BIOMATERIALS EXAM 1 - STRUCTURE AND BONDING
1/35
Earn XP
Description and Tags
lectures 2-3
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
structure - function relationship
structure of a material ultimately decides its properties and as a result, its function
more order → mechanical properties improve
example of structure affecting function:
water and ice: atoms are the same but they are arranged differently
ice - crystalline structure, hydrogen bonds hold molecules together
water - liquid state, not crystalline
mechanical properties change
what kind of atomic bonding is there?
primary and secondary
what bonds are primary?
ionic, covalent, metallic
what bonds are secondary?
hydrogen, vander waals
rank the types of bonds from weakest to strongest
hydrogen/van der waals < covalent < metallic < ionic
rank the types of bonds from strongest to weakest
ionic > metallic > covalent > hydrogen/van der waals
what is the strongest bond
ionic
what is the weakest bond
hydrogen/van der waals
what is stronger, covalent or metallic?
metallic
what is weaker, covalent or metallic?
covalent
ionic bonding:
strong, electrostatic between anions and cations
bonding energy between 600 and 1500 kJ/mol
thermal and electric insulator
high strength and high melting temp
covalent bonding:
sharing electrons between two atoms
bonding energy between 17.94 and 71.77 kJ/mol
directional, high bonding energy possible
low electrical conductivity
metallic bonding:
cations that interact with floating electrons
bonding energy between 70 and 850 kJ/mol
thermal and electric conductor, good mechanical properties including ductility
which materials have which bonds?
ionic - ceramics
covalent - polymers
metallic - metals
hydrogen bonding:
attractive force between a hydrogen atom covalently bonded to a very electronegative atom (such as N, O, or F) and another very electronegative atom
more H bonds = higher viscosity
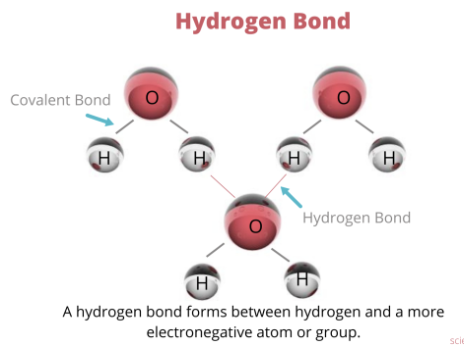
van der waals bonding:
forces created by atomic or molecular dipoles
a bond or molecule whose ends have opposite charges
between biomaterials and proteins
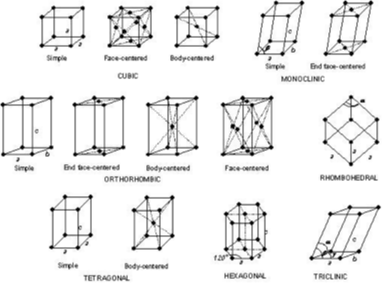
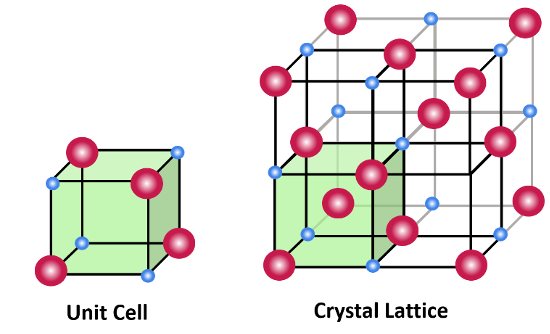
crystallography
crystalline materials are arranged in an ordered fashion
type of crystal structure affects properties
only looking at the first three in picture
