Week 5 - Fastidious Gram Negative Rods
1/117
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
118 Terms
Fastidious Gram Negative Rods Introduction:
Fastidious organisms have complex nutritional requirements, and do not grow readily on routinely used media.
Many require the addition of specific nutritional growth factors to culture medium to make isolation and identification possible
Depending on Genus, fastidious GNR can be facultatively anaerobic (grows in presence or absence of oxygen), while others are strict aerobes or microaerophilic.
Glucose fermenter non-Enterobacteriaceae
Greatly varied in natural habitats and sources of infection
Human infections with fastidious GNR relatively uncommon.
Most commonly recovered organisms include: Haemophilus influenzae, Francisella tularensis, Legionella pneumophila, and Bordetella pertussis
Other organisms discussed in this lecture include: Brucella, the cause of brucellosis (and potential bioterrorism agent); the HACEK group of organisms, which are implicated in sub acute bacterial endocarditis;
Streptobacillus, a slow growing agent of rat-bite fever; as well as misc fastidious including Bartonella, Pasteurella & Capnocytophaga species.
Haemophilus spp: General Characteristics:
Slow growing Facultative anaerobes
Usually require enriched medium that contains fresh blood or some of it’s components
Most need Heme (X factor) or NAD (V factor) for growth
Factor X can diffuse from intact red blood cells and is available in sheep blood agar; Factor V cannot diffuse from intact RBCs and is only released due to hemolysis
Chocolate agar is recommended growth medium; due to fact that it is high in concentration for both factors because sheep blood is added to hot liquid agar causing lysis of the red cells and release of the factors
Some require 5-10% CO2
Short, pleomorphic (many forms) GNR
Sometimes assume coccobacillary morphology or cocci-like appearance on Gram stain
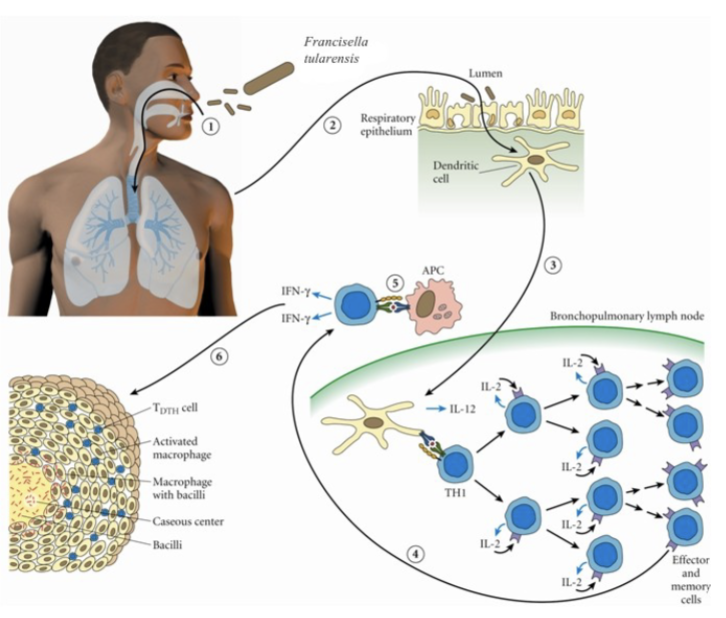
How do you acquire Francisella Tularensis:
Enters the respiratory tract and (2) the lamina propria of the respiratory bronchioles via M cells; (3) Digested antigen is taken up by dendritic cells; the dendritic cells travel to regional lymph nodes and present F. tularensis antigens to T-helper 1 cells; (4) T-helper 1 cells proliferate; they may return to site of initial infection; (5) re-stimulation by local antigen presenting cells results in interferon-γ production and macrophage activation; (6) Failure to clear the organism results in granuloma formation.
Francisella Tularensis History:
1911- Tularemia first described by McCoy- cause of “plague like disease”.
Initially named Bacterium tulerense after the Tulare county, California.
1912-1925- Edward Francis- studying “deerfly-fever” and made the connection between “plaque like disease” and “deerfly fever”.
Studied the causative agent, modes of transmission and coined the name “tularemia”.
Nobel Prize, 1959 and the name was changed from Bacterium tularense to Franciscella tularensis.
Francisella Tularensis General Characteristics:
Does not grow on most routine media
Grows best on cysteine supplemented agar
Blood-cysteine-glucose agar- Best growth & isolation
Enriched chocolate agar
Buffered charcoal yeast extract (same used for Legionella)
Highly infectious (BSL-2 safety precautions)
Due to its highly infectious nature, physicians should notify the laboratory if Francisella is suspected.
Clinical Laboratories (operating at Biosafety level 2) will only presumptively identify F. tularensis and will then forward to BSL-3 laboratory (such as state public health laboratories) for confirmation.
Identification of F. tularensis: (Biochemically)
relatively inert biochemically
• Full biochemical ID not recommended for identification due to
its infectious nature
Presumptive identification occurs based on growth requirements and observations, microscopic morphology and few preliminary biochemical reactions.
Final Identification includes serology methods including immunofluorescence staining, and testing with specific anti-sera.
Francisella Tularensis microscopic morphology:
Extremely small, intracellular gram-negative coccobacillus
Highly pleomorphic
Stains poorly on Gram stain
Typically missed in direct smears from tissue specimens
Francisella Tularensis colony morphology:
Examine plates under laminar flow hood
Small and transparent colonies
Pinpoint colonies after 24 hours
1 to 2 mm after 48 hours
Facultative anaerobe
Require cysteine for growth
Francisella Tularensis if using biochemicals:
Nonmotile
Oxidase, urease, indole and ornithine negative
Weakly positive catalase
Beta-lactamase positive
Direct fluorescence antibody and agglutination techniques are used for identification
Isolates can be tested for presence of polysaccharide antigen and at least one protein antigen
Serum antibody testing commonly used to make diagnosis if patients history and clinical manifestations are compatible with diagnosis of tularemia.
Antibody titer >160 in single specimen highly suggestive of F. tularensis
Four-fold increase in antibody titer in paired serum samples (taken 2 weeks apart) strongly indicative of active disease
Francisella Tularensis clinical significance:
Etiological agent of tularemia, a disease also known as glandular
fever, tick fever, and occasionally as deer fly fever, rabbit fever (zoonoses) and market men’s fever.
Present in a wide variety of wild animals, birds, and some fish or amphibians
Potential Bioterror Agent (Category A infectious agent).
Extremely virulent, disease occurs at low infective dose ( 10 colony forming unit)
Nationally reportable disease- presumptive ID requires notification of local health department, state health department, as well as national.
Most common reservoir of Francisella Tularensis:
Wild rabbits, muskrats and squirrels. Ticks & deerflies are most common arthropod vectors.
Hunters, Veterinarians, and taxidermists are at increased risk.
