Chapter 6 - Energy, Enzymes, and Biological Reactions
1/141
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
142 Terms
Slide 2 - 6.1 Energy, Life, and Laws of Thermodynamics
Define Energy
defined as the capacity to do work
Slide 2 - 6.1 Energy, Life, and Laws of Thermodynamics
Different forms of energy
chemical,
electrical,
thermal,
and radiant energy (including light, gamma rays, and X-rays)
Slide 2 - 6.1 Energy, Life, and Laws of Thermodynamics
Energy can be converted from one form to another – in plants, the radiant energy of sunlight is transformed into __________ in the form of ________
chemical energy; organic molecules
Slide 3 - Kinetic Energy and Potential Energy
All forms of energy can exist in one of two states namely _______ or ___________, which are interconvertible
kinetic or potential
Slide 3 - Kinetic Energy and Potential Energy
Kinetic energy - Define and provide examples
energy of an object in motion
Ex: a falling rock, electricity and light
Slide 3 - Kinetic Energy and Potential Energy
Potential energy - Define and provide examples
is stored energy
Examples: a rock at the top of a hill, chemical energy, gravitational energy, and stored mechanical energy
Slide 4 - Energy Flow in Natural Systems
What is Thermodynamics?
study of energy and its transformations
Slide 4 - Energy Flow in Natural Systems
When discussing thermodynamics, scientists refer to a ________, which is the object under study – everything outside it is the _______.
system
surroundings
Slide 4 - Energy Flow in Natural Systems
What are the three types of systems?
All living organisms are _____ systems.
isolated, closed, and open systems
open
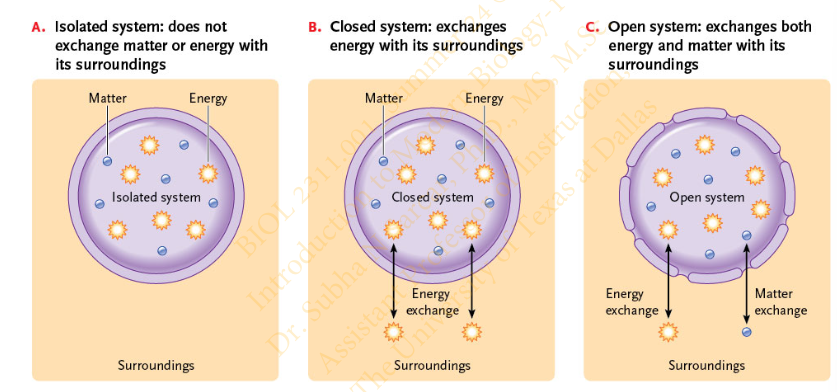
Slide 5 - Systems in Thermodynamics
does not exchange matter or energy with its surroundings. Which system?
