COMBINED Biomolecules Test Bio12AP
1/105
Earn XP
Description and Tags
MCQ (28 marks), mole recognition and 8 descriptions (4 marks), written (14 marks, point form). Main topics: properties of water, dehydration synthesis/hydrolysis sketch (amino acids/carbs), levels of protein structure, phospholipids and bilayer (polarity)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
106 Terms
Lipids
Energy-rich organic compounds, such as fats, oils, and waxes, that are made of carbon, hydrogen, and oxygen. (Are mostly non-polar)
function in energy storage, cell membranes, insulation
how a lipid functions in a cell depends on the lipid’s saturation level
3 major groups of lipids
Fats (Triglycerides):
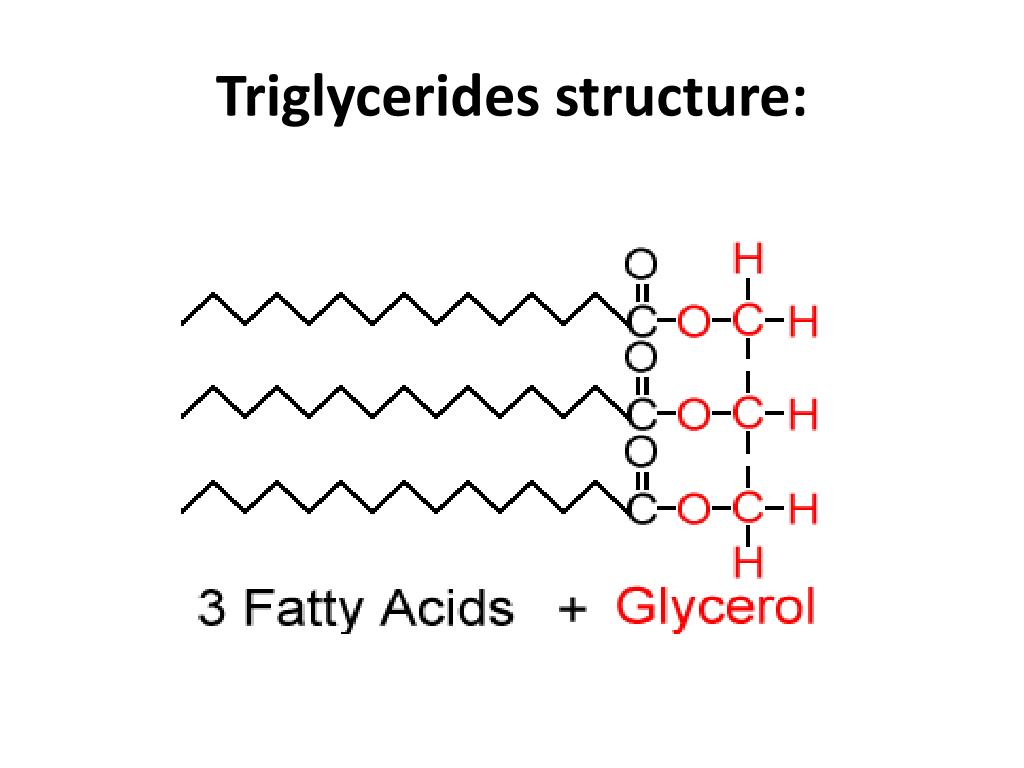
Made of glycerol and three fatty acids.
Primarily used for long-term energy storage.
Steroids:
Lipids with a structure of four fused carbon rings.
Includes hormones like cholesterol and sex hormones.
Phospholipids:
Composed of a glycerol, two fatty acids, and a phosphate group.
Key components of cell membranes, forming a bilayer due to their hydrophilic head and hydrophobic tail.
Components + characteristics of lipids
carbon, hydrogen, and oxygen
completely hydrophobic or amphipathic (presence of long hydrocarbon chains/rings, which are nonpolar)
ratio of hydrogen to oxygen in lipids is much higher than carbohydrates
Glycerol
A three-carbon alcohol to which fatty acids are covalently bonded to make fats and oils.
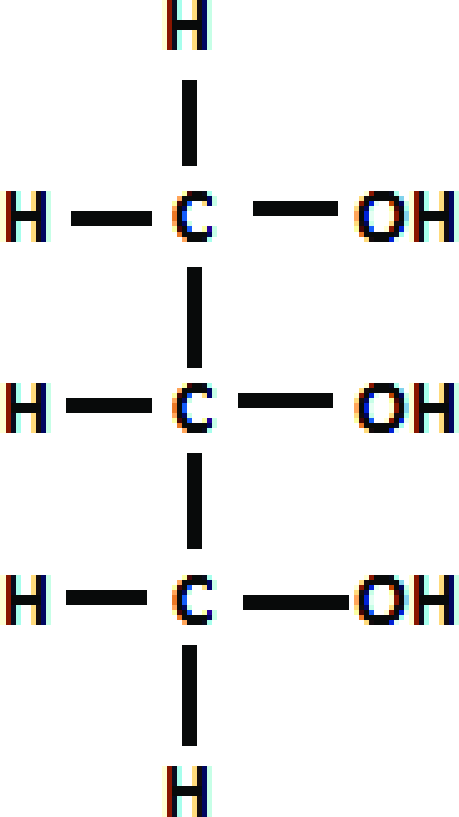
Triglyceride
a lipid made of three fatty acid molecules and one glycerol molecule
deoxyribose vs ribose

Ribose has a hydroxyl group (-OH) on the 2' carbon, while deoxyribose lacks this hydroxyl group, having a hydrogen (-H) instead. This structural difference makes ribose more reactive and less stable than deoxyribose.
saturated fatty acid
A fatty acid in which all carbons in the hydrocarbon tail are connected by single bonds, thus maximizing the number of hydrogen atoms that can attach to the carbon skeleton.