Transmission occurs through either (1) bite of an arthropod (2) direct contact with an infected animal, (3) ingestion of contaminated meat or water
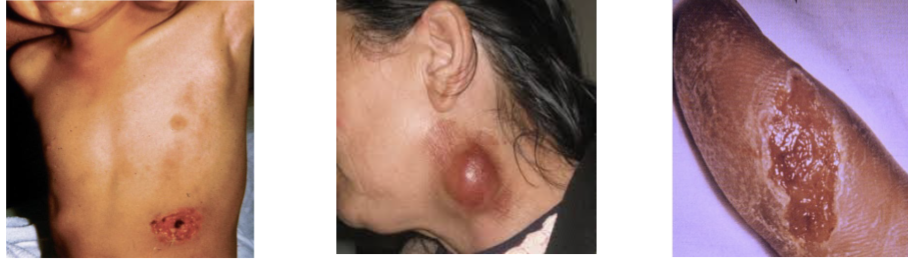
Ulceroglandular:
Associated with Tularemia
Formation of an ulcer at the site of infection, swelling of the lymph glands, fever, chills, headaches, fatigue
Osculoglandular:
Associated with Tularemia
Eye pain, redness of the eyes, discharge from the eye, formation of ulcer in the inside of the eyelid
Oropharyngeal:
Associated with Tularemia
fever, pharyngitis, mouth ulcers, vomiting, diarrhea
Pneumonic:
Associated with Tularemia
cough, chest pain, shortness of breath
Typhoidal:
Associated with Tularemia
high fever, extreme fatigue, vomiting, diarrhea, splenomegaly, hepatomegaly, pneumonia
Franscisella Tularensis infection:
The lymph node adjacent to the site of inoculation become enlarged and necrotic.
Organism is the blood stream cause high fever, headaches, and become systemically ill with chills and generalized aches.
Range from mild to self-limiting to fatal include ulceroglandualr, oculoglandualr. Oropharyngeal and pneumonic form.
The organism is destined to be Biosafety level 3. One of the most common cause of laboratory acquired infection.
F. philomiragia:
Rare cause of pneumonia
F. novicida:
Rare pathogen, closely related to F. tularensis
Rare cause of pneumonia, muscle pain, and fever
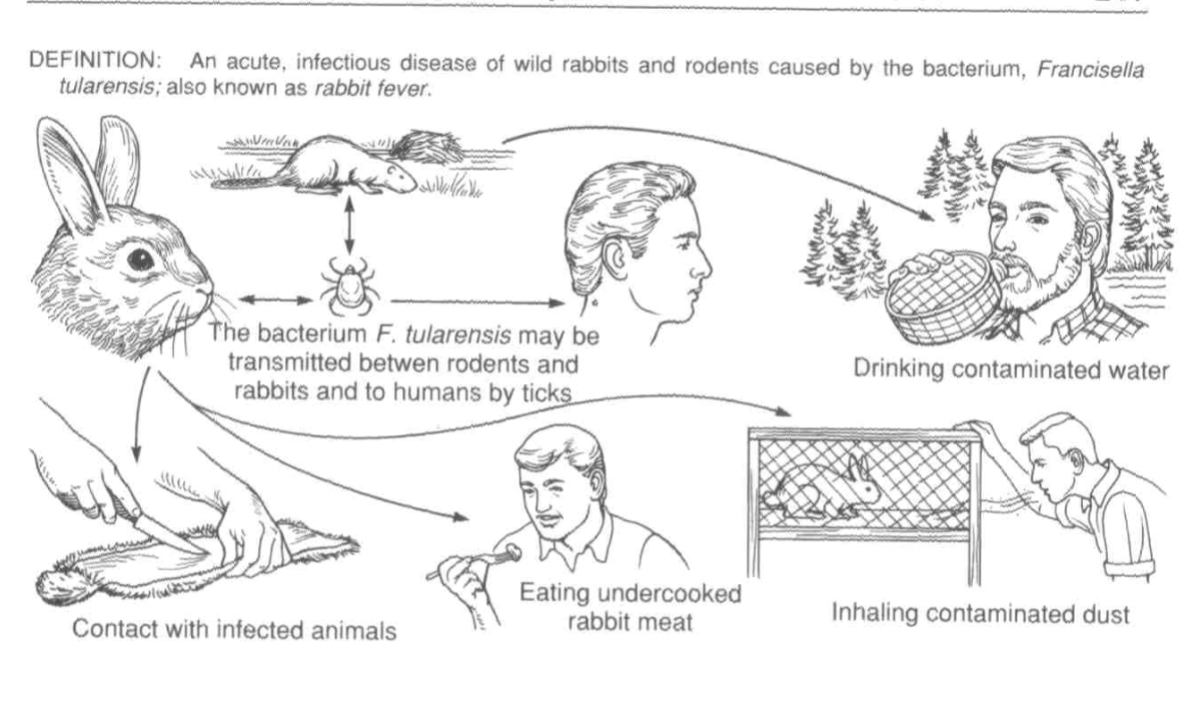
Ways to contract Tularemia
Summary of Francisella tularensis
Legionella Pneumophila general characteristics:
Aerobic GNR
Requires iron salts, cysteine, and high humidity for growth
BCYE agar is best
Buffered charcoal yeast extract
Slow grower
Blood cultures may require 2 weeks for growth
Culture and testing done in Class II biological safety cabinet
Legionellae ubiquitous environment:
warm and moist conditions prevail
Linked with various outbreaks with water tanks/cooling systems in hospitals and other environments
2015 outbreaks in NYC & Bronx NY. Organisms isolated from greater than a dozen water cooling tanks throughout city.
Recovered from lakes, streams, mud, and soil in many parts of the world
Nosocomial infection- nebulizer filled with tap water.
No known animal reservoir
More than 40 species of Legionella identified, but L.pneumophila is by far the most common cause of disease in humans
Legionella Pneumophilia typical specimens:
Bronchial washings
Lung biopsies
Pleural fluid
Blood
Sputum not optimal choice for culture due to abundance of
normal upper respiratory flora
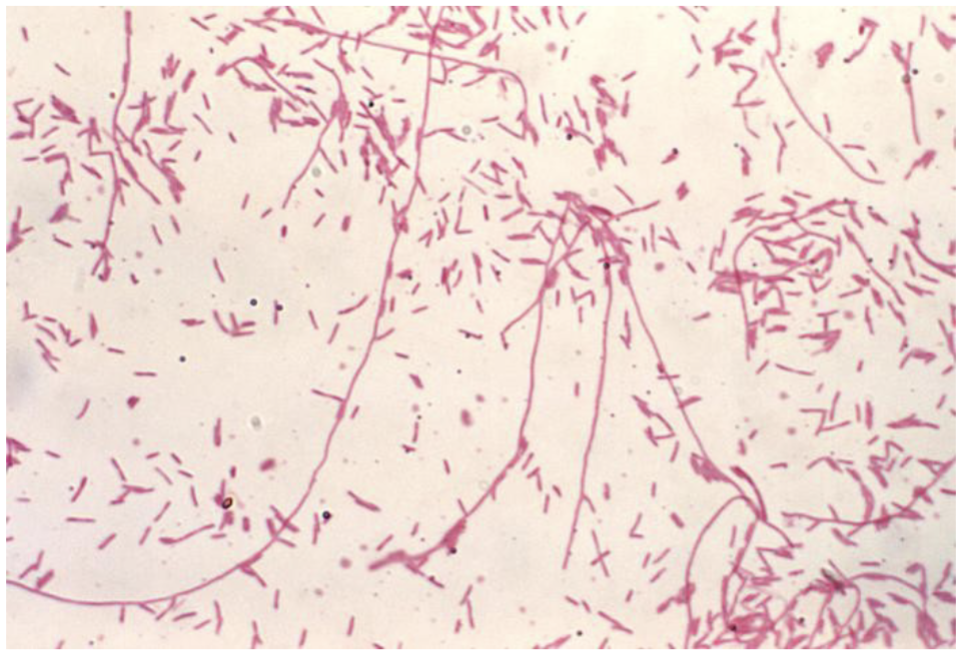
Legionella Pneumophilia microscopic morphology:
Relatively thin rods
Do not stain well with Gram stain
Basic fuchsin can be substituted for safranin; counterstain for up to 3 minutes
Small, coccobacillary and occasional filamentous forms are seen more readily on Gimenez stain
Direct fluorescence antibody testing can be useful, but
has low sensitivity compared to culture on BCYE agar
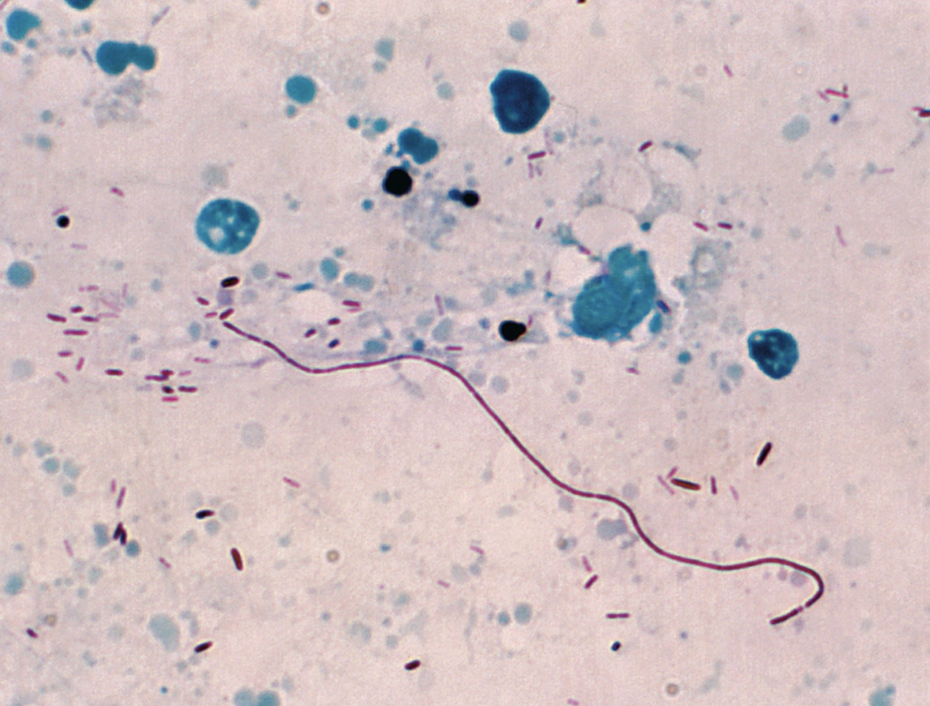
