Chemistry of Life: Elements, Bonds, and Water Properties
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Characteristics of life
Traits defining living organisms, such as metabolism.
Homeostasis
Maintenance of stable internal conditions in organisms.
Metabolism
Chemical processes for energy and nutrient use.
Inheritable information
Genetic material passed to offspring for survival.
Reproduction
Process of producing new individuals or organisms.
Organic molecules
Compounds primarily composed of carbon atoms.
Liquid water
Essential solvent, most abundant in living organisms.
Element
Substance that cannot be chemically broken down.
Atomic number
Number of protons in an atom's nucleus.
Atomic mass
Average mass of an element's isotopes.
Mass number
Total protons and neutrons in an atom.
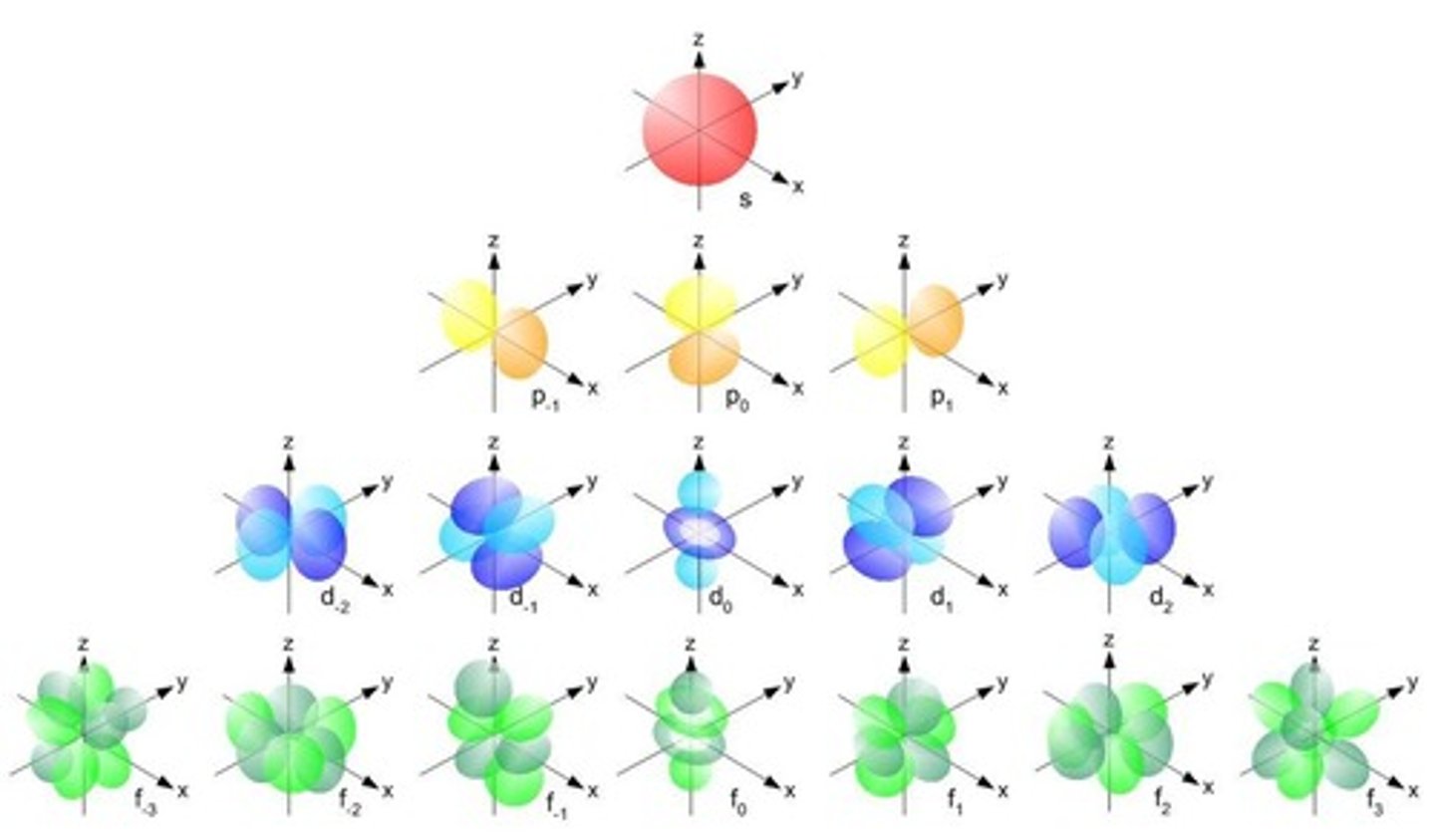
Electron orbitals
Regions where electrons are likely found.