Isolated
Slide 5 - Systems in Thermodynamics
exchanges both matter and energy with its surroundings
Open System
Slide 5 - Systems in Thermodynamics
exchanges energy with its surroundings
Closed System
Slide 5 - Systems in Thermodynamics - Diagram
Slide 6 - First Law of Thermodynamics
State First law of thermodynamics
Energy can be transformed from one form to another, or transferred from one place to another, but it cannot be created or destroyed
Also called the principle of conservation of energy
Slide 6 - First Law of Thermodynamics
In any process that involves energy change, the total amount of energy in a system and its surroundings ______________
remains constant
Slide 7 - Energy Flow from the Sun
For most organisms, the ultimate source of energy is the _____
sun
Slide 7 - Energy Flow from the Sun
plants capture kinetic energy of __________and convert it to the __________ of complex organic molecules
light ; chemical potential energy
Slide 7 - Energy Flow from the Sun
________________ energy stored in sugars and other organic molecules is used for ________, ________, and other work of living organisms
Chemical potential
growth; reproduction
Slide 7 - Energy Flow from the Sun
Most of the solar energy absorbed by green plants is converted into _______ energy (which is largely unusable by living organisms). and ________ into space
heat
radiated
Slide 7 - Energy Flow from the Sun - Diagram

Slide 8 - Second Law of Thermodynamics
Second law of thermodynamics
The total disorder (entropy) of a system and its surroundings always increases (although the total energy in the universe does not change)
Slide 8 - Second Law of Thermodynamics
Second law of thermodynamics - what is entropy
The total disorder (entropy) of a system and its surroundings
Slide 8 - Second Law of Thermodynamics
Living organisms seem to _______ in entropy as they grow. When nutrients and waste products are considered, total energy _________ (increases / decreases / remains constant ) and entropy ________ (increases / decreases / remains constant )
decrease ;
remains constant
increases
Slide 9 - 6.2 Free Energy and Spontaneous Reactions
A chemical or physical reaction that occurs without an input of energy is a
A spontaneous reaction
Slide 9 - 6.2 Free Energy and Spontaneous Reactions
Two factors related to the first and second laws of thermodynamics that must be considered to determine whether a reaction is spontaneous are:
(1) the change in energy content of a system, and
(2) its change in entropy
Slide 10 - Energy Content and Entropy
Reactions tend to be spontaneous if the products have [more/less] _______ energy than the reactants
less
potential
Slide 10 - Energy Content and Entropy
What is enthalpy (H)?
The potential energy in a system is its enthalpy (H)
Slide 10 - Energy Content and Entropy
Reactions that release energy are ______
exothermic
Slide 10 - Energy Content and Entropy
Reactions that absorb energy are _______
endothermic
Slide 10 - Energy Content and Entropy
A reaction where the products have less potential energy than the reactants is an ___________ (exothermic / endothermic) reaction.
endothermic
Slide 10 - Energy Content and Entropy
In an endothermic reaction, the products have ______(more/less) __________energy than the reactants
more
potential
Slide 10 - Energy Content and Entropy - Please verify this question
when the products are less ordered (more random) than the reactants, the reactions tend to be __________
spontaneous
Slide 10 - Energy Content and Entropy - Please verify this question
Reactions tend to occur spontaneously if the entropy of the products is _____________(greater / lesser) than the entropy of the reactants
greater
Slide 11 - Change in Free Energy (1 of 2)