Gimenez stain of Legionella pneumonphilia
Legionella Pneumophilia: Colony morphology:
Cultures should be handles under laminar flow hood (BSC-II)
• Buffered charcoal-yeast extract agar (BCYE) is best for isolation
Francisella tularensis can also grow on BCYE agar and found in same respiratory specimens as L.pneumophila which is another reason why these plates should only be examined under laminar flow hood
Visible colonies appear after 3 to 4 days incubation
May be round or flat with entire edges, glistening, and convex
Ground glass speckling like a shattered windshield
Pigment varies from colorless, grayish, pale green to iridescent pink or blue
May also be translucent
Legionella Pneumophila: Key biochemical and other reactions:
Catalase positive
Gelatinase and beta-lactamase positive
Oxidase and hippurate positive
Growth on BCYE and pale yellow-green fluorescence under long- wave fluorescent light (Wood’s lamp)
Legionella Pneumophila immunologic methods:
Urine antigen testing
Serum antibodies can be tested but offer low sensitivity
Antibodies against organism slowly rise and often do not reach peak levels until 4-8 weeks after infection
Many patients retrospectively diagnosed by indirect fluorescent
antibody testing
Four-fold rise in anti-Legionella antibody to a titer of 128 or higher is considered positive
Legionella Pneumophia clinical significance:
serogroup 1 is the major cause of Legionnaire’s disease.
Disease most common in males over age of 50, with risk factors of chronic respiratory conditions or smoking
Asymptomatic infection possible among all age groups
Organism commonly acquired through inhalation of aerosols created by contaminated air conditioners, humidifiers, shower- heads, and other similar sources
Disease ranges from mild short term febrile illness, to acute purulent pneumonia, typically affecting those older individuals or immunocompromised the worst. Fatality rate is as high a 10%
L. pneumophila can also cause Pontiac fever, an influenza-
like illness that initially occurred during an outbreak in Michigan.
Nationally reportable disease
Legionella Pneumophila treatment:
Erythromycin drug of choice (penetrates WBCs to reach intracellular orgs) or Rifampin when delayed response to treatment
Legionella Pneumophila epidemiology:
Organism is found worldwide and spread via
Air conditioning
Cooling towers and waters systems ( hot tubs).
Nosocomial setting spread through nebulizer with water
Survive in most environment and in the presence of disinfectant such as chlorine.
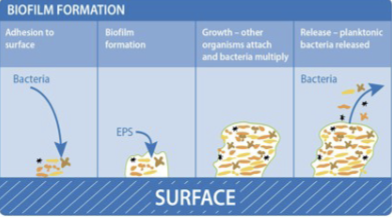
Bacteria form biofilms.
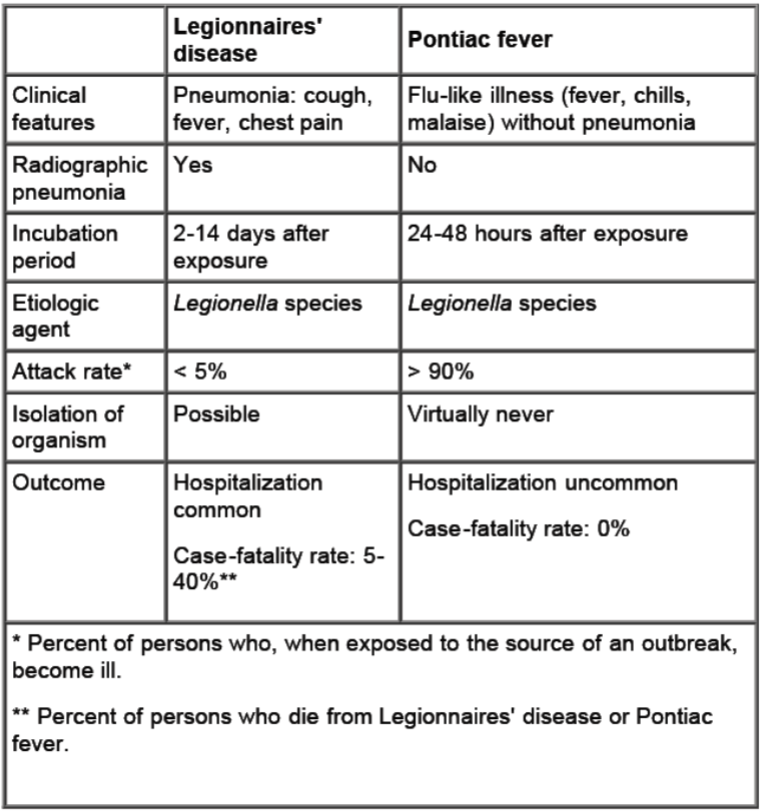
Legionnaires’ disease vs. Pontiac Fever: Legionnaires’ disease
More severe causes morbidity and death unless
treated properly
Incubates for 2 to 10 days followed by symptoms of
fever, chills, dry nonproductive cough, headache
Pneumonia is the primary manifestation
Often present as multi-organ disease, including the GI tract, CNS, liver, and kidneys.