- NO DOUBLE BONDS BETWEEN CARBONS
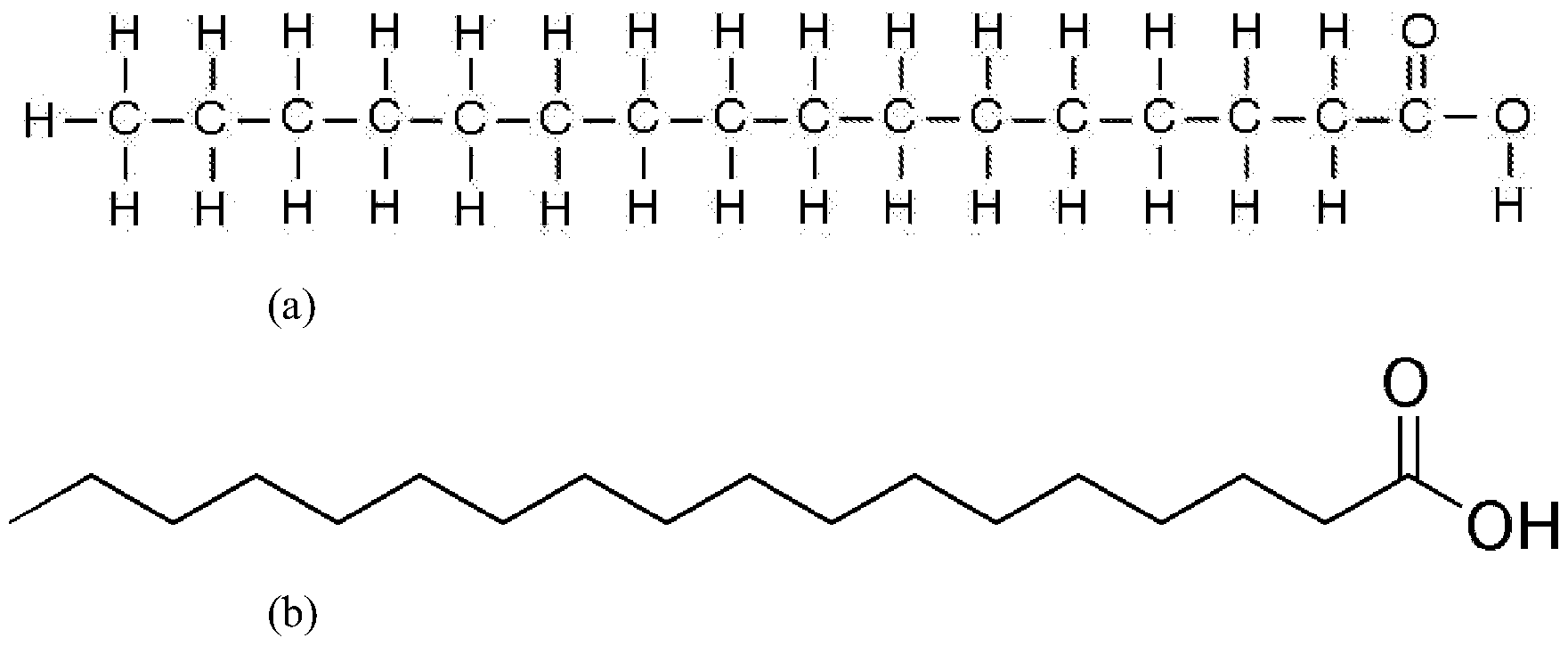
Unsaturated fatty acids
Fatty acids that contain one or more double bonds between the carbon atoms in the hydrocarbon tail. These double bonds reduce the number of hydrogen atoms attached to the carbon skeleton, resulting in a bent structure. Unsaturated fatty acids are typically liquid at room temperature and are commonly found in plant oils and fish oils.
Phospholipids
type of lipid molecule that is a fundamental component of cell membranes. It consists of two main parts:
Hydrophilic (water-attracting) head: This part contains a phosphate group, which is polar and interacts well with water.
Hydrophobic (water-repelling) tails: These are made up of two long fatty acid chains, which are non-polar and do not interact well with water.
Function: Forms bilayers in cell membranes, with polar heads facing outward and non-polar tails facing inward, maintaining cell integrity.
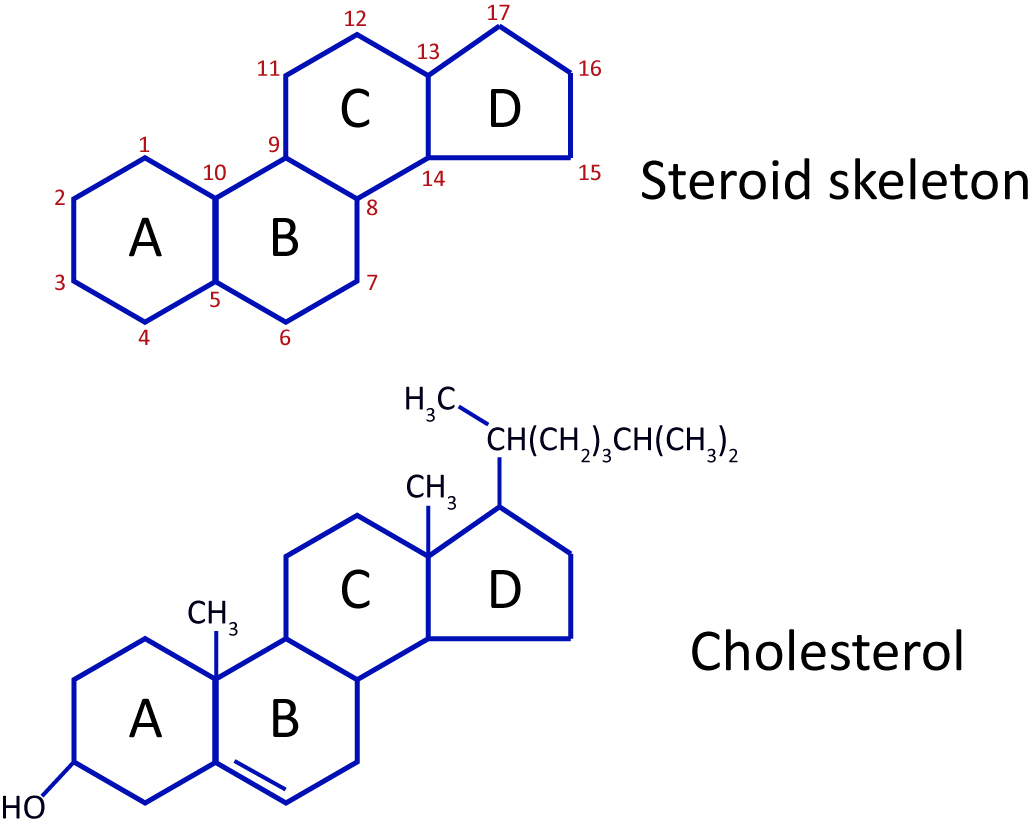
Cholesterol
- sterol molecule
- not soluble in water
- contains C,H,O arranged in rings
- precursor (building block) to sex hormones (estrogen and testosterone)
-component of lipid membrane

Saponification
The chemical reaction between a fat (or oil) and a strong base (such as sodium hydroxide or potassium hydroxide) that produces glycerol and the salt of a fatty acid, commonly known as soap. This process involves the hydrolysis of the fat or oil, resulting in the formation of soap molecules that can emulsify fats and oils in water.
Why will soap mix with water where a fat or other oily substances won't?
