Group 7: Groups in the periodic table: Chemistry: GCSE (9:1)
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Name of Group 7 elements
The halogens
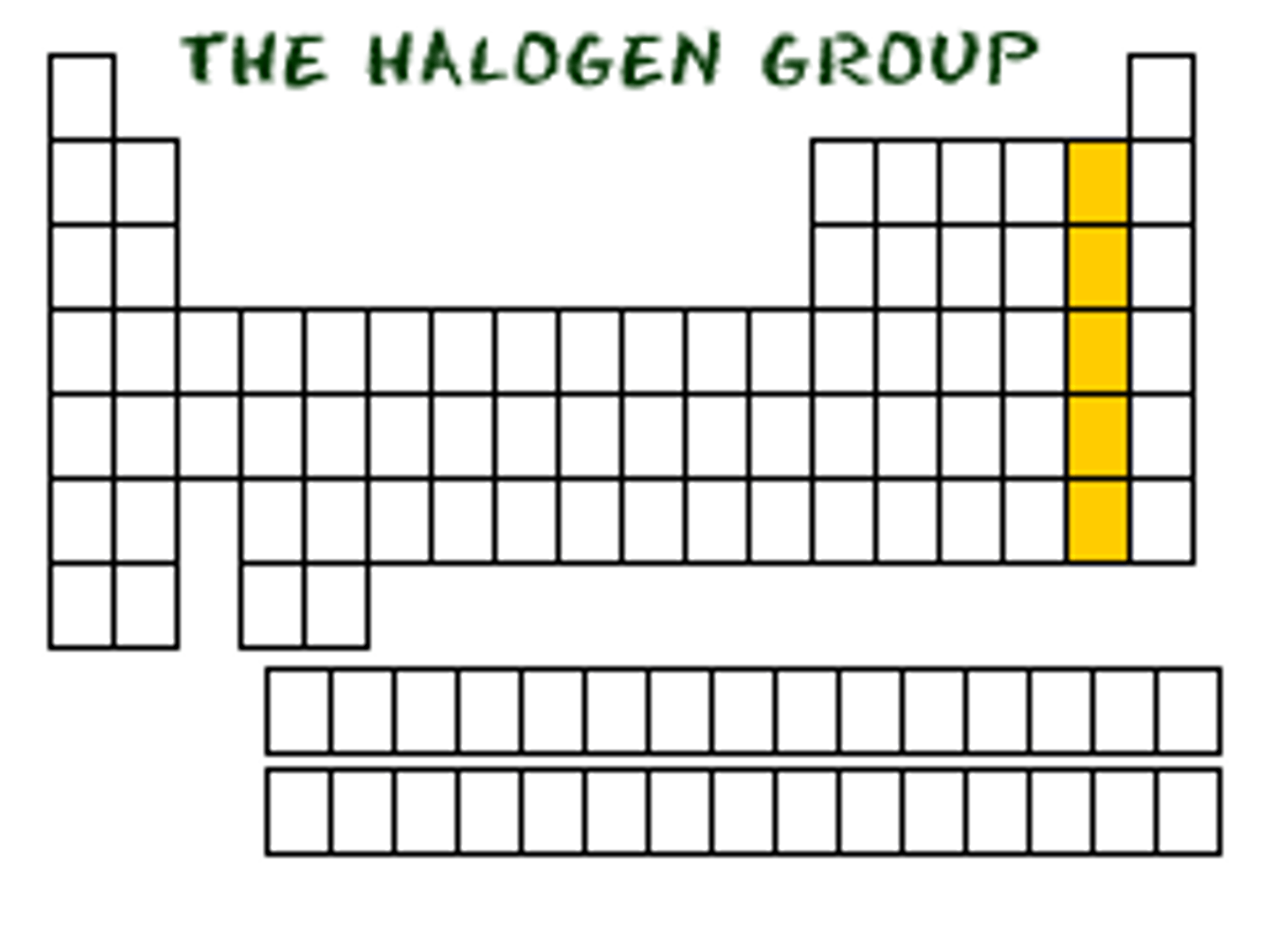
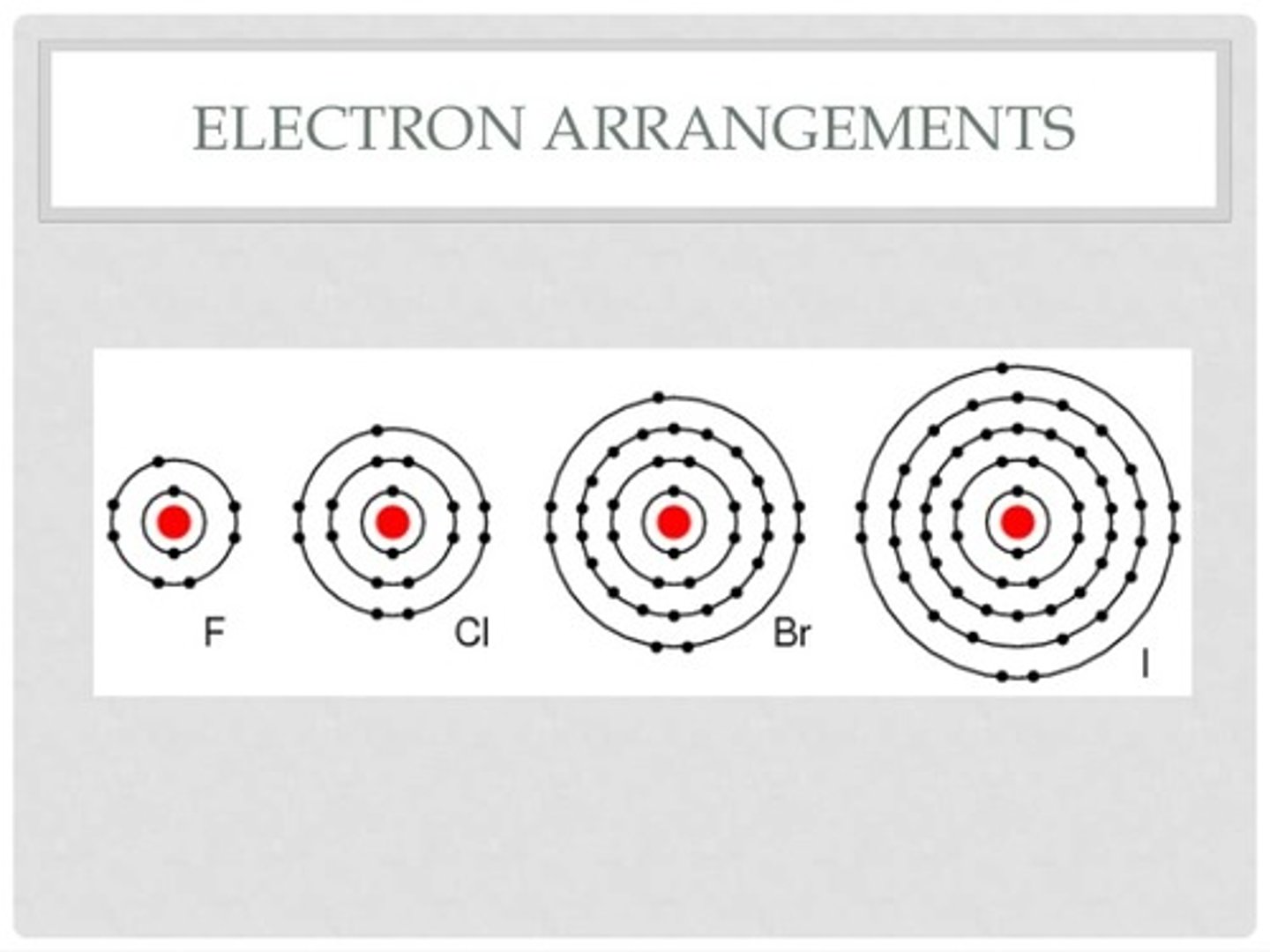
Group 7 elements have similar chemical properties
