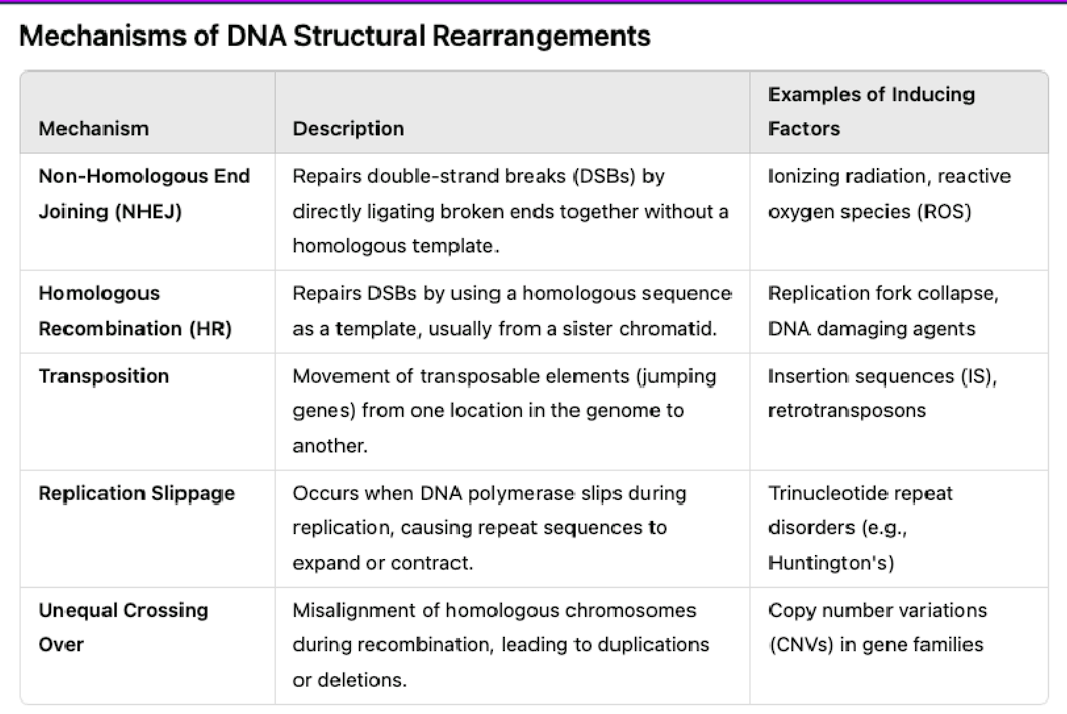
what is the first mechanism of structural rearrangement?
non-homologous end joining (NHEJ)
REPAIRS double break strands by directly ligating broken ends together without a homologous template.
what is the second mechanism of structural rearrangement?
homologous recombination (HR)
repairs a DSB by using a homologous sequence as a template, usually from a sister chromatid.
what is the third mechanism of structural rearrangement?
transposition
move as transposable elements (jumping genes) from one location into the genome to the other
what is the fourth mechanism of structural rearrangement?
replication slippage
occurs when DNA polymerase slips during replication, causing repeat sequences to expand or contract
what is the fifth mechanism of structural rearrangement?
unequal crossing over
misalignment of homologous chromosomes during recombination, leading to duplications or deletions.
Describe the function of RecA in homologous recombination and in the SOS response.
(2. Function of RecA in the SOS Response).
2. Function of RecA in the SOS Response
The SOS response is a global, inducible DNA damage repair system in bacteria. RecA's role here is not enzymatic in the traditional sense, but allosteric—it acts as a cellular alarm signal and co-protease.
The Process:
Activation by DNA Damage:
The same signal that initiates recombination—ssDNA gaps caused by replication forks encountering DNA damage—also activates RecA for its SOS role.
RecA forms its active filament on this ssDNA, but in the context of the SOS response, this filament is now called the RecA filament* ("RecA-star"). The * signifies that it is activated by its association with ssDNA and ATP.
LexA Autocleavage (The "Switch"):
In an undamaged cell, the LexA repressor protein binds to the promoter regions (SOS boxes) of about 40 genes, repressing their transcription. These genes include DNA repair proteins (e.g., UvrA, UvrB for nucleotide excision repair), error-prone translesion polymerases (e.g., UmuC/D, also called DNA Pol V), and RecA itself.