Soap has both a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail. The hydrophilic head interacts with water, while the hydrophobic tail binds to oils and fats. This allows soap to form micelles, where the hydrophobic tails surround and trap the oil, making it possible for the oil to mix with water and be washed away.
Key points:
Soap is amphipathic (has both hydrophilic and hydrophobic parts)
The hydrophobic tail binds to oil, while the hydrophilic head binds to water
Soap allows fats/oils to be suspended in water for removal
Emulsification
physical process of breaking up large fat globules into smaller globules, thereby increasing the surface area that enzymes can use to digest the fat
Soaps act as emulsifiers because they have both water-repelling and water-attracting parts. This lets them interact with water and oils, breaking up and suspending oil droplets in water, making emulsification possible
Saturated fats are typical of......
Animal fats
Unsaturated fats are typical of...
Plant fats
Four important uses of lipids in body
-insulation and protection
-long term energy storage
-structure of cell membrane
- basis of hormones (ex. Sex hormones)
How are phospholipids different from neutral (uncharged) fats
- instead of a 3rd fatty acid there is a phosphate group
two fatty acid tails are non polar
phosphate “head” is polar (neg charge attracts to a water molecule)
- not electrically neutral (non polar)
- soluble in water (have polar "head")
Proteins contain
carbon, hydrogen, oxygen, nitrogen, sulfur
Carbon Helps Organisms Nurture Structures
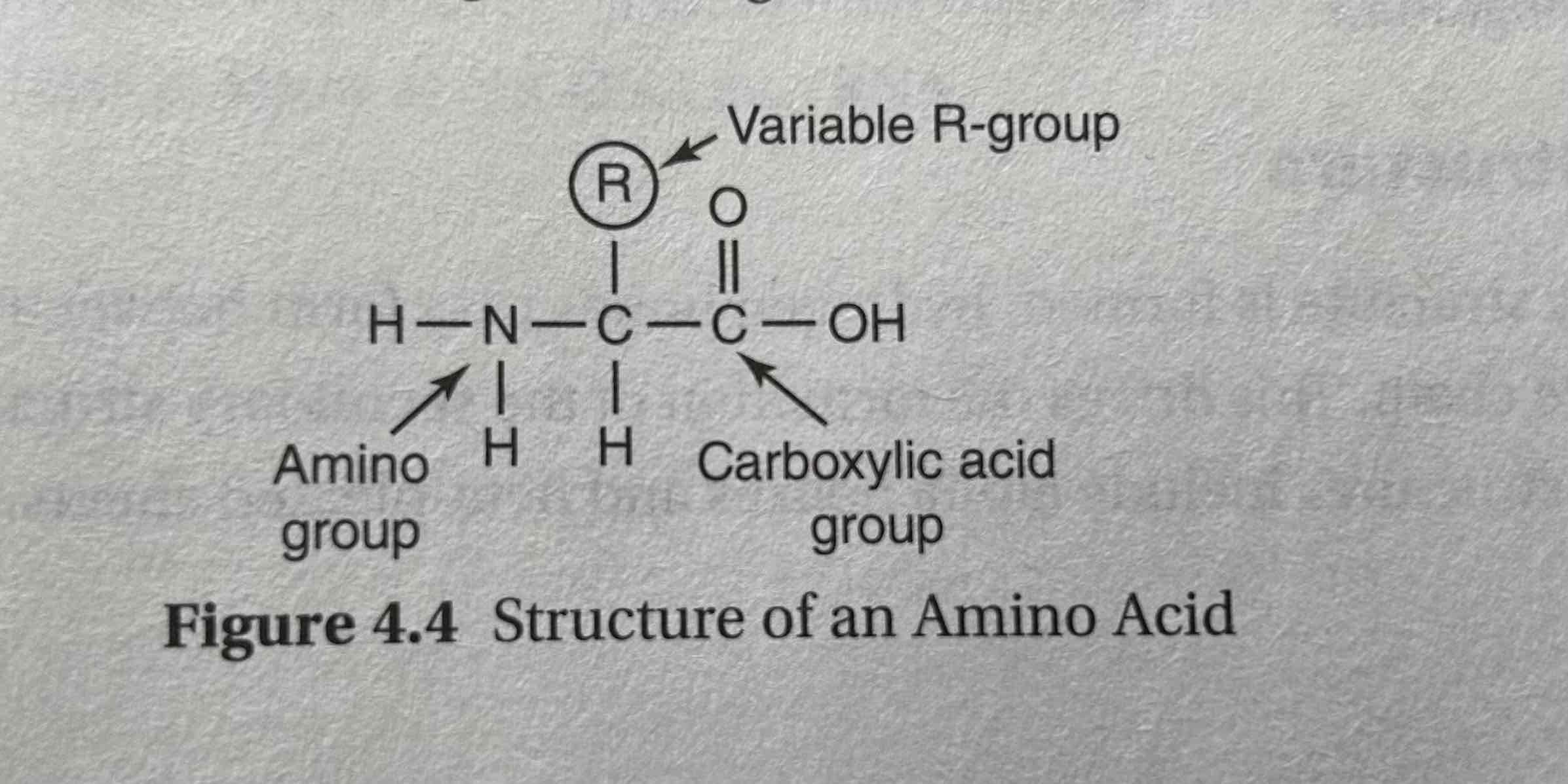
Amino acid
organic compounds that serve as the building blocks of proteins.
Each amino acid contains a central carbon atom, an amino group (–NH₂), a carboxyl group (–COOH), a hydrogen atom, and a variable side chain or R group that determines the specific characteristics of the amino acid. T
There are 20 standard amino acids, each with unique properties, which combine in various sequences to form proteins essential for biological functions, including structure, function, and regulation of the body’s tissues and organs.
parts of an amino acid
Hydrogen atom, a carboxyl group (−COOH), an amino group (−NH2), and a R-group.