Pulmonary function deteriorates in patients with no treatment
Pontiac Fever:
Self-limiting with symptoms of fever, chills, muscle pain, general
body weakness and lethargy (malaise), headache, flu-like
No evidence of pneumonia
Symptoms develop over a 12 hour period and then stick around for 2
to 5 days, then spontaneously disappears without antibiotic treatment.
• There have been no deaths reported
• Hypersensitivity reaction maybe the cause of pathology
Who should be tested for Legionnaires’ disease?:
Hospitalized patients with enigmatic pneumonia
Patients with enigmatic pneumonia sufficiently severe to require care in the ICU
Compromised host with pneumonia
Patients with pneumonia in the setting of a legionellosis outbreak
Patients who fail to respond to treatment to a ß- lactam or cephalosporin
Patients that have traveled away from their home within two weeks before the onset of illness
Patients suspected of nosocomial pneumonia with unknown etiology
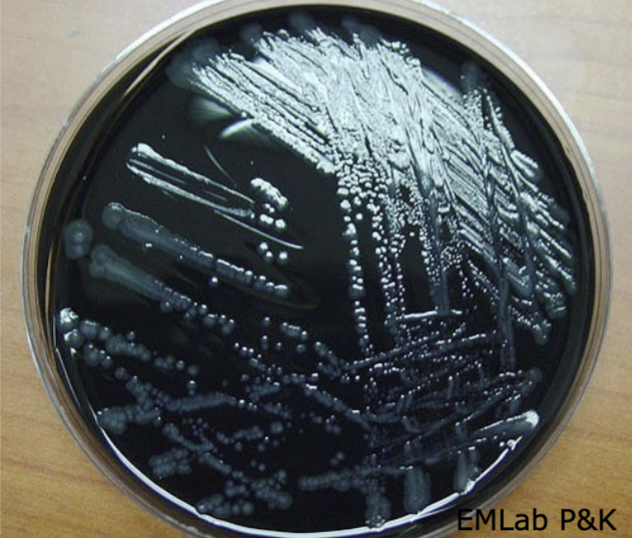
Legionella on BCYE agar
Colonies are small with a crystalline- like, ground glass appearance
L-Cysteine, soluble ferric pyrophosphate, and alpha- ketoglutarate enhances the growth of Legionella species
Activated charcoal removes toxic metabolic products
Protein and other growth nutrients are supplied by yeast extract
Legionella Pneumophila lab diagnosis: Culture is the gold standard: BCYE alpha
Nonselective
Buffered charcoal yeast extract medium suppl. with alpha-ketoglutaric acid
Adding specific supplements like glycine, vancomycin, polymyxin B, and cycloheximide (GVPC) makes it more selective for Legionella, but it should be used alongside non-selective BCYE because some Legionella species may be missed.
Should be used together with a non-selective BCYE because it inhibits some Legionella sp that do not produce beta lactamase. The medium is not fully selective, as other bacteria like Pseudomonas or Bacillus might still grow.
Legionella Pneumophila lab diagnosis: Culture is the gold standard: MWY (Modifed Wadowsky-Yee medium)
includes similar additives and is another selective option( contains glycine, vancomycin, polymyxin B, and anisomycin)
If bacteria grow on BCYEα but not on other media (e.g., BAP or BCYEα without cysteine), they may be Legionella and should be further tested.
Confirm suspected Legionella using Direct Fluorescent Antibody (DFA) testing or by sending samples to a reference lab.
Biochemical test are of limited use
Hippurate, β-lactamase production, gelatinase, oxidase, motility, autofluorescence.
Legionella Pneumophila: Flourecence:
Detection by DFA, direct fluorescent antibody
specific antibodies conjugated to FITC (fluorescein
isothiocyanate)
Fluorescence microscope
some false-positives
Sensitivity is low
Urine antigen test:
Detect soluble lipopolysaccharide antigens
High sensitivity with serogroup 1
lower sensitivity with other serogroups and species
Antigens persists in the urine of treated patients
50% are positive after 1 month
25% are positive after 2 months
Immunosuppressed patients are positive for up to a year
Serology of Legionella Pnuemophila:
Diagnosed with IFA, indirect fluorescent assay
test for a serologic response to infection
A level of 1:128 or greater (4-fold) is considered diagnostic
In the first week of disease an increase in titer can be seen in 25-40% of patients
Because high titers can persist, one positive test cannot be used to define disease
Nucleic Acid Amplification Assays (NAA or PCR): Legionella Pnuemophila:
Highly specific and sensitive
Not widely available
Anticipated to be the diagnostic method of choice in the future
False-negatives are possible
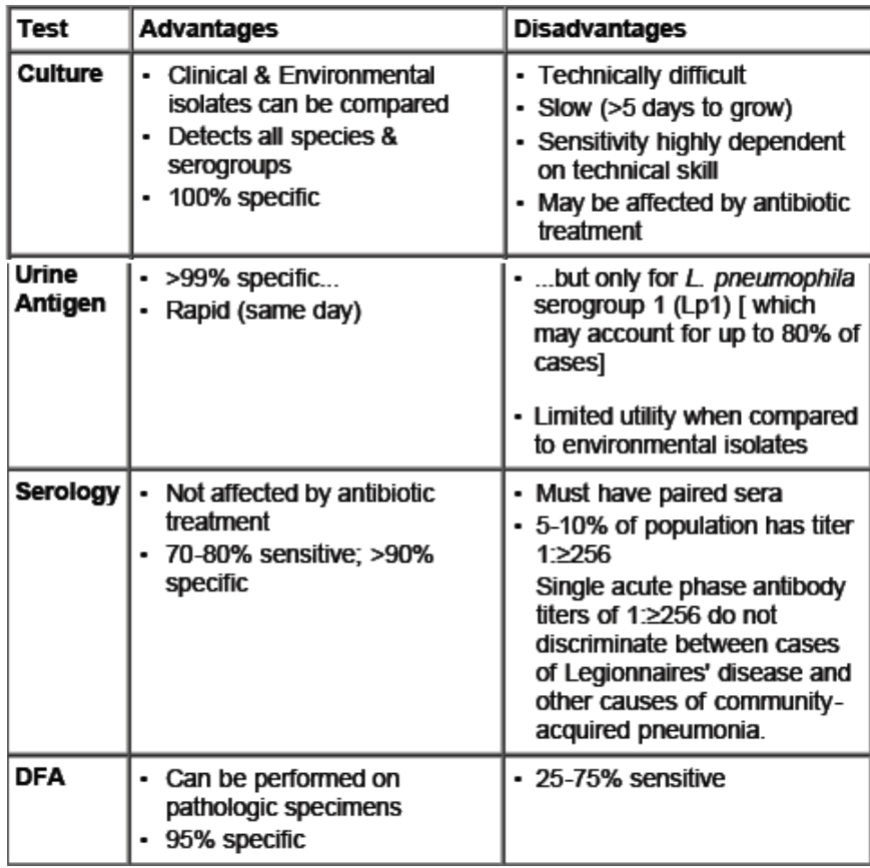
Advantages and Disadvantages of Legionella Pnuemophila tests
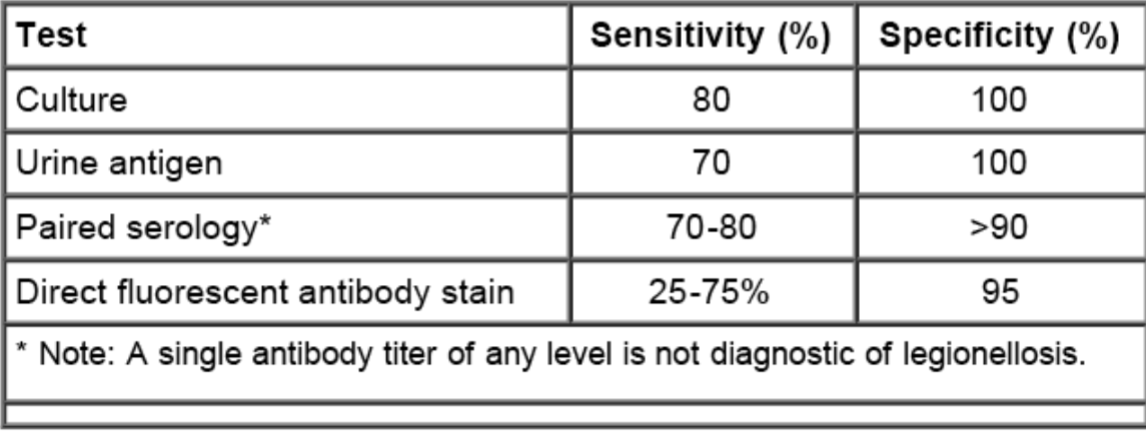
Diagnostic tests for Legionella
Urinary antigen assay AND culture of respiratory secretions on selective media are the preferred diagnostic tests for Legionnaires’ disease
Bordetella spp.
