atomic theory hard cards
1/14
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Periodic table trend principles (3)
e- in large orbits = farther from the nucleus, less attraction to p+ bc more shielding
More p+ = more attraction for e- from nucleus→ effective nuclear charge (Zₑff)
e- in the same subshell repel each other
Atomic Radius (3)
Measure of the radius from the nucleus to the Ve- in an atom
Down group → AR increases: More shells (higher n value) + shielding
Across period → AR decreases: Zeff & Protons increases (Added e- joins same orbital)
Ionic Radius (4)
AR of an ion
Cations = smaller than neutral
Loses electrons so AR decreases
Anions = larger than neutral
Gains elections so AR increases
Ionization Energy (IE) (6)
Amount of energy needed to remove an e-
Trend = opposite to AR, small AR → high IE
Elements on left need to get rid of e- to become stable
Elements on right need to gain e- to become stable
Exceptions: O = lower IE₁ than N bc O wants to lose 1e- to become half filled
Electronegativity (EN) (5)
The ability to attract e-
Measurement of an atom’s Zeff on OTHER atoms
Atoms w/ high EN pull bonded e- closer to their nuclei
Noble gasses have no EN value (shells stable)
EN has the same trend as IE
Intramolecular interactions (2)
Occur WITHIN molecules/compounds/crystals
Includes: ionic, covalent, metallic bonds
Ionic Bonds (5)
Forms when large difference between EN and IE of both atoms (EN >1.8)
Metal w/ low IE/EN transfers Ve- → Non-metal w/ high IE/EN
Electrostatic attraction-both atoms become ions after transfer
Sometimes forms into a crystal lattice (Ex. NaCl)
Makes compound have a high melting point (Caused by a vast number of attractive forces in structure)
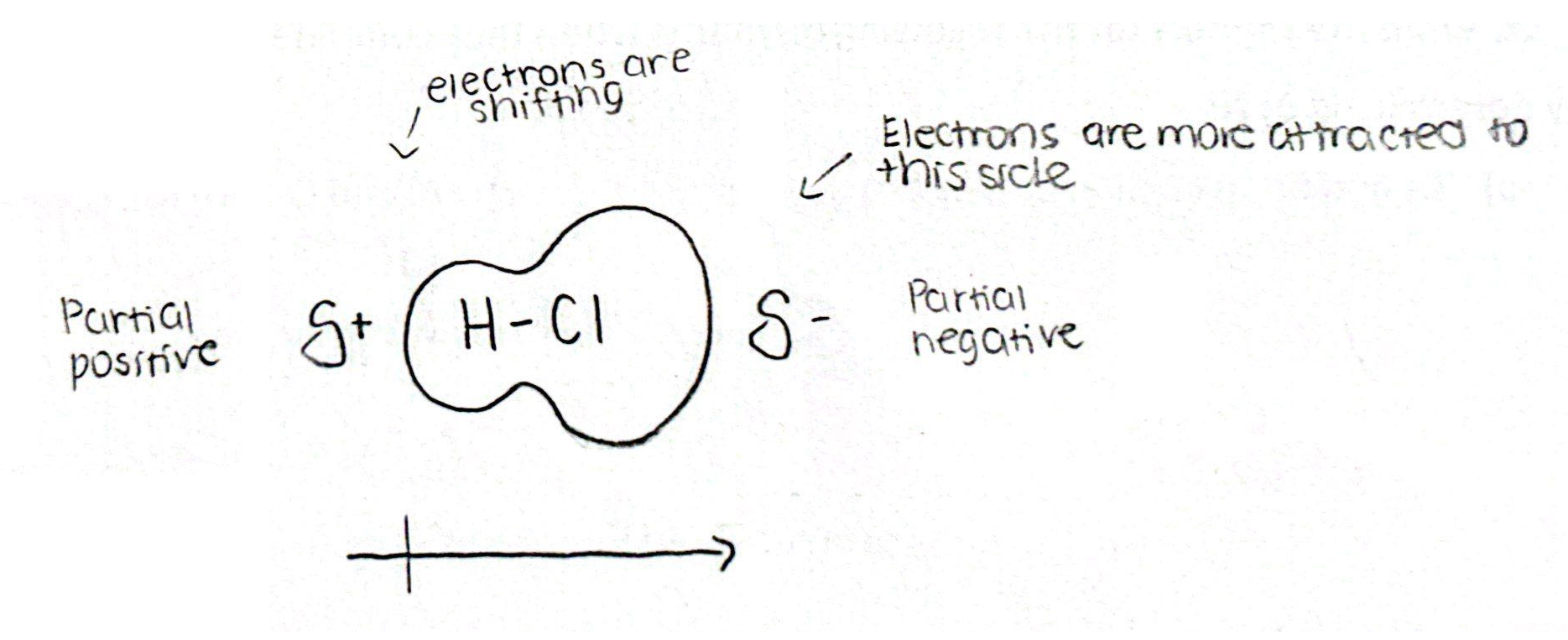
Polar Covalent Bonds (3)