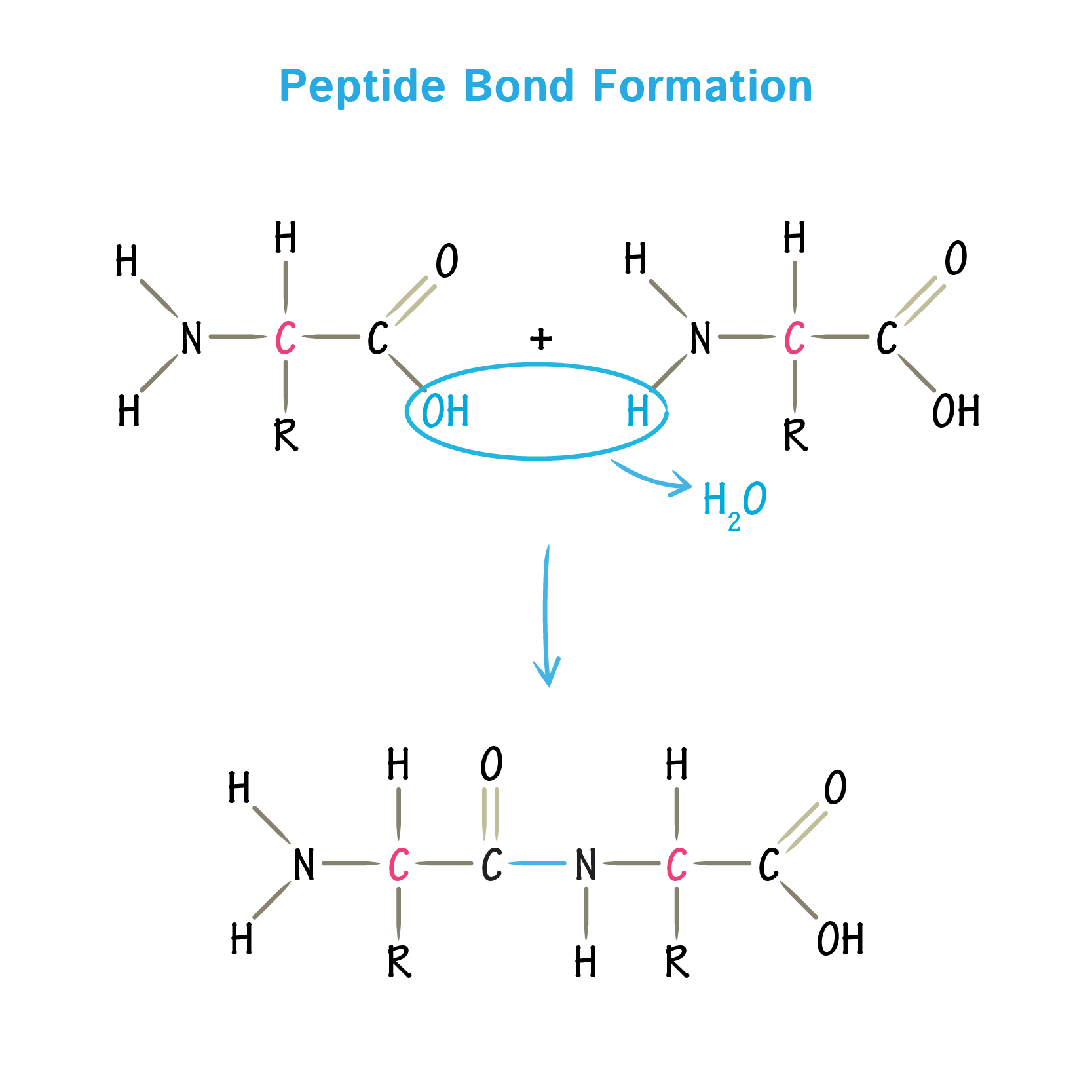
Peptide bonds
covalent bond that links two amino acids together, forming a dipeptide. It is created through a dehydration synthesis (or condensation) reaction, where the carboxyl group (–COOH) of one amino acid reacts with the amino group (–NH₂) of another, releasing a molecule of water (H₂O).
peptide bond formation
amide bond formed by amino group joining with carboxyl group and removing an H2O molecule
- results in C - N bond
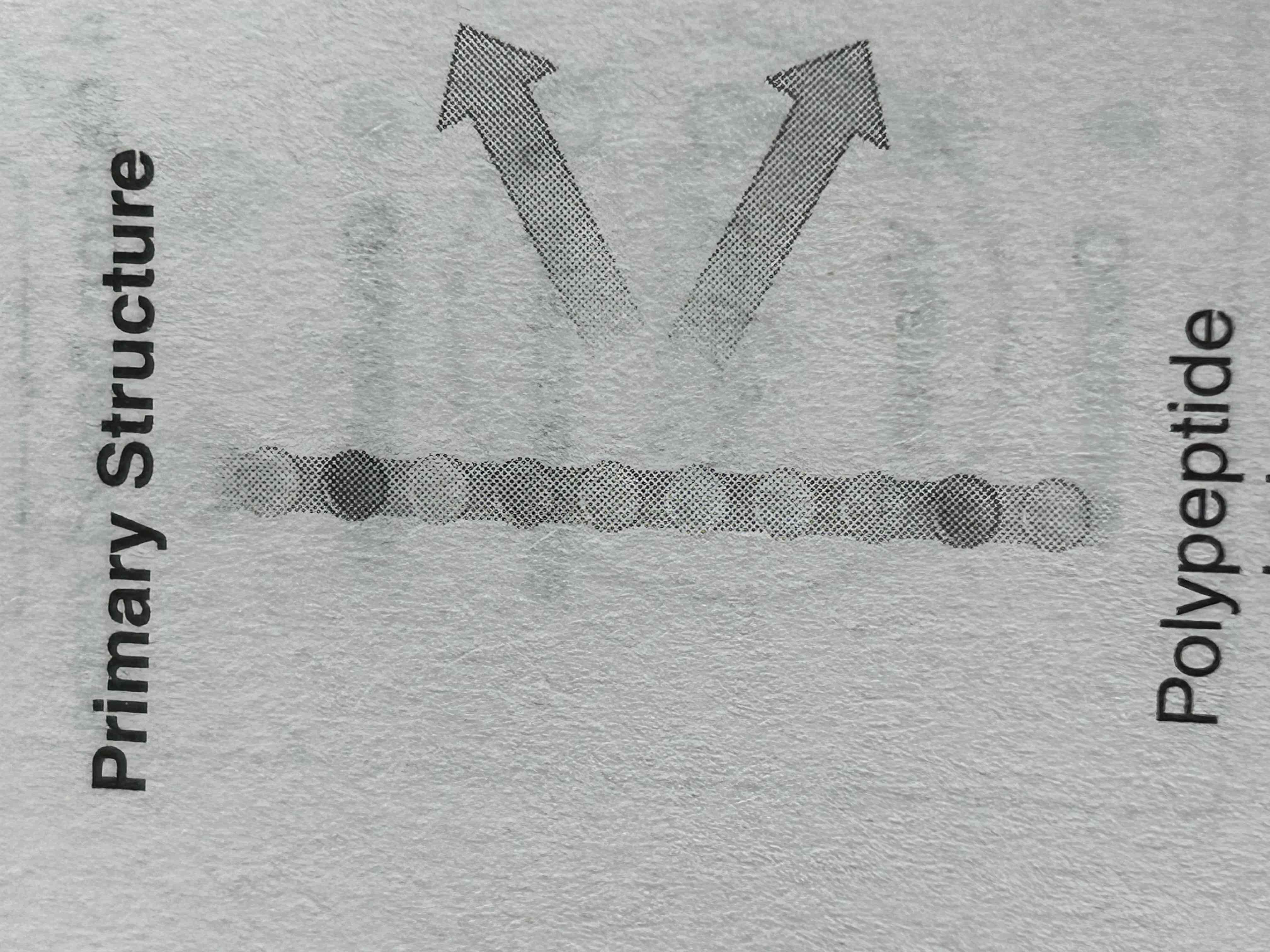
Primary protein structure
sequence of a chain of amino acids joined by peptide bonds
resulting polypeptide has directionality, with amino (NH2) terminus and a carboxyl (COOH terminus)
order of amino acids in chain determines primary structure of protein
secondary protein structure
Folding or coiling of a polypeptide chain.