B. pertussis
B. parapertussi
B. bronchiseptica
B. avium
Bordetella samples:
Nasopharyngeal aspirate
Cotton swab inhibit growth of B. pertussis
Transport media
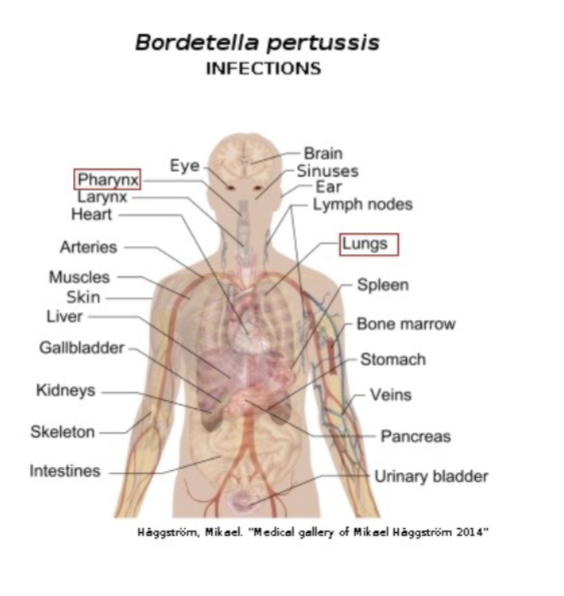
Bordetella Pertussis: Pertussis or whooping cough:
In the 20th century, was one of the most common childhood diseases and a major cause of childhood mortality in the United States.
Remains a major health problem among children in developing countries.
very contagious and is transmitted person to person
According to CDC, 40 percent of babies sick with whooping cough catch it from their mothers.
Pertussis vaccination:
reemergence of pertussis among highly vaccinated children
seems to be due to lower protection by acellular vaccines.
Only key to preventing the disease is vaccination
Whooping cough vaccination is a must for all
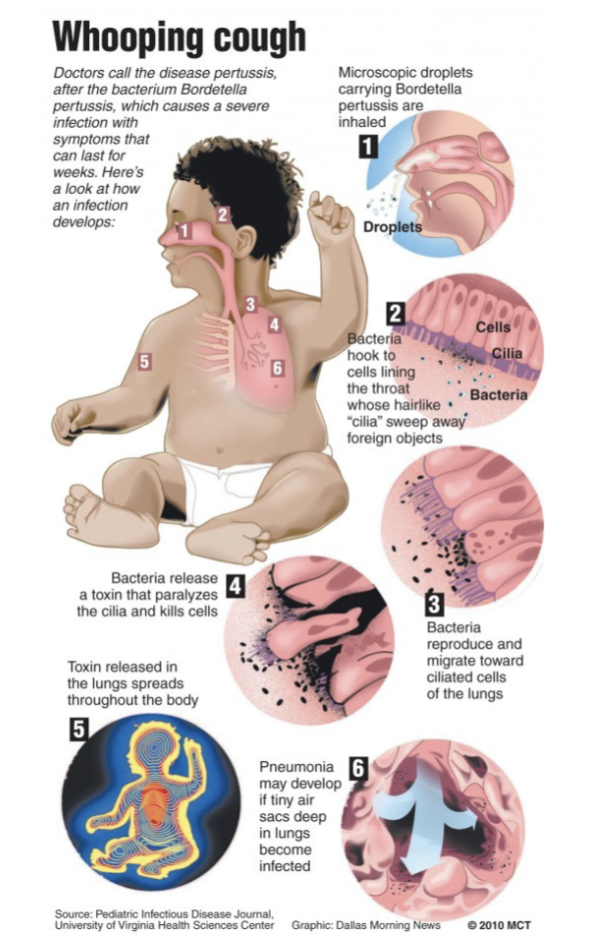
How to acquire Whooping cough
Pertussis toxin: mechanism of action:
PT is secreted from the bacteria and binds to a sialoglycoprotein host cell receptor,
PT is then endocytosed and trafficked through the Golgi apparatus to the endoplasmic reticulum (ER).
In the ER, the B pentamer binds to ATP and dissociates from the A subunit.
The A subunit is then transported on exosomes to the cytoplasmic membrane
There, PT ADP-ribosylates the α- subunit of heterotrimeric G proteins.
This modification inhibits G proteins function, including their ability to inhibit cyclic AMP (cAMP) formation.
The result of these modifications is high cAMP levels and an overall inhibition of immune cell recruitment to the site of infection
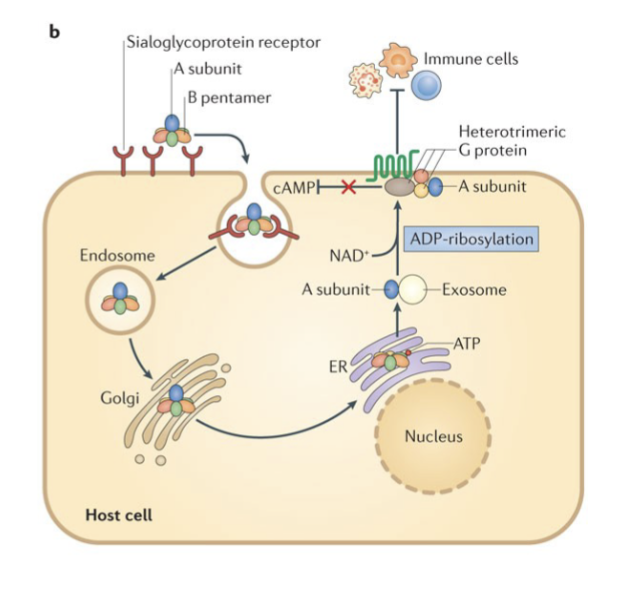
Bordetella Pertussis: General Characteristics:
Etiologic agent of Whooping cough
Humans only known source of the organism
Nonmotile, strict aerobe requiring rich medium for growth
Best cultured on charcoal-containing medium
Bordet-Gengou can also be used (potato-sheep blood- glycerol including penicillin)
Regan-Lowe used for transport
Requires 3 to 7 days incubation in moist container
Faintly stains with Gram stain
Most cases occur in children under age of 5, and most deaths in infants.