The portion of a system’s energy that is available to do work is called _____ energy (G)
free
Slide 11 - Change in Free Energy (1 of 2)
The change in free energy, ΔG can be calculated for any chemical reaction from the formula
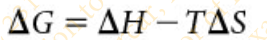
Slide 11 - Change in Free Energy (1 of 2)
ΔH is the change in ___________
enthalpy
Slide 11 - Change in Free Energy (1 of 2)
T is the _________
absolute temperature in degrees Kelvin (K)
Slide 11 - Change in Free Energy (1 of 2)
ΔS is the________
change in entropy
Slide 12 - Change in Free Energy (2 of 2)
For a reaction to be spontaneous, ΔG must be __________
negative
Slide 12 - Change in Free Energy (2 of 2)
In some processes, such as the combustion of methane, the large loss of potential energy, ___________, dominates in making a reaction spontaneous
negative enthalpy (ΔH)
Slide 12 - Change in Free Energy (2 of 2)
In other reactions, such as the melting of ice at room temperature, a __________ dominates
decrease in order (ΔS increases)
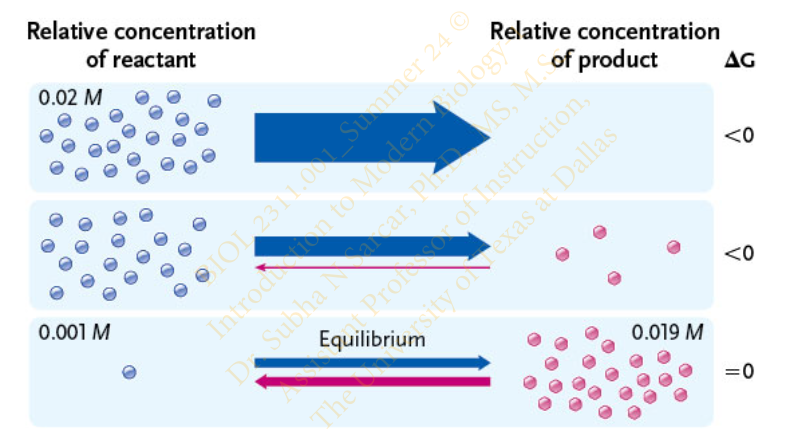
Slide 13 - Equilibrium Point
In many spontaneous biological reactions reactants _______ (may / may not) convert completely to products even though the reactions have a negative ΔG
may not
Slide 13 - Equilibrium Point
The reactions run in the direction of completion (toward reactants or toward products) until they reach the ___________
equilibrium point
Slide 13 - Equilibrium Point
Define Equilibrium Point
a state of balance between the opposing factors pushing the reaction in either direction
Slide 14 - Equilibrium Point
As a system moves toward equilibrium, what happens to its free energy when the system c
its free energy becomes progressively lower and reaches its lowest point when the system achieves equilibrium (ΔG = 0)
Slide 14 - Equilibrium Point
Can moving away from equilibrium be spontaneous?
To move away from equilibrium requires free energy and thus will not be spontaneous
Slide 14 - Equilibrium Point
The more negative the ΔG, the _______ completion the reaction will move before equilibrium is established
closer to
Slide 15 - Equilibrium Point of a Reaction
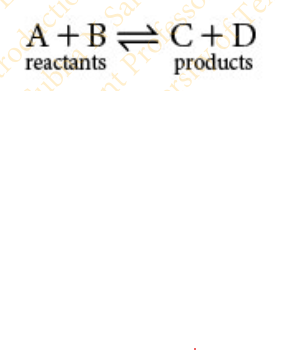
Slide 16 - Reversible Reactions
Many reactions have a ΔG that is near zero and are readily _________
reversible
Slide 16 - Reversible Reactions
Reactions are readily reversible by adjusting the concentration of ______________
products and reactants slightly
Slide 16 - Reversible Reactions
Reversible reactions are written with a double arrow:
Slide 17 - Reactions in Living Organisms
Many reactions in living organisms never reach equilibrium because of the following:
living systems are open – the supply of reactants is constant and products do not accumulate
Slide 17 - Reactions in Living Organisms
The ΔG of life is always __________ (positive / negative)
negative
Slide 17 - Reactions in Living Organisms
Why is ΔG of life is always negative
organisms constantly take in energy-rich molecules and use them to do work
Slide 17 - Reactions in Living Organisms
At what stage do organisms reach equilibrium?
only when they die
Slide 18 - Exergonic and Endergonic Reactions
What are the characteristics of an Exergonic reaction?
Reaction that releases free energy
ΔG is negative because the products contain less free energy than the reactants
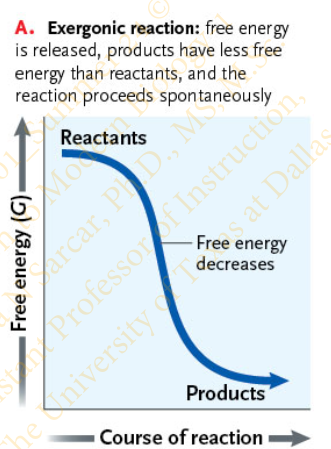
Slide 18 - Exergonic and Endergonic Reactions
What are the characteristics of an Endergonic reaction?
Reactants must gain free energy from the surroundings to form the products
ΔG is positive because the products contain more free energy than the reactants
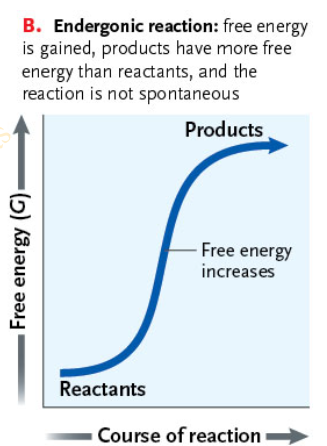
Slide 18 - Exergonic and Endergonic Reactions
In which of the reactions the ΔG is positive?