Compound
Substance formed from two or more elements.
Molecule
Smallest unit of a compound retaining properties.
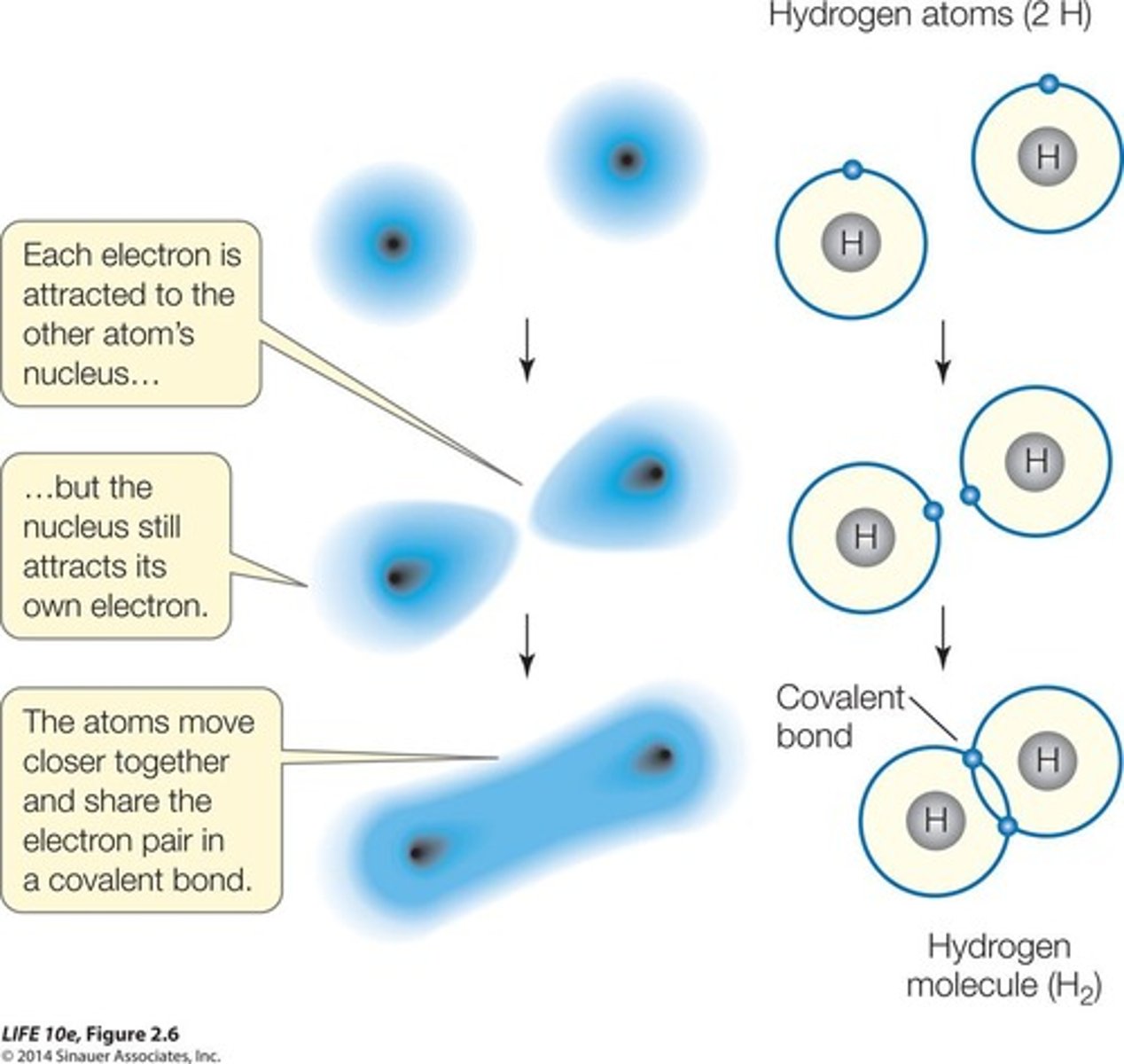
Covalent bond
Atoms share pairs of electrons in a bond.
