OCR A level chemistry equations
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
how to calculate reaction ∆H
bond enthalpy of products - bond enthalpy of reactants
2
New cards
Gibbs free energy
∆G = ∆H - T∆S
3
New cards
predicting feasibility based on different parts of equation
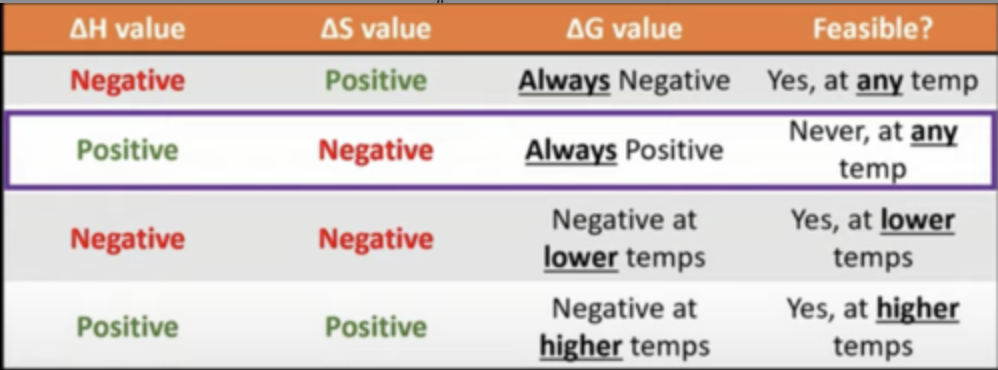
4
New cards
Arrhenius plot
lnk = (-Ea/RT) + lnA
5
New cards
equation for rate constant using half life
k = ln2 / t1/2
6
New cards
expression for Kw
Kw (1 × 10-14) = [OH-][H+]
7
New cards
electrode potential of cell
E° cell = E°positive - E°negative