Endergonic
Slide 18 - Exergonic and Endergonic Reactions
In which of the reactions the energy is released?
Exergonic reaction
Slide 19 - Metabolic Pathways
What is a metabolic pathway?
a series of reactions in which the products of one reaction are used immediately as the reactants for the next reaction in the series
Slide 19 - Metabolic Pathways
In which pathway, the energy is released by the breakdown of complex molecules to simpler compounds;
catabolic pathway (or a single catabolic reaction)
Slide 19 - Metabolic Pathways
In a catabolic pathway, the overall ΔG is ____________(positive / negative)
negative
Slide 19 - Metabolic Pathways
In which pathway, the energy is used to build complicated molecules from simpler ones;
anabolic pathway (or an anabolic reaction or biosynthetic reaction)
Slide 19 - Metabolic Pathways
In a anabolic pathway, the overall ΔG is ____________(positive / negative)
positive
Slide 20
ATP stands for
adenosine triphosphate
Slide 20 - Adenosine Triphosphate (ATP): Energy Currency of the Cell
The nucleotide adenosine triphosphate (ATP) consists of the
__________ linked to the ___________ base adenine and a chain of _________
five-carbon sugar ribose
nitrogenous
three phosphate groups
Slide 20 - Adenosine Triphosphate (ATP): Energy Currency of the Cell
What makes the bonding arrangement in Phosphate groups unstable?
The negative charges of the phosphate groups repel each other strongly, making the bonding arrangement unstable
Slide 20 - Adenosine Triphosphate (ATP): Energy Currency of the Cell
Removal of one or two phosphate groups is a _________ reaction that releases ___________
spontaneous
large amounts of free energy
Slide 21 - Hydrolysis of ATP
The breakdown of ATP is a [hydrolysis/dehydration synthesis] reaction
hydrolysis
Slide 21 - Hydrolysis of ATP
Hydrolysis of ATP results in ?
results in the formation of adenosine diphosphate (ADP) and a molecule of inorganic phosphate (Pi )
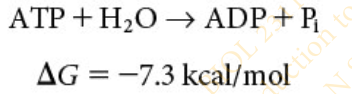
Slide 21 - Hydrolysis of ATP
ADP can be hydrolyzed to _______
adenosine monophosphate (AMP)
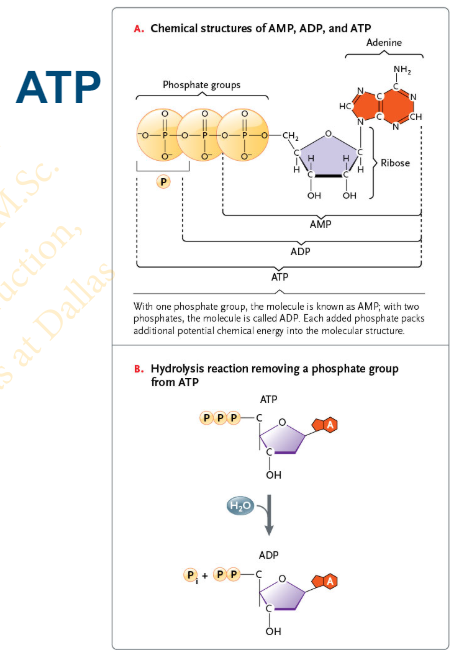
Slide 21 - Energy Coupling, using ATP
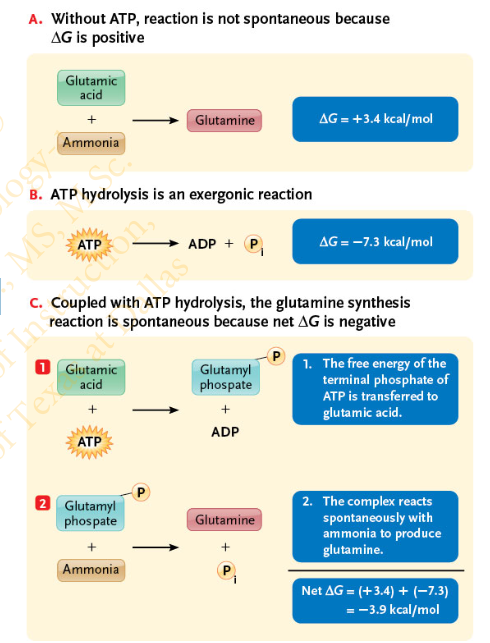
In the process of energy coupling, when ATP is hydrolyzed, the _______ group is transferred to a __________ involved in an ________ reaction
terminal phosphate
reactant molecule
endergonic
Slide 21 - Energy Coupling, using ATP
What is phosphorylation?