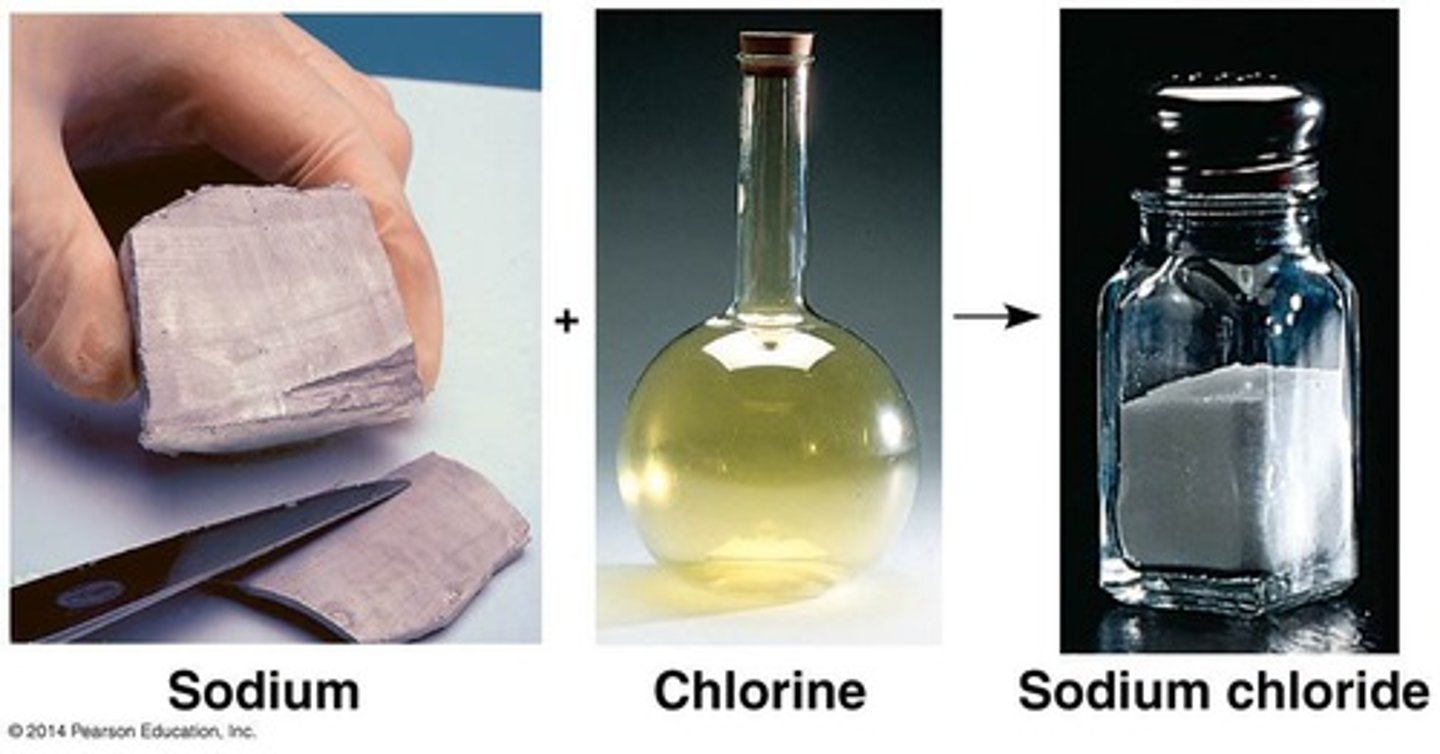
Ionic bond
Electrical attraction between oppositely charged ions.
Cation
Positively charged ion with more protons than electrons.
Anion
Negatively charged ion with more electrons than protons.