Cause: Hydrogen bonding between backbone atoms of amino acids.
Forms: Alpha helices (coils) or beta-pleated sheets (folds).
Stabilized by: Hydrogen bonds.

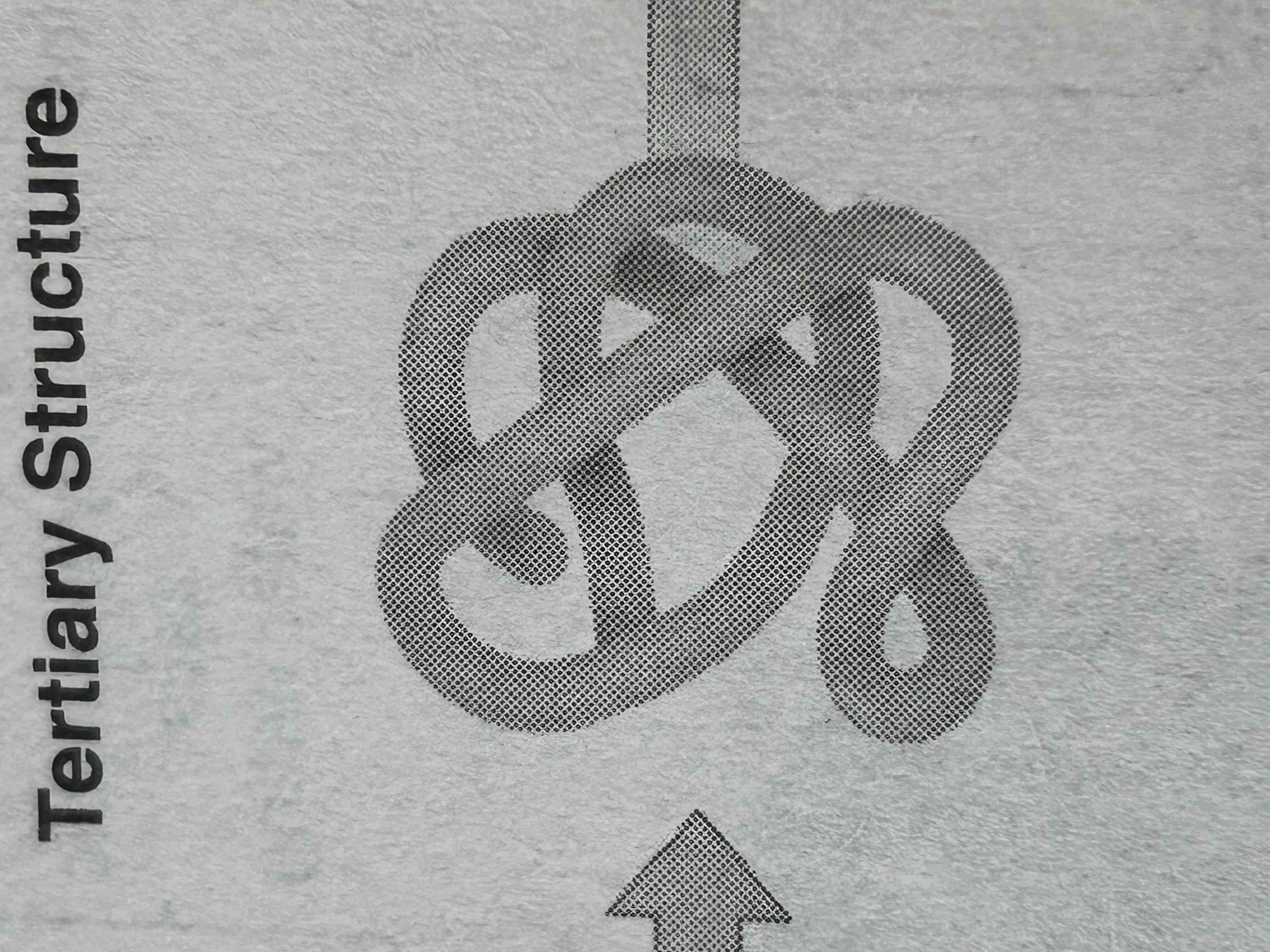
tertiary structure of protein
he three-dimensional shape formed by the folding of the secondary structure (alpha helices and beta sheets), driven by interactions between the R groups (side chains) of amino acids. These interactions include hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions. This structure is critical to the protein’s function.
Key Point: Tertiary structure = 3D shape, stabilized by various interactions (ionic, hydrophobic, etc.).
quaternary structure of a protein
multiple polypeptide chains (called subunits) come together and interact in a protein. The subunits are held together by non-covalent bonds like hydrogen bonds, ionic bonds, and hydrophobic interactions. An example is hemoglobin, made of four subunits.
Key points:
Made of multiple polypeptide chains.
Held by non-covalent bonds (e.g., hydrogen, ionic, hydrophobic).
Dipeptide
a molecule formed when two amino acids are joined by one peptide bond
product of a rxn between 2 amino acids, single peptide bond links them together
peptide bond = BOND ITSELF, dipeptide = molecule formed when 2 amino acids are linked
What causes protein denaturation?