The activated RecA* filament does not cleave LexA itself. Instead, it facilitates the self-cleavage (autoproteolysis) of LexA.
RecA* acts as a catalytic cofactor, inducing a conformational change in LexA that stimulates its latent protease activity to cut itself.
Derepression of SOS Genes:
Cleaved LexA can no longer dimerize or bind DNA.
This de-represses all the SOS genes, leading to a massive surge in the production of DNA repair enzymes.
This includes the error-prone polymerases, which can replicate past damaged DNA bases but at the cost of increased mutations—a last-resort "translesion synthesis" strategy for cell survival.
Switching Off the Response:
Once the DNA damage is repaired, ssDNA gaps are no longer present.
RecA* filaments disassemble, as they lack their ssDNA scaffold.
Without RecA*, LexA no longer undergoes autocleavage. Newly synthesized LexA repressor accumulates and re-binds the SOS boxes, shutting down the response.
In summary for the SOS response: RecA acts as a damage sensor and signal transducer. Its filament form signals the severity of DNA damage and directly triggers the dismantling of the LexA repressor, turning on the cell's emergency repair systems.
Mention the function of other important recombination proteins.
While RecA is the central player in homologous recombination, it does not work alone. A cast of other critical proteins prepares the DNA, regulates the process, and resolves the final structures. Here are the functions of other important recombination proteins, primarily in the context of the well-studied E. coli model.
1. Proteins for Initiating and Processing DNA Ends
These proteins generate the single-stranded DNA (ssDNA) tails that RecA needs to form its active filament.
RecBCD Complex (Exonuclease V)
Function: A multi-functional enzyme complex that is the primary initiator of homologous recombination in E. coli, especially for repairing double-strand breaks.
Unwinds and Degrades: It binds to a blunt or nearly blunt end of double-stranded DNA and uses its potent helicase and nuclease activities to unwind and degrade both strands.
Chi Site Recognition: When it encounters a specific 8-base-pair sequence called a Chi site (5'-GCTGGTGG-3'), its activity changes dramatically. It continues unwinding but now preferentially degrades the 3'→5' strand, leaving the 5'→3' strand intact.
Recruitment of RecA: This processing creates the 3' single-stranded DNA tail onto which RecA can load, initiating homologous recombination.
Analogy: RecBCD is the "DNA shredder" that, upon a specific signal (Chi), stops shredding and becomes a "preparation tool" for RecA.
RecFOR Pathway
Function: An alternative pathway to load RecA onto ssDNA. While RecBCD processes double-strand breaks, the RecFOR pathway loads RecA onto single-stranded gaps that are often bound by single-strand binding protein (SSB). These gaps can arise from damaged bases or incomplete replication.
Proteins:
RecQ: A helicase that unwinds DNA to create a ssDNA region.
RecJ: A nuclease that degrades the ssDNA strand to create a defined end.
RecF, RecO, RecR: Work together to displace SSB and facilitate the loading of RecA onto the ssDNA gap.
2. Proteins for Branch Migration and Resolution
After RecA-mediated strand invasion forms a Holliday junction, these proteins process the intermediate.
RuvAB Complex
Function: The primary branch migration complex in E. coli.
RuvA: A tetrameric protein that binds specifically to the center of the Holliday junction, recognizing its four-way structure and stabilizing it in an open, square-planar conformation.
RuvB: A hexameric, ATP-dependent motor protein that assembles onto two opposite arms of the junction. It acts as a molecular pump, driving the exchange of DNA strands and moving the branch point (branch migration) rapidly and efficiently.
Analogy: If the Holliday junction is a four-way intersection, RuvA is the roundabout that organizes the traffic, and RuvB is the engine in the cars that drives them through.
RuvC
Function: The resolvase that cuts (resolves) the Holliday junction to produce two separate DNA molecules.
It is an endonuclease that binds to the RuvAB-junction complex.
It makes two symmetric nicks in two of the four DNA strands at the junction.
Depending on which strands are cut, the resolution can result in either patch recombinant molecules (with only a short heteroduplex region) or splice recombinant molecules (where the DNA on either side of the junction has been exchanged).
RecG
Function: A helicase that can also catalyze branch migration, often acting as a backup to RuvAB or on specific types of stalled replication forks. It can also "reverse" model replication forks, converting them back into Holliday junction-like structures for repair.