because they all have 7 outer shell electrons
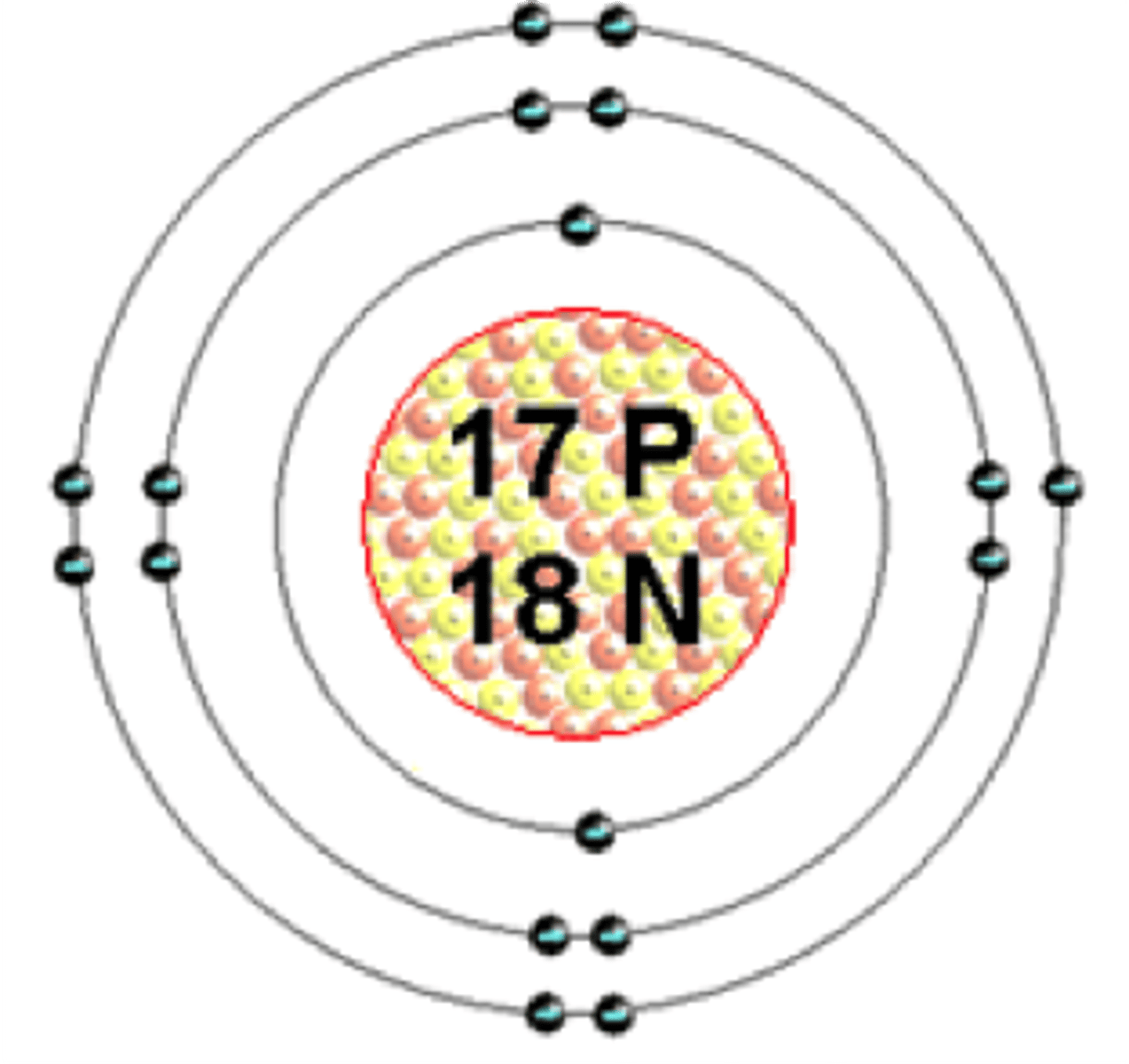
Diatomic molecule
A molecule consisting of two atoms
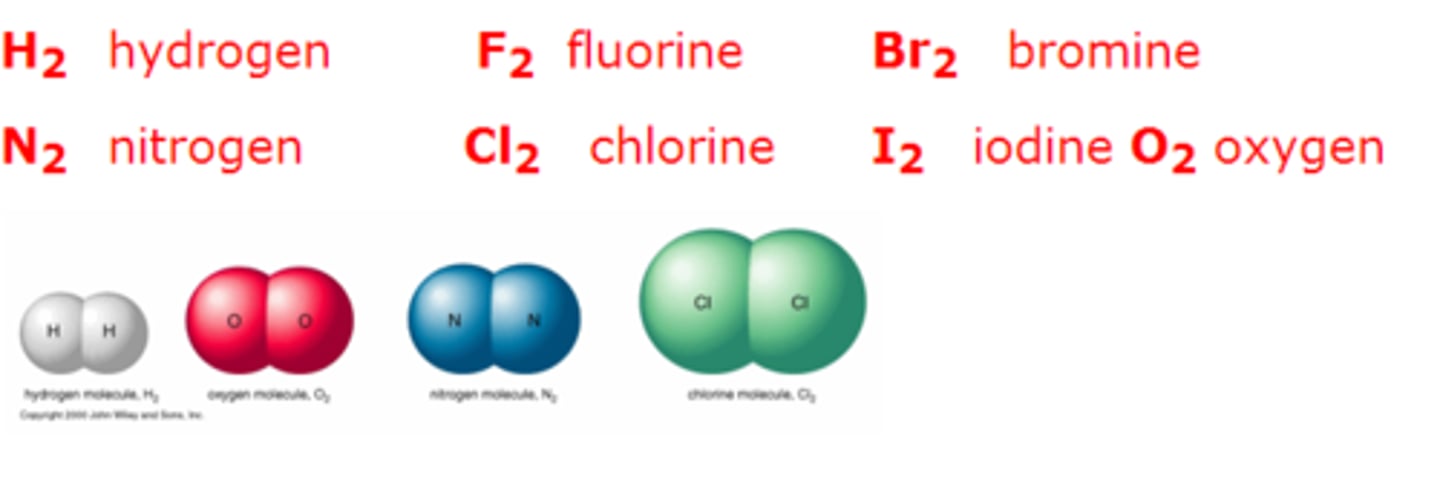
Chemical structure of group 7
Diatomic molecules

Pattern in melting points and boiling points down group 7
Melting and boiling points decrease