- extreme high temperatures
- non-optimum ph
-addition of heavy metals (Pb, Hg)
nucleic acids
biological macromolecules that store and transmit genetic information in living organisms. They are polymers made up of nucleotides, each consisting of a nitrogenous base, a five-carbon sugar (either ribose or deoxyribose), and a phosphate group. The two main types of nucleic acids are DNA and RNA
macromolecules containing hydrogen, oxygen, nitrogen, carbon, and phosphorus (CHONP)
types of nucleic acids
DNA, RNA
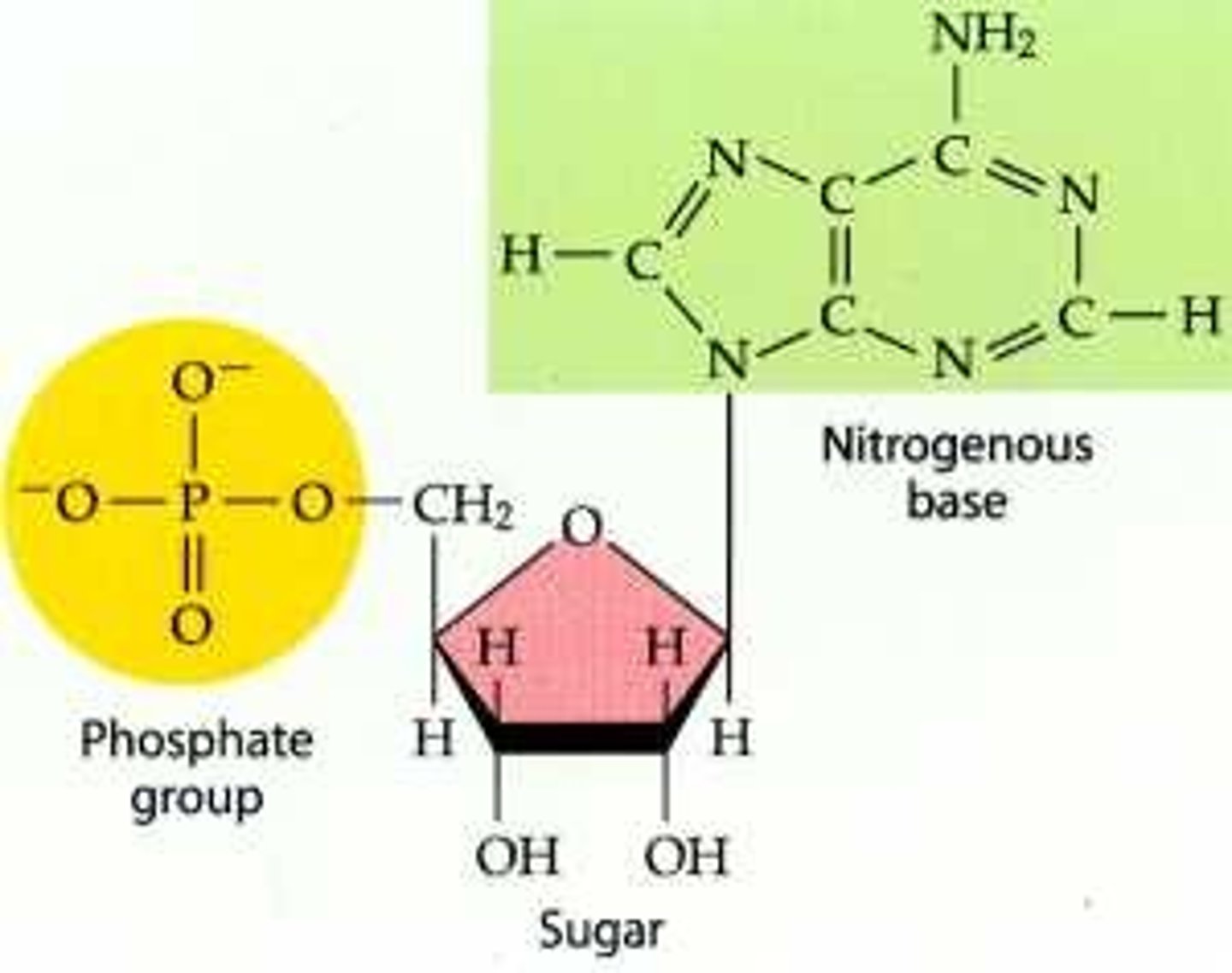
nucleotide
monomer of nucleic acids made up of a 5-carbon sugar (deoxyribose or ribose), a nitrogenous base (adenine, thymine, cytosine, guanine or uracil), and a phosphate group
when nucleotides link together in a chain, they form nucleic acids → essential for storing/transmitting genetic information
directionality of nucleotides
phosphate group is always attached to the 5’ carbon in sugar
3’ carbon always has a hydroxyl group to which new nucleotides may be added
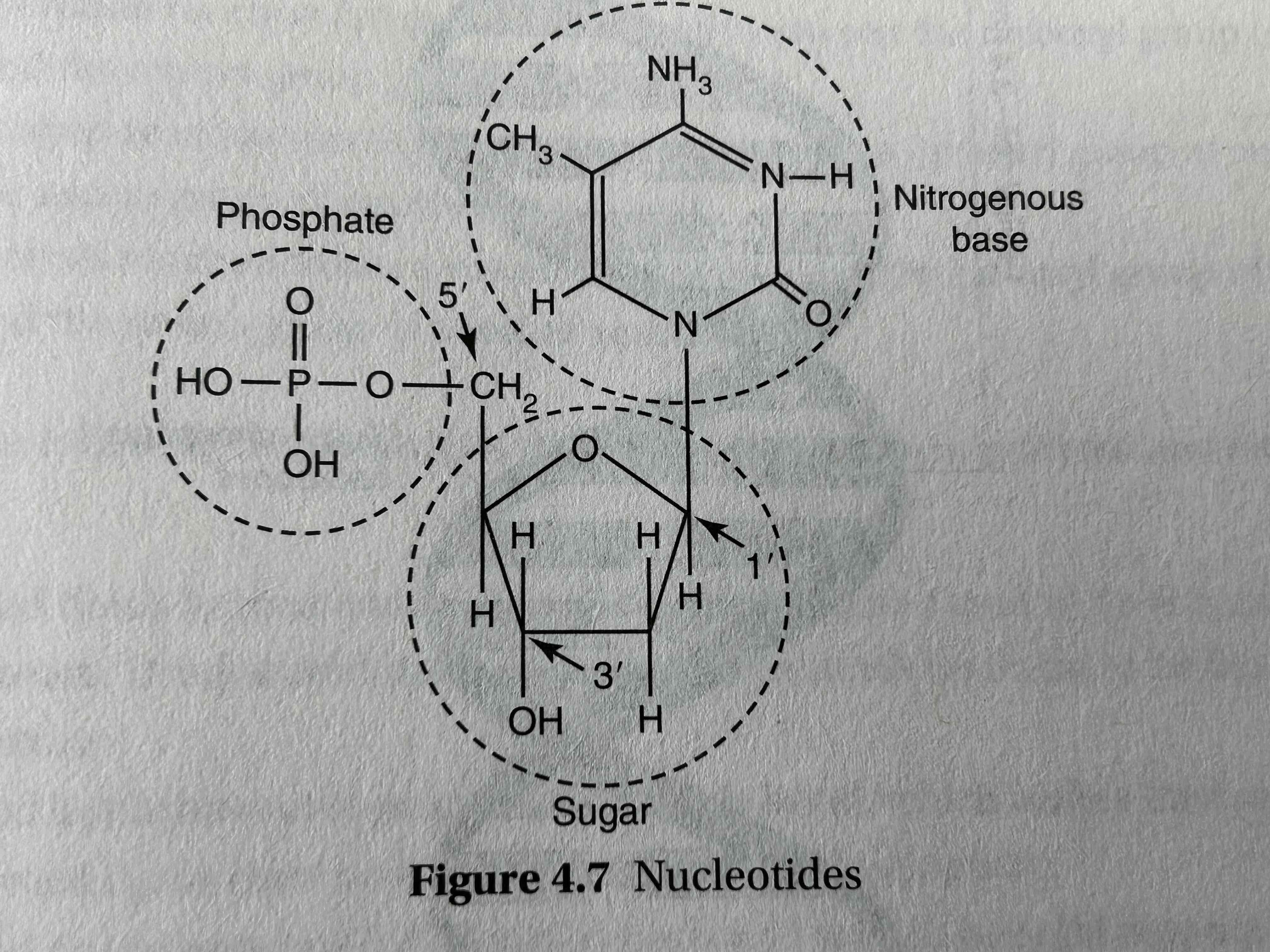
general nucleic acid strand has...