3. Key Supporting ProteinsSingle-Strand Binding Protein (SSB)
Function: Binds tightly and cooperatively to ssDNA, preventing it from forming secondary structures or being degraded. While it protects the ssDNA, it also blocks RecA binding. RecBCD and the RecFOR pathway are needed to help load RecA and displace SSB from the DNA.
DNA Polymerase I & DNA Ligase
Function: After strand invasion and branch migration, there are often gaps in the DNA backbone. DNA Polymerase I fills in any remaining single-stranded gaps using its polymerase activity, and DNA Ligase seals the nicks in the sugar-phosphate backbone to complete the repair.
Summary Table of Key Recombination Proteins
Protein Complex | Primary Function |
|---|---|
RecBCD | Processes double-strand breaks; unwinds and nucleolytically degrades DNA until a Chi site, then loads RecA. |
RecFOR | Loads RecA onto ssDNA gaps (e.g., those covered by SSB). |
RecA | Forms a nucleoprotein filament on ssDNA; catalyzes homology search and strand invasion. |
RuvA | Binds and stabilizes the Holliday junction. |
RuvB | An ATPase motor that drives branch migration. |
RuvC | An endonuclease that resolves the Holliday junction by cutting it. |
RecG | A helicase that can also catalyze branch migration, particularly on replication forks. |
SSB | Protects ssDNA from degradation and prevents secondary structure formation. |
In conclusion, homologous recombination is a highly coordinated ballet of enzymes. RecA is the star performer that executes the crucial strand exchange, but it relies on a dedicated team to set the stage (RecBCD/RecFOR), manage the performance (RuvAB), and bring it to a proper conclusion (RuvC).
Mention the function of other important recombination proteins.
While RecA is the central player in homologous recombination, it does not work alone. A cast of other critical proteins prepares the DNA, regulates the process, and resolves the final structures. Here are the functions of other important recombination proteins, primarily in the context of the well-studied E. coli model.
1. Proteins for Initiating and Processing DNA Ends
These proteins generate the single-stranded DNA (ssDNA) tails that RecA needs to form its active filament.
RecBCD Complex (Exonuclease V)
Function: A multi-functional enzyme complex that is the primary initiator of homologous recombination in E. coli, especially for repairing double-strand breaks.
Unwinds and Degrades: It binds to a blunt or nearly blunt end of double-stranded DNA and uses its potent helicase and nuclease activities to unwind and degrade both strands.
Chi Site Recognition: When it encounters a specific 8-base-pair sequence called a Chi site (5'-GCTGGTGG-3'), its activity changes dramatically. It continues unwinding but now preferentially degrades the 3'→5' strand, leaving the 5'→3' strand intact.
Recruitment of RecA: This processing creates the 3' single-stranded DNA tail onto which RecA can load, initiating homologous recombination.
Analogy: RecBCD is the "DNA shredder" that, upon a specific signal (Chi), stops shredding and becomes a "preparation tool" for RecA.
RecFOR Pathway
Function: An alternative pathway to load RecA onto ssDNA. While RecBCD processes double-strand breaks, the RecFOR pathway loads RecA onto single-stranded gaps that are often bound by single-strand binding protein (SSB). These gaps can arise from damaged bases or incomplete replication.
Proteins:
RecQ: A helicase that unwinds DNA to create a ssDNA region.
RecJ: A nuclease that degrades the ssDNA strand to create a defined end.
RecF, RecO, RecR: Work together to displace SSB and facilitate the loading of RecA onto the ssDNA gap.
2. Proteins for Branch Migration and Resolution
After RecA-mediated strand invasion forms a Holliday junction, these proteins process the intermediate.
RuvAB Complex
Function: The primary branch migration complex in E. coli.
RuvA: A tetrameric protein that binds specifically to the center of the Holliday junction, recognizing its four-way structure and stabilizing it in an open, square-planar conformation.
RuvB: A hexameric, ATP-dependent motor protein that assembles onto two opposite arms of the junction. It acts as a molecular pump, driving the exchange of DNA strands and moving the branch point (branch migration) rapidly and efficiently.
Analogy: If the Holliday junction is a four-way intersection, RuvA is the roundabout that organizes the traffic, and RuvB is the engine in the cars that drives them through.
RuvC
Function: The resolvase that cuts (resolves) the Holliday junction to produce two separate DNA molecules.
It is an endonuclease that binds to the RuvAB-junction complex.
It makes two symmetric nicks in two of the four DNA strands at the junction.