Transmission by inhalation of contamination aerosols from individuals with early stages of disease
Communicability rates of 30-90%
After 2 weeks- “catarrhal stage” occurs where patient only has symptoms of mild cough and sneezing, but is highly infectious with large number of organisms in the respiratory droplets
Explosive “paroxysmal stage” follows with severe cough and “whooping” sound during inhalation
Bordetella Pertussis useful biochemical tests:
Biochemical tests usually not performed in clinical laboratory
• Acid, but no gas, from glucose and lactose
Bordetella Pertussis: Microscopic morphology:
Very small, gram-negative coccobacilli
Singles or pairs
Faintly stains with Gram stain
can use basic fuchsin or safranin O as counterstain
Bordetella Pertussis colony morphology:
Bordet-Gengou medium with 20% blood
Diffuse zone of hemolysis after 7 days
Smooth, mucoid, and convex
Shiny “Mercury drops” with a pearly luster
White colonies with a mother-of-pearl opalescence on Regan Lowe medium

Bordetella Pertussis key reactions:
Specimens included nasal swab or nasopharyngeal swab
Biochemical testing not routinely utilized
Outdated practice included “Cough Plate” inoculation- where an agar plate would be inoculated by droplets expelled during paroxysmal coughing (No longer performed).
Identify with:
DFA and slide agglutination tests with culture
Direct fluorescent antibody (DFA) must be performed in tandem with culture due to low sensitivity (50%)
Serologic Tests with antibodies not very useful for rapid diagnosis because agglutination properties do not appear until third week of illness
Molecular PCR- most sensitive method of ID
Bordetella Pertussis: Treatment:
Erythromycin effective when administered by catarrhal stage, or as prophylaxis for unimmunized patients. Resistance has been reported
The recommended antimicrobial agents for treatment or chemoprophylaxis of pertussis are azithromycin*, clarithromycin, and erythromycin. Clinicians can also use Trimethoprim-sulfamethoxasole. Clinicians should choose the antimicrobial after consideration of the:
Potential for adverse events and drug interactions
Tolerability
Ease of adherence to the regimen prescribed
Cost
Bordetella Pertussis: Immunization:
Several vaccines available and are usually administered with toxoids from Corynebacterium diptheriae and Clostridium tetani, otherwise known as the DTaP vaccine
Infants should receive three injections of vaccine during first year of life, followed by two booster injections
Adults who do not receive booster immunization have decreased immunity and are primary reservoir for the organism.
Nationally reportable disease
Bordetella Pertussis: Antigenic and biologically active products:
Pertussis toxin
Filamentous hemagglutinin (FHA)
Agglutinogens
Adenylate cyclase
Pertactin
Tracheal cytotoxin
These products are responsible for the clinical features and an immune response to one or more produces immunity following infection. Some observational studies suggest that pertussis infection can provide immunity for 4 to 20 years, but that it is not life-long.

Bordetella spp. Oxidase & Urea
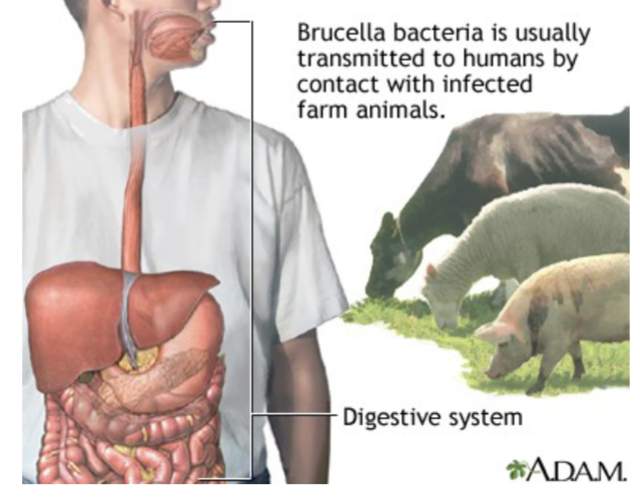
Brucella: General Characteristics:
Intracellular bacteria
usually found within animals, humans accidental hosts
DNA analysis identified only one true species, B. melitensis, with a number of biovars; however, they generally are still referred to by original “species” designation.
Four biovars known to infect humans, with typical animal host for each are: B.melitensis (goats and sheep), B.suis (swine), B.abortus (cattle), and B.canis (dogs)
Strict aerobe
Complex growth requirements
amino acids, vitamins, salts, and glucose for culture continuum
For direct isolation from patients, trypticase soy agar or blood culture medium is used.
Slow growing on BAP or Choc; incubation in presence of 5-10% CO2
B. abortus is only biovar with strict requirement for CO2
Brucella: Microscopic morphology:
GNR
Stain irregularly and pale with Gram stain
Coccobacillary forms dominate in early cultures
Bipolar staining sometimes observed
Brucella: Colony morphology:
Small, smooth, convex, transparent and nonhemolytic colonies on enriched medium after 3 to 5 days of incubation
Brucella: Useful biochemical tests:
Relatively inactive metabolically
• Catalase positive
• Oxidase positive
• Urease positive
• Many strains also H2S +
• Reduces Nitrates to nitrites.
Brucella: Key biochemical and other reactions
Presumptive ID with Gram stain, and positive oxidase and urease (Most clinical laboratories)
H2S, CO2 requirement and susceptibility to dyes used to identify biovars (Reference laboratories)
Serological testing may offer additional basis of identification
IgG titers >80 indicates active infection
If titers negative & strong clinical presentation of brucellosis suggested, additional tests may be performed to determine “blocking antibodies” of IgA class.
Brucella: Clinical Significance:
Causative agent of undulant fever
In animals, the brucellae localize in the pregnant uterus causing “contagious abortion” (erythritol).
Highly invasive organism spreads to other animals or humans in
contact with aborted fetus. Organism can also survive in dry soil up
to 60 days
Ingestion of unpasteurized milk or cheeses and other products that contain unpasteurized milk is an important route of transmission for in humans
Brucellae localize in mammary glands in animals and can be shed in
milk for years
Farmers, veterinarians, and slaughterhouse workers are at greatest risk (zoonoses) but is also the most common laboratory acquired
infection
Potential bioterror agent
• Nationally reportable disease
• Appropriate authorities must be notified from local health departments, through state and national laboratories. Original specimens must be preserved for possible criminal investigation.
Brucella: Pathogenicity
Fever, malaise, weakness and nonspecific aches pains major
manifestations of brucellosis. Different degrees of disease severity are
associated with different biovars.
Incubation period ranges from 1 to 6 weeks, with slow insidious onset of disease. Bacteria disseminated via the lymphatics and bloodstream to many parts of the body.
Granulomatous nodules form, and may progress into abscesses- Bone marrow.
CNS symptoms, and lymph node enlargement commonly noted as well
Undulant fever- daily periodicity with extreme sweating associated
Generalized symptoms usually subside over period of weeks to
months, although localized symptoms may persist longer, even for
years.