Forms when EN = 0.5-1.8
When e- are pulled more towards one atom bc of higher EN, one side of the moc becomes more negative than the other side (unequal sharing of e-)
Causes dipole (One side more negative)
Non-polar Covalent Bonds (7)
Forms when EN < 0.4
Between identical/same charged non-metals
EN value = same; ΔEN = 0
Collision of atoms = electron clouds overlap
Attractive forces (nucleus) may begint o overlap repulsive forces (e-)
Pair of Ve- shared between both
The electrons will not be more attracted to one nuclei over the other (equidistant)
Metallic Bonds (3)
Protons swarmed by electrons
“sea” of electrons makes metal malleable + conductive
Forms metallic crystals
Octet Rule (5)
All atoms form stable octet when bonding
Stable + low-energy configuration
EXCEPTIONS (sometimes):
H makes 1 bond
B takes less e-
S & P take more e-
Intermolecular Forces (4)
Occurs BETWEEN covalent molecules
Strength/weakness affects state of matter + chemical properties
Strong IMF = High BP (solid/liquid)
Weak IMF = Low BP (liquid/gas)
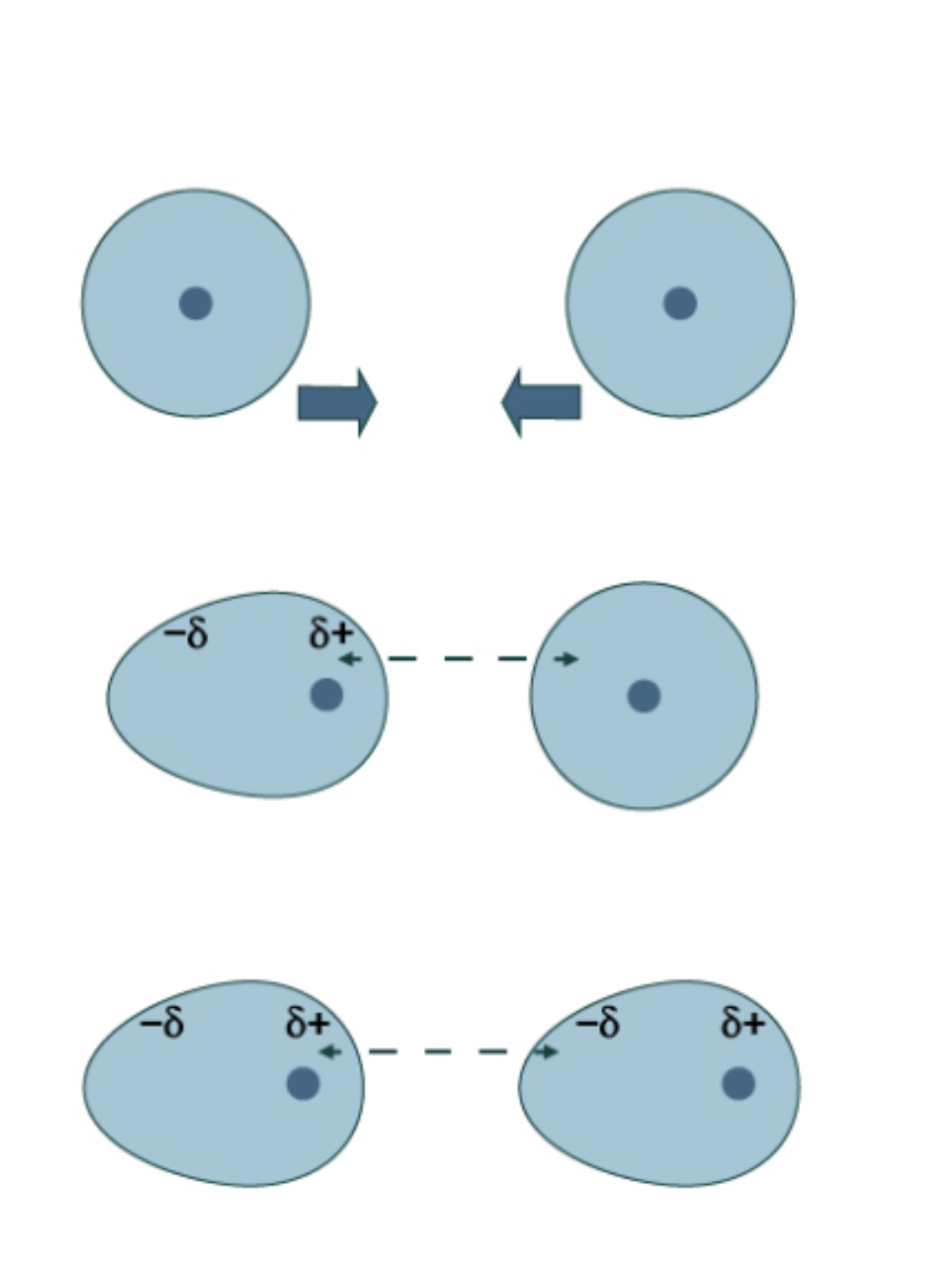
London Dispersion Forces (10)
Weakest force but Always present
Between 2 non-polar molecules
Creates temporary dipole
When e- in moc 1 shift to one side → temporary dipole forms
Can induce a dipole in a nearby molecule
They form a temporary dip-dip before floating away
Attraction increases w/ atomic number
Dipole increases when more e- is added
Attraction increases w/ larger molecules
More spaces to attract
If only LDF present → low BP + MP
Dipole-dipole (4)
2nd strongest force
Between 2 polar molecules
δ+ from moc1 attracted to δ- from moc2
Stronger bonding = Higher BP + MP
Hydrogen Bonding (3)
Strongest IMF
Type of dipole-dipole (only for H)
H bonded with atoms with high EN (N, O, F)