EKQu's| Biological Molecules Carbs
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
What elements are present in carbohydrates?
Carbon, hydrogen, oxygen.
What is the name of the bond formed when two monosaccharide join together?
Glycosidic bond.
What type of reaction forms a glycosidic bond? What else is formed
Condensation reaction. A water molecule is formed.
What type of reaction breaks a glycosidic bond? What is used
Hydrolysis. water
Name 3 disaccharides and their monomers
Sucrose – Alpha glucose and Fructose
Maltose - Alpha glucose x 2
Lactose - Alpha glucose and Galactose
What is the difference between an α-glucose and a β-glucose molecule?
The OH group on carbon 1 is ‘down’ in α-glucose and ‘up’ in β-glucose.
Draw the simple structure of alpha glucose
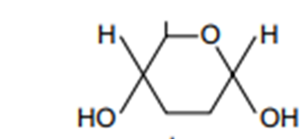
Glycogen is a branched molecule. What type of bond allows this?
1,6 glycocidic bond
Cellulose is made up of parallel strands of β-glucose molecules.
Name the bond which holds these strands together.
Hydrogen Bonds
Explain the test for a non-reducing sugar
Test with benedicts and heat – negative result
Add hydrochloric acid to break the glyosidic bond, neutralise with alkali
Retest and get an orange positive result
Describe how the structures of starch and cellulose molecules are elated to their functions.
Starch (max 3)
1. Helical/ spiral shape so compact;
2. Large (molecule)/insoluble so osmotically inactive;
Accept: does not affect water potential/ψ.
3. Branched so glucose is (easily) released for respiration;
Ignore: unbranched.
4. Large (molecule) so cannot leave cell/cross cell-surface membrane;
Cellulose (max 3)
5. Long, straight/unbranched chains of β glucose;
6. Joined by hydrogen bonding;
Note: references to 'strong hydrogen bonds' disqualifies this mark point.
7. To form (micro/macro)fibrils;
8. Provides rigidity/strength
Describe two differences between the structure of a cellulose molecule and a glycogen molecule
1. Cellulose is made up of β-glucose (monomers) and glycogen is made up of α-glucose (monomers);
2. Cellulose molecule has straight chain and glycogen is branched;
3. Cellulose molecule has straight chain and glycogen is coiled;
4. glycogen has 1,4- and 1,6- glycosidic bonds and cellulose has only 1,4- glycosidic bonds;
Describe and explain two features of starch that make it a good storage molecule.
1. Insoluble (in water), so doesn’t affect water potential;
2. Branched / coiled / (α-)helix, so makes molecule compact;
3. Polymer of (α-)glucose so provides glucose for respiration;
4. Branched / more ends for fast breakdown / enzyme action
5. Large (molecule), so can’t cross the cell membrane
Explain how cellulose molecules are adapted for their function in plant cells.
1. Long and straight chains;
2. Become linked together by many hydrogen bonds to form fibrils;
3. Provide strength (to cell wall).
Describe a test you could use to check if food in the diet contained starch.
1. Add iodine / potassium iodide solution;
2. Blue-black colour (with starch);
Explain how you would test for the presence of starch, proteins, and reducing sugars in a sample. (6 marks)
Starch Test:
Add iodine solution (iodine in potassium iodide) to the sample.
A positive result is indicated by a colour change from brown to blue-black.
Protein Test:
Add Biuret reagent to the sample.
A positive result is indicated by a colour change from blue to purple/lilac.
Reducing Sugar Test:
Add Benedict’s reagent to the sample and heat it.
A positive result is indicated by a colour change from blue to brick-red/orange precipitate.
1. Describe the biochemical tests you would use to confirm the presence of lipid, non-reducing sugar, and amylase in a sample. (6 marks)
Lipid Test:
Add ethanol to the sample and shake.
Add water and shake again.
A positive result is indicated by a white emulsion forming.
Non-Reducing Sugar Test:
Perform the Benedict’s test; if negative (solution remains blue), proceed.
Add dilute hydrochloric acid to the sample and boil it to hydrolyse the non-reducing sugar.
Neutralise the solution with sodium hydrogencarbonate.
Re-perform the Benedict’s test; a positive result is indicated by a brick-red/orange precipitate.
Amylase Test:
Add starch solution to the sample and incubate.
At intervals, remove samples and add iodine solution.
A positive result is indicated by a colour change from brown to blue-black; disappearance of this colour over time indicates starch breakdown by amylase.