Topic 2: Ionic bonding
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Last updated 9:58 AM on 5/22/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
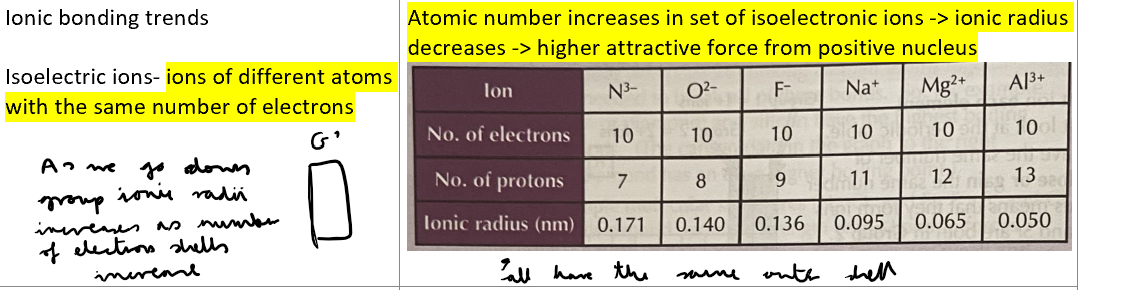
What is the ionic bonding trend amongst isoelectronic ions with increasing atomic numbers?
2
New cards
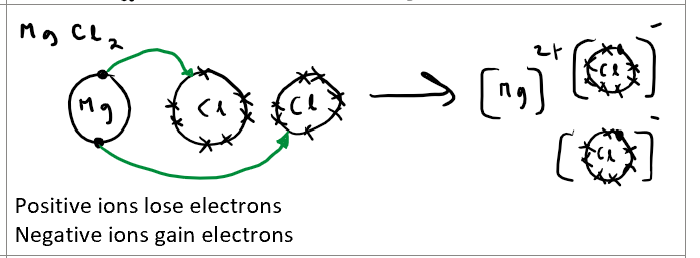
Draw the dot and cross diagram for magnesium chloride
3
New cards
What is ionic bonding?
The strong electrostatic attraction between two oppositely charged ions
4
New cards
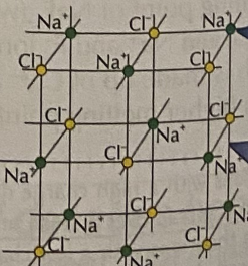
What structure do ionic compounds have?
Giant ionic lattice
5
New cards
Are ionic compounds soluble, how?

6
New cards
Can ionic compounds conduct electricity, how?

7
New cards
What melting point do ionic compounds have, why?

8
New cards
Why are ionic compounds brittle?

9
New cards
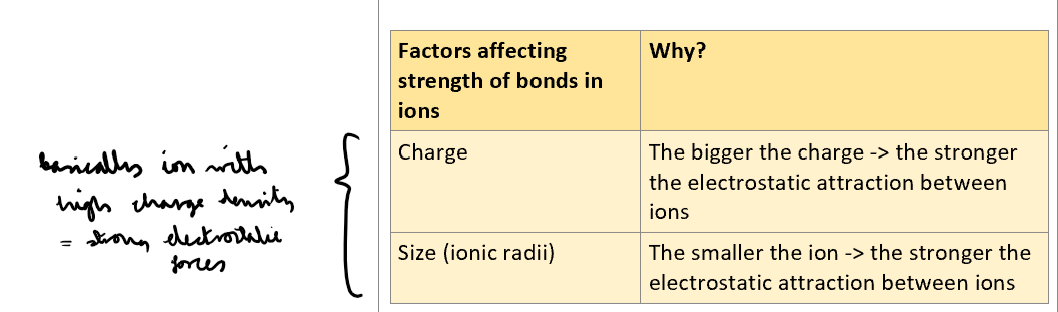
What are factors which affect strength of bonds in ions? Explain how they affect strength of bonding in ions?