The addition of a phosphate group to a molecule is called phosphorylation (the modified molecule is phosphorylated)
Slide 22 - Energy Coupling, using ATP
what are the features of the enzyme that energy coupling requires, and why?
Energy coupling requires an enzyme with a specific site that binds both ATP and the reactant molecule to bring the ATP and reactant molecule into close association
Slide 23 - Regeneration of ATP
ATP synthesis from ADP and Pi is an _________ reaction that uses energy from the _____________, ___________, and ________(food)
endergonic
exergonic breakdown of carbohydrates,
proteins
fats
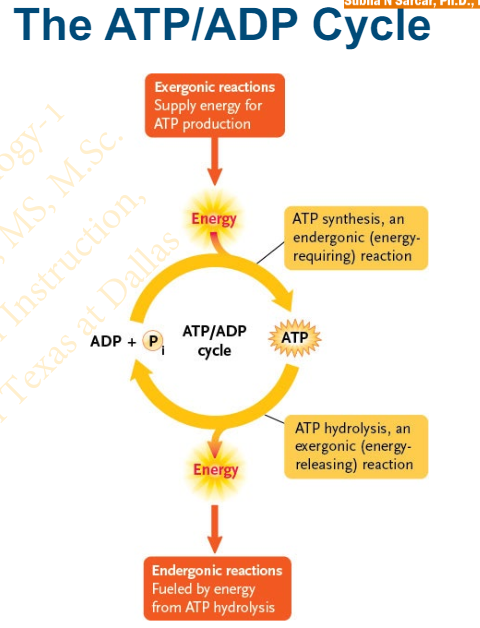
Slide 23 - Regeneration of ATP
The continual hydrolysis and resynthesis of ATP is called the ________
ATP/ADP cycle
Slide 23 - Regeneration of ATP
Approximately how many ATP molecules are hydrolyzed and resynthesized each second in a typical cell
10 million
Slide 24 - 6.4 Role of Enzymes in Biological Reactions
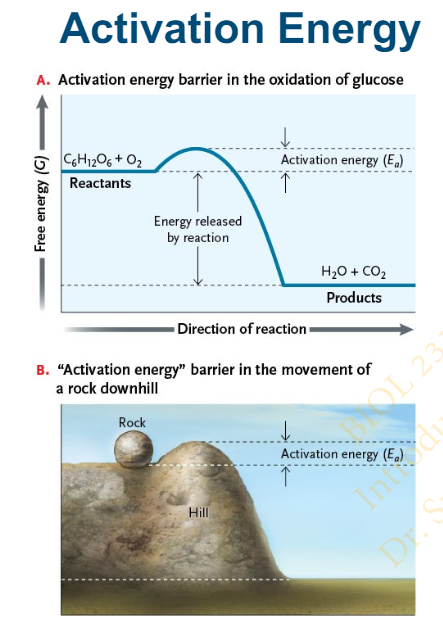
Even when a reaction is spontaneous (negative ΔG), the reaction will not start unless ____________
a small amount of activation energy (Ea) is added
Slide 24 - 6.4 Role of Enzymes in Biological Reactions
What are the effects of Activation energy on bonds. Explain with an example
unstable and ready to be broken (the transition state)
Example: Activation energy is like the effort required to raise a rock over the rim of a depression and start it rolling downhill
Slide 25 - Activation Energy Diagram