a sugar-phosphate backbone and nitrogenous bases projecting to the side

Pyrmidines
Pyrimidines: "CUT the PYe"
Cytosine
Uracil
Thymine
bases with a single ring structure
thymine, uracil, cytosine
purines
Bases with a double-ring structure.
Adenine and Guanine
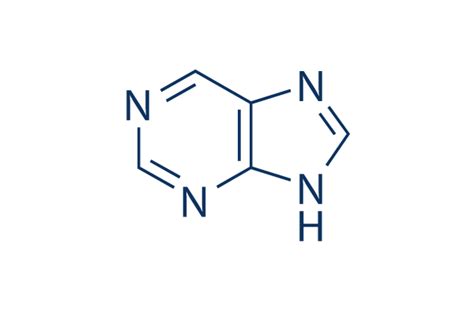
RNA
A single-stranded nucleic acid that contains the sugar ribose. RNA is typically shorter than DNA and plays crucial roles in protein synthesis and gene expression. It consists of nucleotide units made up of a nitrogenous base (adenine, uracil, cytosine, or guanine) and a phosphate group.
3 kinds of RNA
mRNA, tRNA, rRNA
mRNA
messenger RNA; type of RNA that carries instructions from DNA in the nucleus to the ribosome
tRNA
transfer RNA; type of RNA that carries amino acids to the ribosome
rRNA
ribosomal RNA; type of RNA that makes up part of the ribosome
ribosome
site of protein synthesis
nitrogenous bases of RNA
Adenine, Uracil (instead of thymine), Cytosine, Guanine
DNA vs RNA
DNA:
five carbon sugar: deoxyribose
nitrogenous bases: adenine, thymine, cytosine, guanine
strands: double stranded helix
function: holds genetic info
location: usually in nucleus
RNA
five carbon sugar: ribose
nitrogenous bases: adenine, uracil, cytosine, guanine
strands: usually single stranded but can form 3D structure when folded
function: translates + regulates expression of genetic info
location: in both nucleus and cytoplasm
ATP
(adenosine triphosphate) main energy source that cells use for most of their work
- "energy currency"
- used for large number of energy demanding reactions
ADP
Adenosine Diphosphate (ADP): The compound formed when ATP loses a phosphate group, releasing energy.
"Low-energy" form of ATP.
Energy release: Provides free energy, which is used in energetically unfavorable reactions.
nucleotides are…
Composed of a nitrogenous base, sugar, and phosphate group
Building blocks of DNA and RNA
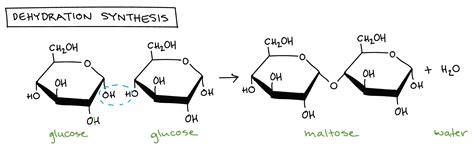
dehydration synthesis
A chemical reaction in which two molecules are bonded together with the removal of a water molecule.
What are the structural differences between glycogen, cellulose and starch?
Glycogen: highly branched
Cellulose: rigid, straight
Starch: slightly branched
Simple sugars (monosaccharides)
building blocks of carbohydrates; glucose, fructose, galactose
all isomers of eachother
Polysaccharides
Carbohydrates that are made up of more than two monosaccharides
hydration shell
water molecules that surround an ion during dissociation (disolving ionic compounds) → stabilizes ions in solution and prevents them from recombining
dissociation
the process by which an ionic compound breaks apart into its individual ions when dissolved in a solvent like water
Starch
A storage polysaccharide in plants made up of long chains of glucose molecules. It consists of two types of molecules: amylose (unbranched) and amylopectin (branched). Amylopectin is the more predominant form, making starch somewhat branched.
Glycogen
storage form of glucose in animals
- branched
Cellulose
Carbohydrate component of plant cell walls. - rigid, straight
Lactose = ______ + ______
glucose + galactose
maltose = __ + __
glucose + glucose
sucrose = __ + __
glucose + fructose
Four types of macromolecules
carbohydrates, lipids, proteins, nucleic acids
Four reasons why water is vital for life
- high heat of absorption (allows large bodies of water to maintain constant temperature)
- freezes from top -> down (protects organisms living in ponds/lakes)
- acts as a solvent, dissolves other polar molecule (it transports nutrients + waste in organisms, facilitaes chemical reactions, maintains structure/function of cells)
- adhesive + cohesive qualities
water's adhesive properties
- Water molecules are polar, with partial positive and negative charges. This polarity allows them to form hydrogen bonds with other polar or charged surfaces, leading to adhesion.
- helps in capillary action, where water moves through narrow spaces against gravity. This is vital for plants, as it allows water to travel from the roots up through the xylem to the leaves, facilitating nutrient and water distribution.
water's cohesive properties
- Water’s attraction to itself, causing molecules to stick together. They're polar and form hydrogen bonds with each other. This results in high surface tension and the tendency of water to form droplets.
- cohesion helps maintain the structure of cellular fluids and supports the formation of cellular membranes.
surface tension
The force that causes the surface of a liquid to act like a stretched elastic sheet, minimizing its surface area.
Why It Happens: Molecules at the surface of a liquid experience stronger cohesive forces inward because they are not surrounded by other molecules on all sides, creating a “skin” that resists external forces.
what's the relationship between water's high specific heat and its resistance to temperature changes?
it can absorb and release large amounts of heat with only small changes in temperature. This property makes water resistant to rapid temperature changes, helping to stabilize temperatures in both the environment and within organisms.
evaporative cooling
The process in which the surface of an object becomes cooler during evaporation, a result of the molecules with the greatest kinetic energy changing from the liquid to the gaseous state.