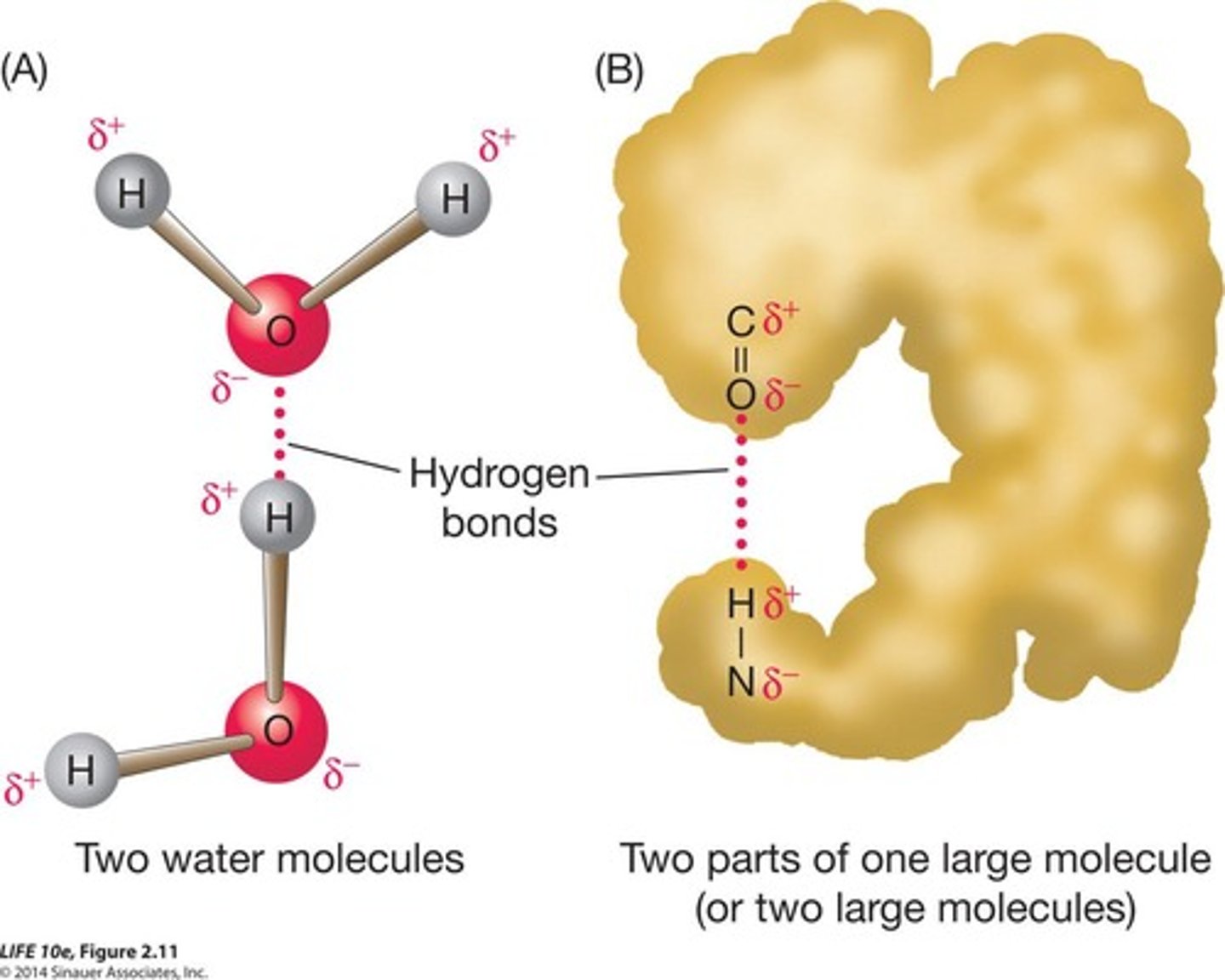
Hydrogen bond
Weak bond between polar molecules due to charge.