Depending on which strands are cut, the resolution can result in either patch recombinant molecules (with only a short heteroduplex region) or splice recombinant molecules (where the DNA on either side of the junction has been exchanged).
RecG
Function: A helicase that can also catalyze branch migration, often acting as a backup to RuvAB or on specific types of stalled replication forks. It can also "reverse" model replication forks, converting them back into Holliday junction-like structures for repair.
3. Key Supporting ProteinsSingle-Strand Binding Protein (SSB)
Function: Binds tightly and cooperatively to ssDNA, preventing it from forming secondary structures or being degraded. While it protects the ssDNA, it also blocks RecA binding. RecBCD and the RecFOR pathway are needed to help load RecA and displace SSB from the DNA.
DNA Polymerase I & DNA Ligase
Function: After strand invasion and branch migration, there are often gaps in the DNA backbone. DNA Polymerase I fills in any remaining single-stranded gaps using its polymerase activity, and DNA Ligase seals the nicks in the sugar-phosphate backbone to complete the repair.
Summary Table of Key Recombination Proteins
Protein Complex | Primary Function |
|---|---|
RecBCD | Processes double-strand breaks; unwinds and nucleolytically degrades DNA until a Chi site, then loads RecA. |
RecFOR | Loads RecA onto ssDNA gaps (e.g., those covered by SSB). |
RecA | Forms a nucleoprotein filament on ssDNA; catalyzes homology search and strand invasion. |
RuvA | Binds and stabilizes the Holliday junction. |
RuvB | An ATPase motor that drives branch migration. |
RuvC | An endonuclease that resolves the Holliday junction by cutting it. |
RecG | A helicase that can also catalyze branch migration, particularly on replication forks. |
SSB | Protects ssDNA from degradation and prevents secondary structure formation. |
In conclusion, homologous recombination is a highly coordinated ballet of enzymes. RecA is the star performer that executes the crucial strand exchange, but it relies on a dedicated team to set the stage (RecBCD/RecFOR), manage the performance (RuvAB), and bring it to a proper conclusion (RuvC).
Discuss examples where recombination plays a biomedical relevant role.
Homologous recombination is not just a theoretical process; it is a fundamental biological mechanism with profound biomedical implications. Its roles can be divided into two broad categories: essential, beneficial functions and pathological consequences when it goes awry.
Here are key examples of its biomedical relevance.
1. Essential for Human Health: Proper DNA RepairA. Repair of DNA Double-Strand Breaks (DSBs)
The Role: Homologous recombination (HR) is the primary error-free pathway for repairing the most lethal type of DNA damage: double-strand breaks. It uses the sister chromatid as a template for precise repair.
Biomedical Relevance:
Cancer Prevention: Defects in HR genes (e.g., BRCA1, BRCA2, PALB2, RAD51) dramatically increase genomic instability. Cells are forced to use more error-prone repair pathways, leading to an accumulation of mutations and a very high risk of cancer.
Example - Hereditary Breast and Ovarian Cancer: Women with inherited mutations in the BRCA1 or BRCA2 genes have a 45-85% lifetime risk of breast cancer and a significantly elevated risk of ovarian cancer. The BRCA2 protein is crucial for loading RAD51 (the eukaryotic equivalent of RecA) onto single-stranded DNA. Without functional BRCA2, HR cannot proceed, and cells become genetically unstable.
B. Restarting Stalled Replication Forks
The Role: When a DNA replication fork encounters a damaged base (a "lesion"), it can stall and collapse. HR mechanisms can use the newly synthesized sister chromatid to rebuild and restart the fork without causing a break.
Biomedical Relevance: Failure to properly restart forks leads to replication stress, a major source of DNA breaks and a hallmark of pre-cancerous cells. Many cancer-prone syndromes are linked to defects in proteins that manage replication fork stability and restart.
2. A Double-Edged Sword: When Recombination Goes WrongA. Chromosomal Translocations and Cancer
The Problem: Sometimes, HR occurs between non-homologous sequences or at the wrong time and place. This can cause large-scale chromosomal rearrangements.
Biomedical Relevance:
Oncogene Activation: A classic example is the Philadelphia chromosome in Chronic Myelogenous Leukemia (CML). An aberrant recombination event (a translocation) between chromosomes 9 and 22 creates a novel fusion gene, BCR-ABL. This gene produces a hyperactive tyrosine kinase that drives uncontrolled cell division. (Note: This is often mediated by the error-prone Non-Homologous End Joining pathway, but homologous recombination between repetitive sequences like Alu elements can also cause translocations).