Slide 25 - Enzymes Reduce Activation Energy Diagram
Slide 26 - Enzymes
A catalyst is a chemical agent that ___________ the rate of a reaction without being ______ by the reaction
accelerates (catalyzes)
changed
Slide 26 - Enzymes
The most common biological catalysts are _____called ________,
proteins
enzymes
Slide 26 - Enzymes
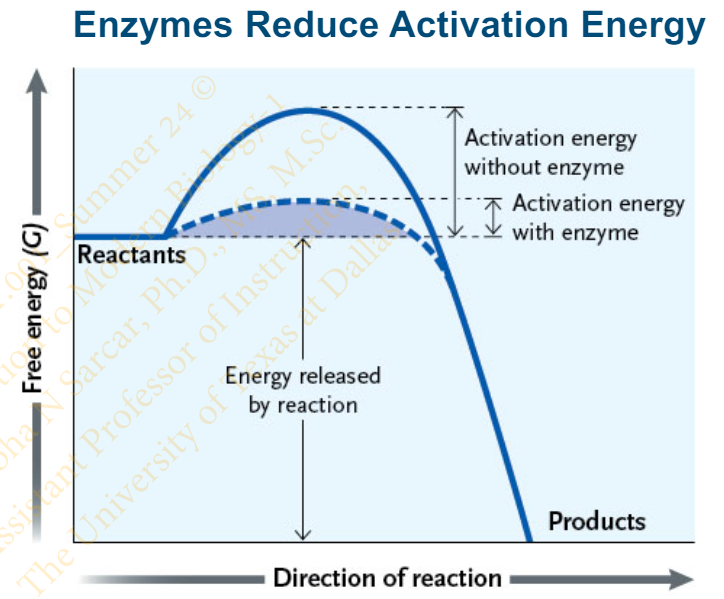
what is the effect of enzymes on the rate of reaction? and how does it do that?
Enzymes increased the rate of reaction by lowering the activation energy of the reaction
Slide 26 - Enzymes
Enzymes _______(do/do not) alter the ΔG of the reaction
do not
Slide 26 - Enzymes
The free energy ___________ (varies / stays the same); the difference is in the path the reaction takes
The free energy stays the same; the difference is in the path the reaction takes
Slide 27 - Enzymes
What determines the function of a protein? (give the term)
The 3-D structure of a protein (its conformation) determines its function
Slide 27 - Enzymes
each enzyme has a _________that catalyzes a _________
specific protein structure
specific reaction
Slide 27 - Enzymes
Cells have thousands of different enzymes, found in different areas inside and outside of the cell
The name of an enzyme typically refers to its ________ or _________, and ends in _______
substrate
type of reaction
–ase (e.g., proteinases)
Slide 28 - An Enzyme and its Substrate
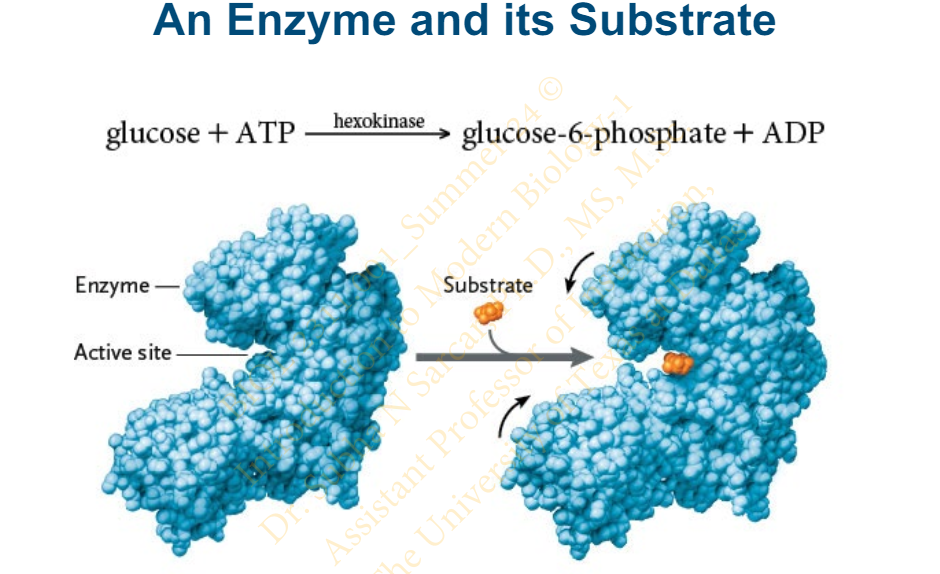
Slide 29 - Enzymatic Reactions
What happens in an enzymatic reaction?