Brucella: Treatment:
Immunity after recovery, although reinfection is possible
Brucellae usually susceptible to tetracycline, streptomycin, and
ampicillin.
Intracellular nature of organisms make them difficult to eradicate;
extended administration of tetracycline may be required.
Brucella: Prevention:
If brucellosis is suspected in a patient, clinicians should note “suspect or rule out brucellosis” on the laboratory submission.
The best way to prevent brucellosis infection is to be sure you do not consume:
• undercooked meat
• unpasteurized dairy products, including:
• milk
• cheese
• ice cream
• Pasteurization is when raw milk is heated to a high temperature for a short period of time.
This heating process destroys harmful bacteria that may make the milk unsafe to consume.
• If you are not sure that the dairy product is pasteurized, do not eat it.
• People who handle animal tissues (such as hunters and animal herdsman) should protect themselves by using:
• rubber gloves
• goggles
• gowns or aprons
• This will help ensure that bacteria from potentially infected animals do not get into eyes or inside a cut or abrasion on the skin.
Brucellosis:
Common laboratory acquired infection
• must work in Biosafety cabinet
• avoid aerosol
• never sniff the plate!
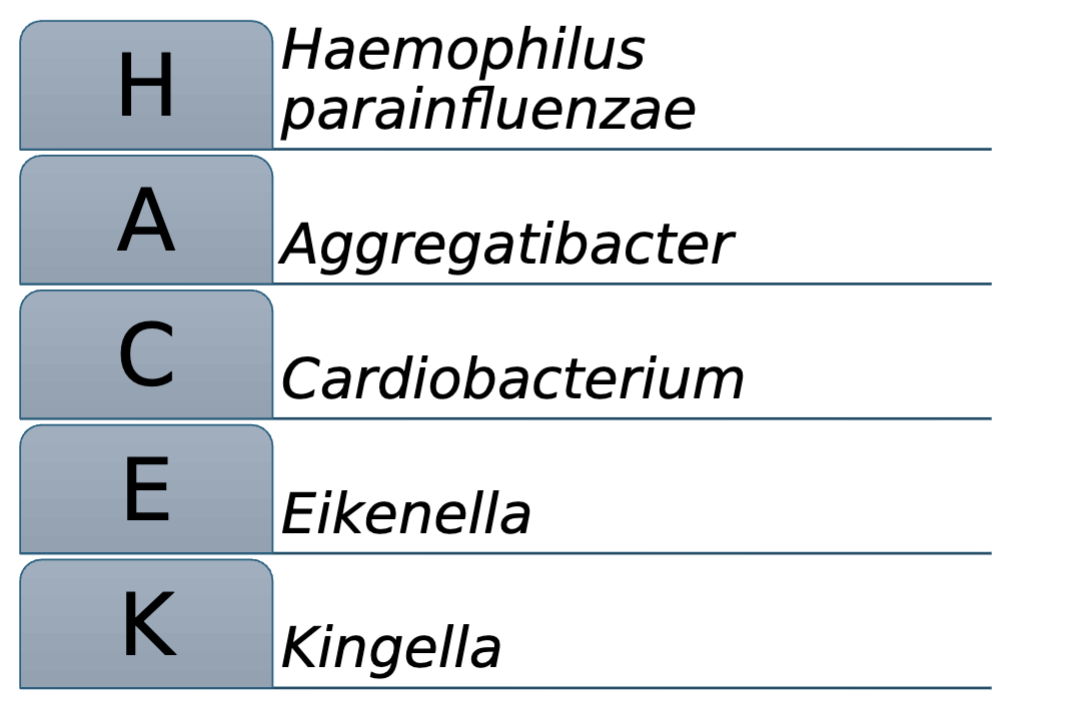
HACEK Group:
Haemophilus parainfluenzae
Aggregatibacter
Cardiobacterium
Eikenella
Kingella
HACEK group: general characteristics:
Group of fastidious Gram negative coccobacilli organisms
Slow growing Gram negative Coccobacilli
• Hold blood cultures up to 2 weeks to isolate
• Require or stimulated by CO2
HACEK: Clinical significance:
Associated with 3% subacute bacterial endocarditis (SBE)
cases
Most cases of SBE caused by viridans streptococci
May play a role in formation of fatty plaque walls in arteries
Occasionally implicated in infections other than SBE
Usually part of the normal oral flora
Infections occur due to poor oral hygiene, dental manipulation
and damaged heart valves are predisposing factors
H. parainfluenzae:
described previously with Haemophilus spp. Normal upper
respiratory flora.
• Requirement of NAD (factor V) for growth (NG on BAP)
Aggregatibacter:
A. aphrophilus (formerly known as H. aphrophilus)
• Coccobacillary
• Round, convex colonies
• Requires CO2
• Requires heme (factor X) for initial isolation (grows on BAP)
• Can be weakly ALA positive
• Nonhemolytic on horse blood agar
• Weak oxidase positive
• Catalase, indole and urease negative
• Glucose and lactose positive
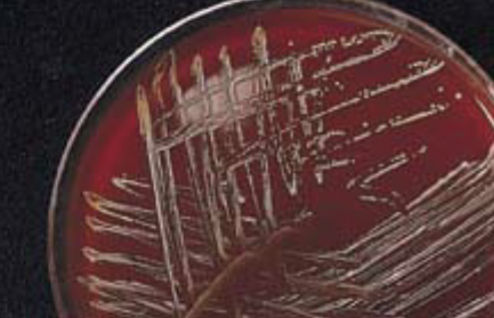
Aggregatibacter actinomycetemcomitans: Macroscopic colony morphology:
• Rough colonies on TSBY agar (Tryptic Soy Broth Yeast Extract agar)
• Irregular edges, rough surface, star-shaped internal structure,
pitting on NA (nutrient agar).
• Smooth colonies on subculture
• Requires CO2
A. actinomycetemcomitans: biochemicals:
Oxidase and catalase positive
• Indole negative
• Glucose and maltose positive
A. actinomycetemcomitans: Clinical significance:
Associated with periodontal disease and can cause other infections such as bacteremia and osteomyelitis.
“Comitans” means accompanying. Aggregatibacter
actinomycetemcomitans is frequently isolated with
Actinomyces israelli
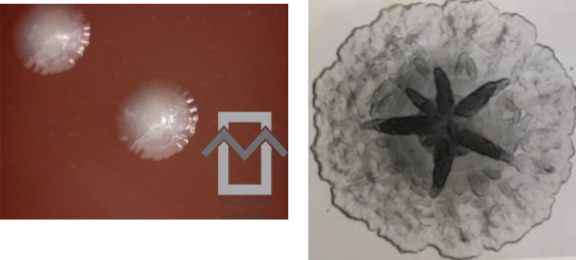
actinomycetemcomitans on blood agar. Note the
star-shaped centers of the colonies
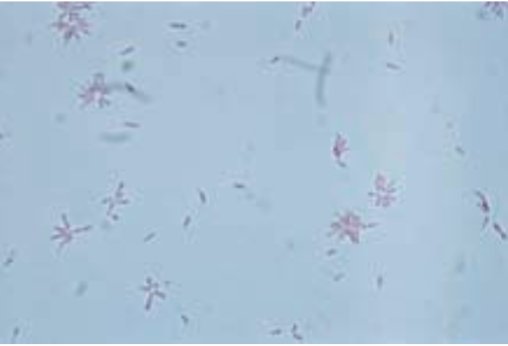
Cardiobacterium: C. hominis:
Facultative anaerobe
Pleomorphic, gram variable
Rosette or flower-like arrangement on Gram stain
Alpha-hemolysis
Cardiobacterium: C. hominis: Biochemicals:
Oxidase, nitrate and indole positive
• Catalase negative
• Glucose, maltose, sucrose positive
• Lactose negative
• It is often seen in patients with underlying heart valve abnormalities, such as those with prosthetic heart valves or congenital heart defects.