Pattern of reactivity down the halogens
They get less reactive
Reason for patterns in reactivity
Larger atomic radius, more shielding, less nuclear attraction, harder to gain a reaction
Group 7 elements react by
gaining 1 electron
Displacement reaction
A reaction in which a more reactive element replaces a similar element in a compound
Potassium bromide + Chlorine -->
Potassium chloride + Bromine
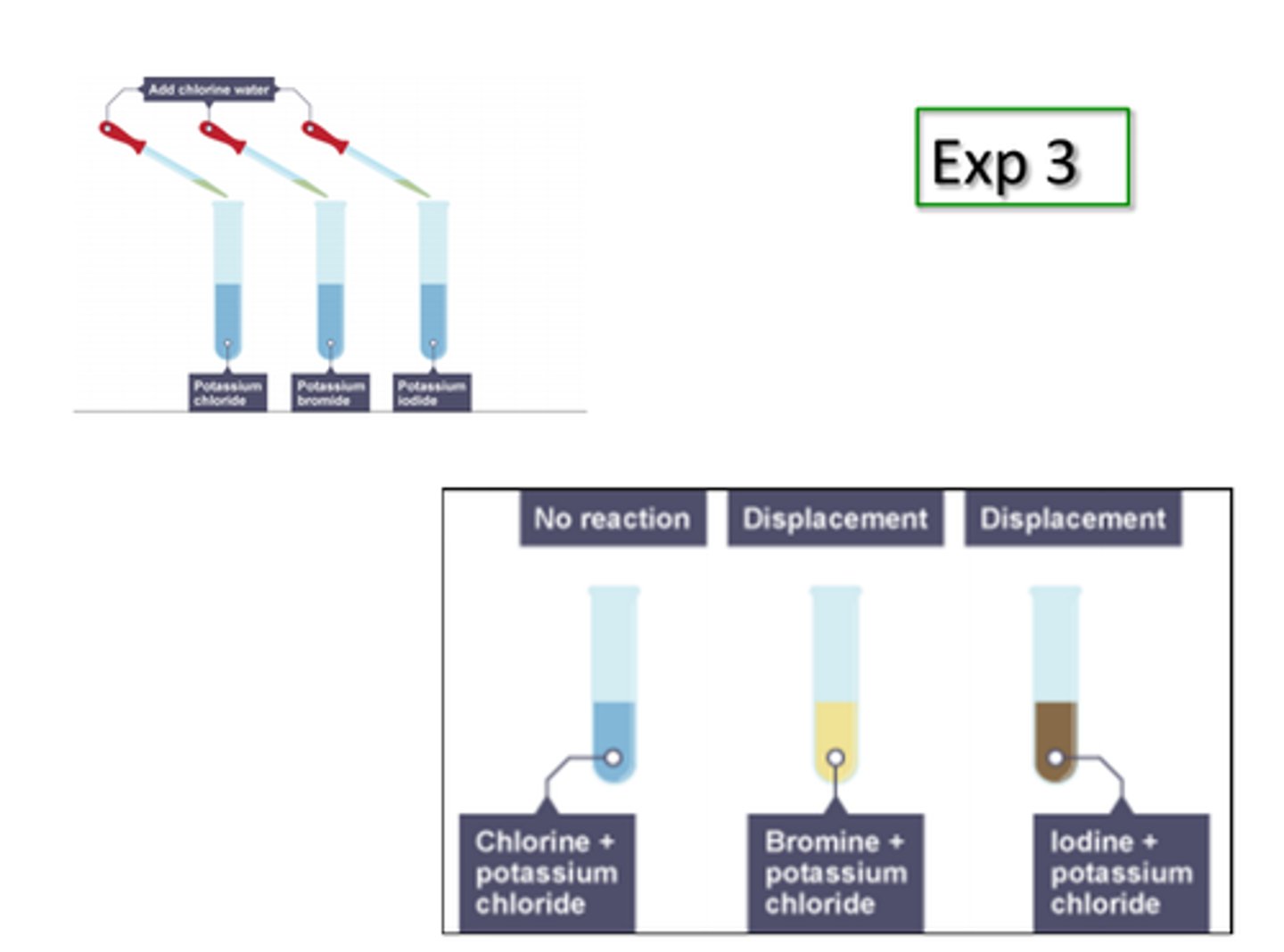
Sodium chloride + Bromine -->
No reaction
Sodium iodide + Bromine -->
Sodium bromide + Iodine
Chlorine
A yellow-green gas which when dissolved in water is used as a disinfectant

Bromine
A corrosive red-brown liquid

Iodine
A shiny grey solid, which sublimes easily forming a purple vapour

Test for chlorine
Bleaches damp litmus paper
Reduced in displacement
More reactive halogen
Oxidised in displacement
Less reactive halogen