-
what allows ice to float on water?
its solid form is less dense than its liquid form due to the arrangement of water molecules in a crystalline structure, which creates more space between them.
solvent
A liquid substance capable of dissolving other substances
solution
A homogeneous mixture of two or more substances
solute
A substance that is dissolved in a solution.
how does water dissolve ionic compounds
The hydrogen in the water pulls off the negative ion, and the oxygen in the water pulls off the positive ion. This causes the ions to split up, and for the compound to become dissolved.
hydrophobic
Having an aversion to water; tending to coalesce and form droplets in water.
hydrophilic
Having an affinity for water.
buffer
compound that prevents sharp, sudden changes in pH
- maintains pH and homeostasis in living beings
carbohydrates contain
carbon, hydrogen, oxygen
Ratio of carbohydrates
1 carbon: 2 hydrogen: 1 oxygen
hydrolysis
Breaking down complex molecules by the chemical addition of water
polysaccharides
Carbohydrates that are made up of more than two monosaccharides
examples of polysaccharides
starch, glycogen, cellulose
when acids dissociate, ___ are released
H+ ions
when bases dissociate, ___ are released
OH- ions
disaccharides
double sugars
formation of these sugars through dehydration synthesis (removal of water to join 2 molecules)
sucrose, lactose, maltose
using surcose and its constituent simple sugars, show a hydrolysis reaction
sucrose + water → glucose + fructose
using maltose and its constituent simple sugars, show a dehydration synthesis reaction
glucose + glucose → water + maltose
Steroids
Lipids with a structure of four fused carbon rings. Examples include cholesterol (maintains cell membrane fluidity) and steroid hormones like testosterone and estrogen, which regulate growth and metabolism.
R group
a side chain attached to a molecule, particularly in amino acids.
unique for each amino acid
Determines amino acid’s identity and whether the amino acid will be non polar, polar, acidic, basic
Function of proteins
enzyme catalysis
maintaining cell structures
cell signaling
cell recognition
example of a pentose sugar
ribose
Hexose sugars derived from the hydrolysis of sucrose:
The two hexose sugars are glucose and fructose
Biomolecule stored in the adipose tissue of mammals
Triglycerides (fats) are stored in the adipose tissue
Number of carbon rings in the distinctive steroid compounds
Steroid compounds have four carbon rings
Chemical used as the building block for all steroid compounds in the human body
Cholesterol is the building block for steroid compounds.
identify this molecule
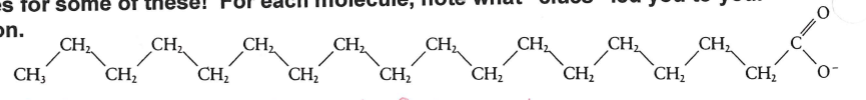
saturated fatty acid
identify this molecule
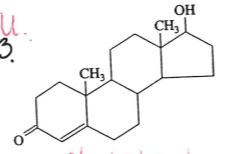
cholesterol
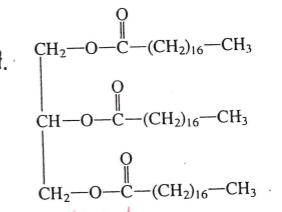
identify this molecule
lipid
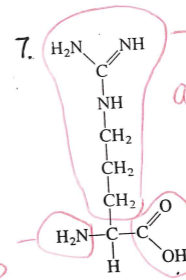
identify this molecule
amino acid
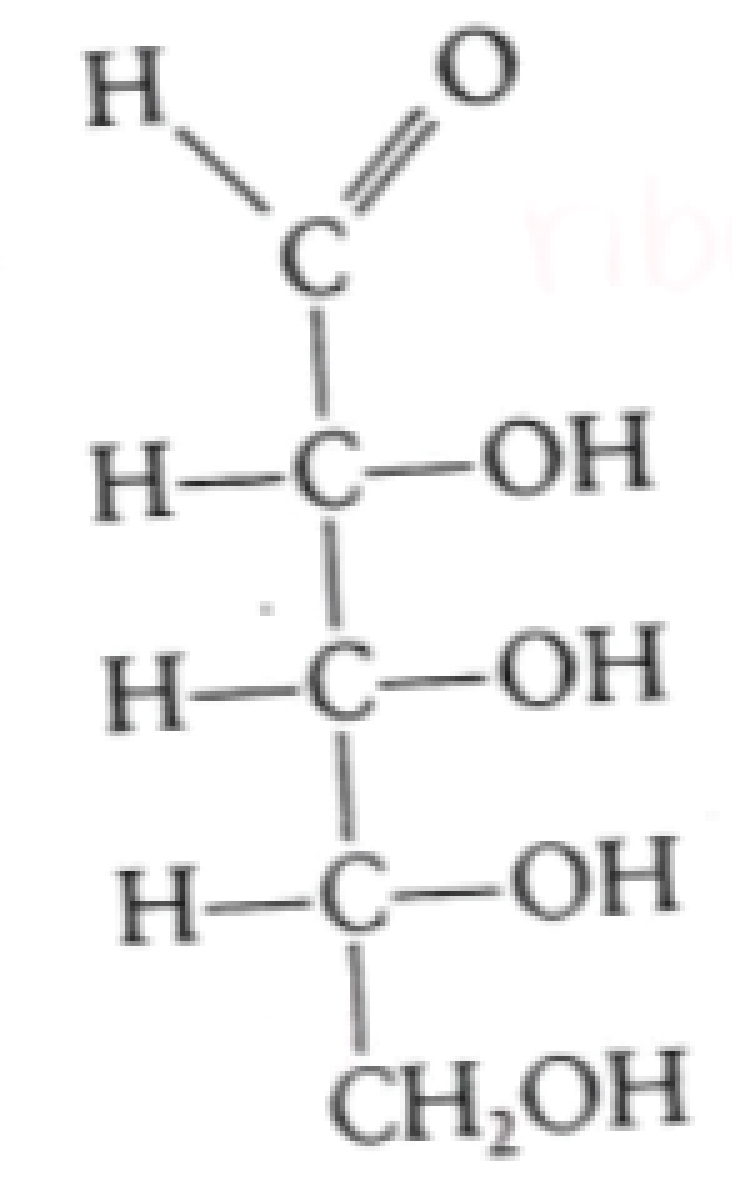
identify this molecule
ribose
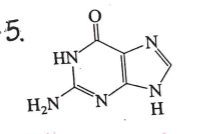
identify this molecule
nitrogenous base
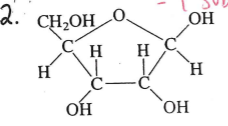
identify this molecule
simpe sugar (fructose) monosaccharide