Rates of reaction y2
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
How to find rate with a colourimeter
Create standard solutions of reactant with colour and find the absorbance of the solutions. Find absorbance at regular times after reaction started. and use these values to plot a conc time graph
Shape of a concentration time graph for 0 order and 1st order reaction
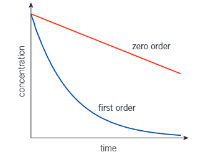
What is special about half life of a first order
Half life is constant
How to find k from a first order graph (2 ways)
Do gradient over concentration at that point
Find half life and do ln2 / half life
Shape of rate concentration graphs
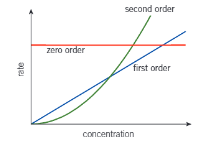
How to find k for zero order from rate time graph and conc time graph
Rate time: k is the y intercept
conc time: k is the gradient
Why are multi step reactions common
It is unlikely that all of the particles will collide in the correct orientation at the same time
What is the rate determining step
The slowest step in the reaction, thereby determining the rate of the whole reaction. The species involved in this step are the ones in the rate equation
What does the exponential factor in the Arrenhius equation represent
proportion of molecules above activation energy
What does the pre exponential factor in the Arrenhius equation represent
The frequency that particles are colliding in the correct orientation (changes with temp but we assume same over small temperature range)