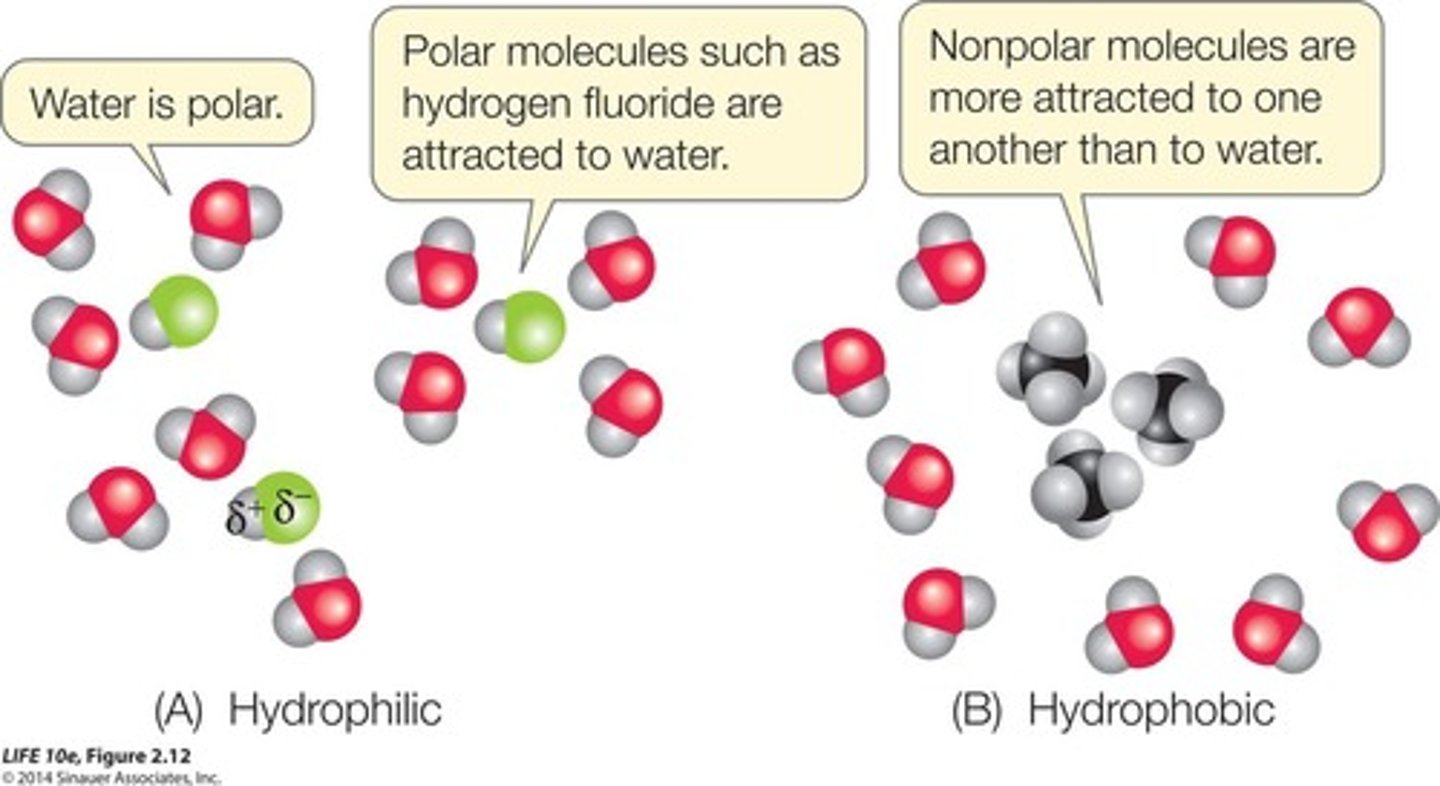
Hydrophobic interactions
Attraction between nonpolar molecules in water.
van der Waals force
Weak attractions due to temporary charge regions.
Chemical reaction
Process of making or breaking chemical bonds.
Molecule shape
Determines biological function of organic compounds.
Organic chemistry
Study of carbon-containing compounds and their properties.
Functional groups
Specific groupings of atoms in organic molecules.
Specific heat
Heat needed to change temperature of 1g by 1°C.
Heat of fusion
Heat required to convert solid to liquid.
Heat of vaporization
Heat needed to convert liquid to vapor.
Aqueous solution
Solution where water is the solvent.
Cohesion
Attraction between like molecules, e.g., water.
Adhesion
Attraction between unlike substances, e.g., water and glass.