an enzyme combines briefly with reacting molecules and is released unchanged when the reaction is complete
Slide 29 - Enzymatic Reactions
The reactant that an enzyme acts on is called the _____
substrate
Slide 29 - Enzymatic Reactions
Each type of enzyme catalyzes the reaction of __________ or ___________. this is called ______
a single type of substrate molecule
group of closely related molecules
enzyme specificity
Slide 29 - Enzymatic Reactions
The substrate interacts with a small pocket or groove in the _________, called the __________
enzyme molecule
active site
Slide 29 - Enzymatic Reactions
When the substrate binds at the active site, both ______ and _________ are distorted
enzyme
substrate molecules
Slide 29 - Enzymatic Reactions
What is an induced fit?
When the substrate binds at the active site, both enzyme and substrate molecules are distorted – this makes the chemical bonds in the substrate ready for reaction (induced fit)
Slide 29 - Enzymatic Reactions
When does a catalysis occur?
What happens in a catalysis?
Catalysis occurs once an enzyme-substrate complex is formed
When catalysis occurs, the substrate is converted into one or more products
Slide 31 - Catalytic Cycle of Enzymes
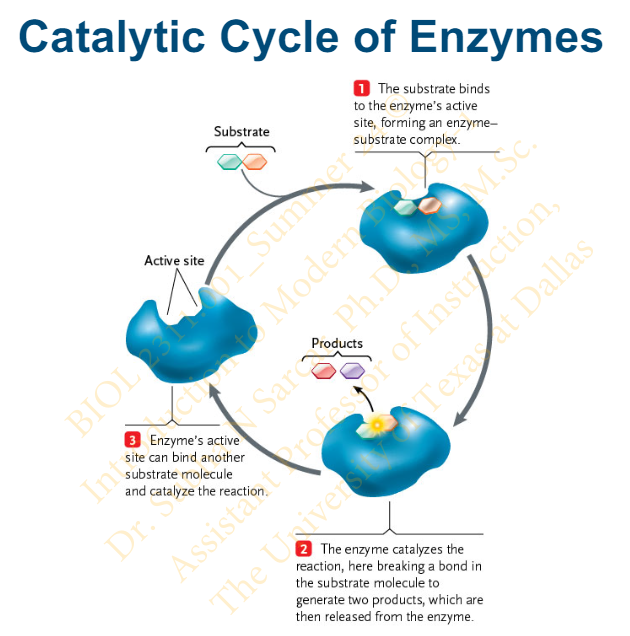
Slide 32 - Cofactors and Coenzymes
What is a cofactor
a nonprotein group that binds to the enzyme, promotes catalytic activity
Slide 32 - Cofactors and Coenzymes
What does a cofactor help with?
Many enzymes require a cofactor, for catalytic activity