Eikenella: Eikeella corrodens:
Facultative anaerobe
Small, straight, GNR
Small, grayish, with slight green discoloration on BAP
Bleach-like odor
Lemon-yellow pigment (observed on swab)
Approximately 50% pit or corrode agar
Most commonly associated in wounds caused by human bites or “fistfights”
Also isolated from skin infections of drug addicts who lubricate needles with saliva before injecting drugs

Eikenella corrodens: Biochemicals:
Oxidase and nitrate positive
Catalase, urease, and indole negative
Carbohydrate fermentation negative
Kingella:
K. kingae, K. dentrificans, K. oralis (oral cavity and upper respiratory commensal)
Kingella kingae: general characteristics:
Short, plump rods with blunt ends
No growth on Thayer-Martin (K.denitricans will)
Growth on Sheep blood agar requires several days in 5-10% CO2
Beta-hemolytic on SBA
Variable colony; Some appear as “fried-egg” with haze around center and pits agar
Kingella kingae: biochemicals:
• Oxidase positive
• Most are nitrate positive
• Catalase negative
• Glucose and maltose positive
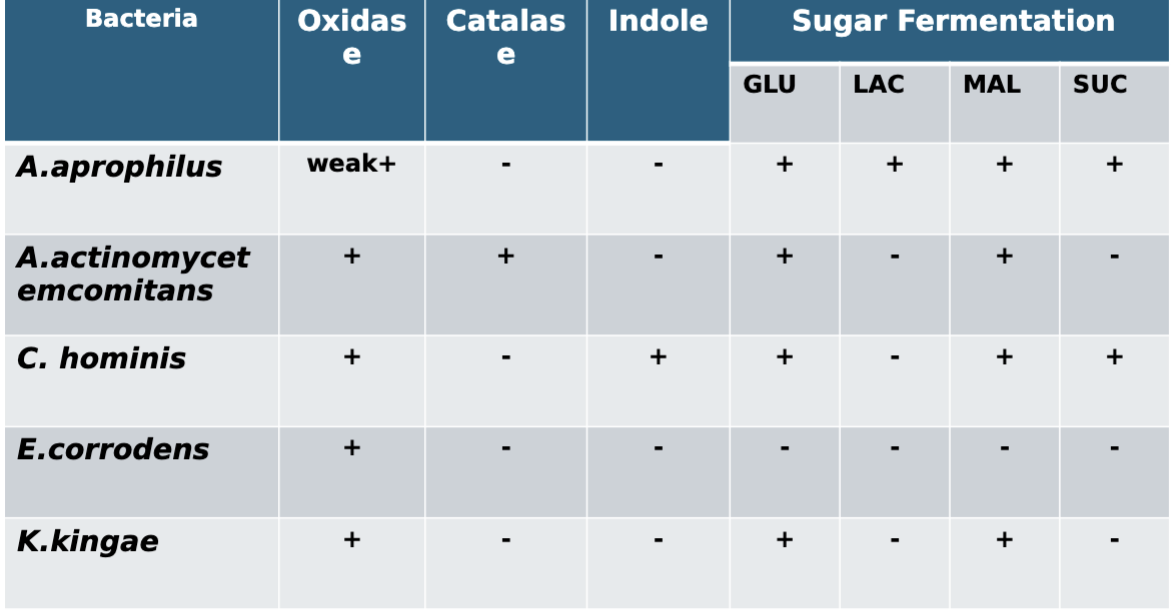
Selected characteristics of HACEK group
Additional Isolates:
Capnocytophaga
• Streptobacillus
• Bartonella
• Pasteurella
Capnocytophaga:
Normal flora of humans, dogs, cats
C. ochracea, C.sputigena, C. gingivalis, C. canimorsus
Associated with dogs and cats bites
Can cause fulminant sepsis with disseminated intravascular coagulation (DIC)
Also cause of periodontitis, septicemia, endocarditis
Isolated together with actinomycetes (like A. actinomycetemcomitants)
Capnocytophaga: General Characteristics:
Normal flora of mouth and oral cavities
Gram negative, fusiform rods
Colonies exhibit a characteristic gliding motility on agar
Similar to that of Proteus spp. but not as extensive
Require increased carbon dioxide for growth
Non-hemolytic and produce a yellow-orange pigment
Capnocytophaga: biochemical reactions:
• Negative for most biochemical reactions
• Hydrolyze esculin and reduce nitrate
Capnocytophagoa: Seven species:
Dog and cat
C. canimorsus and C. cynodegmi
Oxidase and catalase positive
Human
C. gingivalis, C. ochracea, C. haemolytica, C. sputigena and C. granulosa
Oxidase and catalase negative
Capnocytophaga: Clinical Manifestations:
Produce an immunosuppressive factor (protease)- virulence
factor
Most common isolate is C. ochracea
Juvenile periodontal disease, endocarditis and oral ulcers in
granulocytopenic individuals
Capnocytophaga: Laboratory diagnosis:
Slow growth on sheep blood and chocolate agars with 5-10%
carbon dioxide at 35-37°C
No growth on MAC
Blood cultures may take up to 7 days for positive results
Presumptive identification
Yellow-pigmented, spreading colony
Thin, fusiform, gram negative rods
No growth in ambient air
Streptobacillus S. moniliformis general characteristics:
Microaerophile
Pleomorphic GNR forming long irregular chains
Best growth:
Medium supplemented with serum, protein, egg yolk or starch
37°C (organism fail to grow at 22)
“L forms” are readily observed in most cultures
Gram negative bacteria that have lost most of their cell wall, but retained outer membrane and peptidoglycan that are still able to grow and divide
L-form:
Loss of cell wall
Long irregular form
“Lesion” in French- initially described as due to abnormal shape in culture.
Streptobacillus: Useful biochemical and other tests:
Nonmotile; lacks a capsule
• Oxidase, catalase, indole, and nitrate negative
• Serology for serum antibodies (reference laboratories)
Streptobacillus Microscopic Morphology:
String of beads or pearls
Streptobacillus: Colony morphology:
• BHI agar with 20% horse serum:
Small, colorless to grayish, smooth and glistening
• Irregular edges possible
• “L forms” resemble fried eggs
• BA: Cottonlike colonies after 3 days incubation
• Broth: “fluff balls” or “bread crumbs” at bottom of tube
Streptobacillus clinical significance:
Human infection usually occurs following a rat bite, resulting in a
disease known as rat-bite fever ( also caused by spirillum minus)
• Normally found in throats of rats, mice, guinea pigs and other rodents
• When infection occurs via ingestion of contaminated milk, the disease is known as “Haverhill fever”.
• Fever and petechial or blotchy rash present on extremities after 2 day incubation.
• Painful polyarthritis involving knees, ankles, elbows, and other joints in 50% of patients
May cause false positives on VDRL screening test for syphyllis
Bartonella: General characteristics:
Facultative, intracellular gram negative coccobacilli