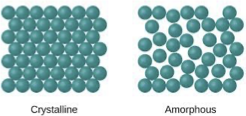
crystalline structure
atoms arranged in a repetitive fashion in a 3D structure over large atomic distances
opposite is amorphous
more order = stronger/stiffer material
think plastic balls in a bucket
even carbon has graphite and diamond
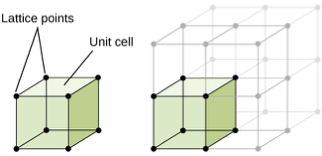
unit cell
smallest repeating unit of a crystalline material
repeated in 3 dimensions to form final material
coordination number
the number of atoms or ions immediately surrounding a central atom in a complex or crystal
atomic packing factor (APF)
fraction of the volume of a unit cell that is occupied by atoms or ions
sum of the sphere volumes of all atoms within a unit cell divided by the unit cell volume
dimensionless
APF formula:
this DO BETTER LATER
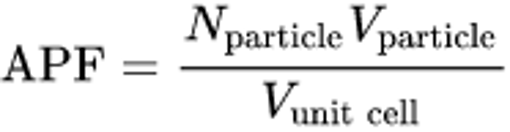
APF for FCC:
74%
derivation of FCC APF
[(8*1/8 + 6*1/2) * 4/3 pi r3] / (2root2r)3 = 0.740 = 74%
![<p>[(8*1/8 + 6*1/2) * 4/3 pi r<sup>3</sup>] / (2root2r)<sup>3</sup> = 0.740 = 74%</p>](https://knowt-user-attachments.s3.amazonaws.com/e98ad140-ffa2-4855-aa53-bc3a5ad1d928.png)
APF for BCC:
68%
derivation of BCC APF:
(1/8*8+1)*4/3 pi r^3/(4/root3r)^3=0.68 or 68%
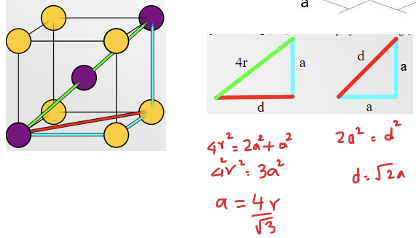
APF for simple cubic
52%
derivation for simple cubic APF:
skip lol
what are the cubic structures called?
lattice systems
what is a lattice?
an ordered array of points describing the arrangement of particles that form a crystal
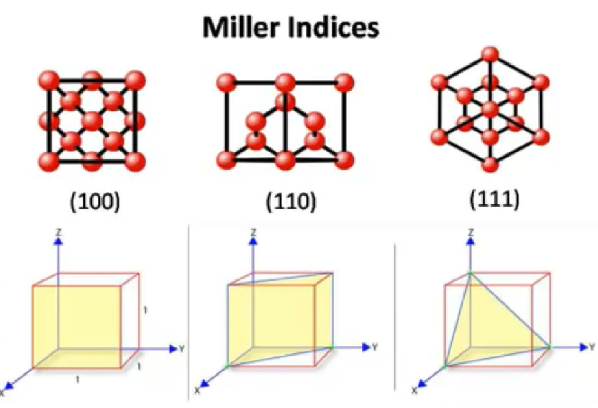
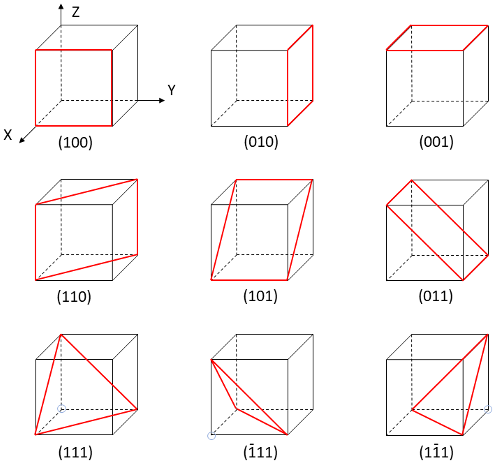
miller indices in lattice structures
group of three numbers that indicates the orientation of a plane or set of parallel planes of atoms in a crystal
what does APF affect
mechanical properties
what do miller indices/orientation of planes affect
chemistry/chemical properties
miller indices notation
(h k l) represent a point
negative numbers and directions are denoted by a bar on top of the number
how to determine miller indices:
determine the points at which the plane intersects the x, y, and z axes.
If the plane is parallel to an axis, the intercept for that axis is taken to be ∞
take the reciprocal of the intercepts
multiply by an integer to clear fractions if needed
record integer indices in parentheses with no commas (h k l)
negative numbers are indicated by a bar over the integer