Genomic Instability: Improper HR between dispersed, repetitive sequences (like LINEs or Alu elements) can lead to deletions, inversions, and duplications, which are common in cancer genomes.
B. Expansion of Trinucleotide Repeats
The Problem: Certain neurological disorders (e.g., Huntington's disease, Fragile X syndrome, myotonic dystrophy) are caused by the expansion of three-nucleotide repeats (e.g., CAG). Evidence suggests that aberrant HR processes can contribute to this expansion during DNA replication or repair.
Biomedical Relevance: Understanding this mechanism is crucial for developing therapies to prevent or slow the progression of these currently incurable degenerative diseases.
3. A Tool for Pathogens: Immune Evasion and Antibiotic ResistanceA. Antigenic Variation in Pathogens
The Role: Some pathogens use programmed, high-frequency recombination events to change the surface proteins (antigens) displayed to our immune system.
Biomedical Relevance:
Trypanosoma brucei (African Sleeping Sickness): The parasite has hundreds of silent genes for its Variable Surface Glycoprotein (VSG). It uses gene conversion (a type of HR) to move a different silent VSG gene into an active expression site, constantly changing its "coat" and staying one step ahead of the host's antibody response.
Neisseria gonorrhoeae (Gonorrhea): This bacterium uses HR to shuffle parts of its pilin gene, altering its attachment pili and evading the immune system.
B. Horizontal Gene Transfer in Bacteria
The Role: Bacteria can acquire new genes from other bacteria through three mechanisms: transformation (uptake of free DNA), transduction (via viruses), and conjugation (via plasmids). In all cases, integrating this new DNA into the chromosome requires homologous recombination.
Biomedical Relevance: This is the primary mechanism for the spread of antibiotic resistance genes. A bacterium that acquires a resistance gene via a plasmid can use its RecA system to integrate that gene into its own chromosome, making the resistance permanent and heritable.
4. Harnessing Recombination for Biomedical TechnologyA. Gene Therapy and Gene Editing
The Application: Technologies like CRISPR-Cas9 are designed to create a precise double-strand break at a specific genomic location. The cell's own homologous recombination repair pathway is then hijacked.
How it Works: By providing a "donor DNA" template along with the CRISPR machinery, scientists can trick the cell into using HR to incorporate a new, therapeutic gene or correct a disease-causing mutation at the break site. This holds promise for curing genetic disorders like sickle cell anemia and cystic fibrosis.
B. Cancer Therapy
The Application: Many standard chemotherapies and radiation work by causing DNA damage, particularly double-strand breaks.
Mechanism: Cancer cells with defective HR (e.g., BRCA-mutated cells) are exquisitely sensitive to these agents because they cannot repair the damage. This concept is called synthetic lethality.
PARP Inhibitors: These are a revolutionary class of drugs used to treat BRCA-deficient cancers. PARP enzymes are involved in a different DNA repair pathway (Base Excision Repair). Inhibiting PARP in an HR-deficient cancer cell creates a catastrophic accumulation of unrepaired DNA damage, leading to cell death. This is a direct therapeutic exploitation of a recombination deficiency.
Summary Table
Context | Role of Recombination | Biomedical Consequence |
|---|---|---|
DNA Repair | Error-free repair of breaks using a sister chromatid. | Prevents cancer. Defects (e.g., in BRCA1/2) cause high cancer risk. |
Genomic Stability | Proper restart of stalled replication forks. | Prevents replication stress and genomic instability. |
Chromosomal Translocations | Aberrant recombination between non-homologous sequences. | Causes cancer (e.g., Philadelphia chromosome in CML). |
Pathogen Evolution | Antigenic variation via gene conversion. | Enables immune evasion (e.g., in Trypanosomes, N. gonorrhoeae). |
Bacterial Evolution | Integration of foreign DNA via horizontal gene transfer. | Spreads antibiotic resistance. |
Gene Editing (CRISPR) | Harnessed to incorporate a donor DNA template. | Potential to cure genetic diseases. |
Cancer Therapy | Targeting HR-deficient cancers (e.g., with PARP inhibitors). | Synthetic lethality provides a targeted treatment strategy. |
In conclusion, homologous recombination is a cornerstone of genomic integrity. Its proper function is essential for preventing cancer, while its dysfunction or manipulation by pathogens and scientists alike has direct and powerful consequences for human health and disease treatment.