electron configuration
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
Explain the term mass number and relative atomic mass
Mass number: total number of protons and neutrons
Relative atomic mass: total weighted average mass of the naturally occurring isotopes of the element relative to 1/12th mass of an atom of carbon
What is the electromagnetic spectrum?
a range of frequencies that covers all electromagnetic radiation and their respective wavelengths and energy
Methods of exciting electrons
Passing an electric discharge or high voltage through a sample of gas
Heating the element into gaseous form/doing a flame test
What is spectroscopy?
The study of the interaction between matter and light
What is the emission spectra?
The range of frequencies or wavelengths or electromagnetic radiation emitted during an electron transition from a higher to a lower energy level
Properties of isotope
They have identical chemical characteristics
but different physical properties
They have same number of protons but different number of neutrons
Qualitatively describe the relationship between energy and frequency in the electromagnetic spectrum
Energy is directly proportional to frequency.
High-frequency light carries more energy than low-frequency light.
Qualitatively describe the relationship between colour and wavelength in the electromagnetic spectrum
Colour is determined by the wavelength of light.
Shorter wavelengths are at the violet end of the spectrum
longer wavelengths are at the red end.
Frequency decreases from violet to red light. Violet has the highest frequency and red the lowest frequency.
Qualitatively describe the relationship between Frequency and wavelength in the electromagnetic spectrum
Frequency is inversely related to wavelength.
High-frequency light has shorter wavelengths,
low-frequency light has longer wavelengths.
c = f x λ
Difference between a continuous and a line spectrum.
A continuous spectrum covers a broad range of wavelengths without gaps
A line spectrum consists of discrete, isolated wavelengths
How does an element’s highest main energy level relate to its period number in the periodic table?
The period number in the periodic table corresponds to the highest main energy level
Deduce the maximum number of electrons that can occupy each energy level.
2n^2.
n is the energy shell.
(For the second energy level (n=2), the maximum number of electrons it can hold is 2 * (2^2) = 8 electrons.)
How does emission spectra provide evidence for the existence of different materials ?
Emission spectra show distinct patterns of emitted light when materials are heated or excited.
Each element or compound has unique energy levels for its electrons.
Electrons release energy as light when they transition from higher to lower energy levels.
The emitted light produces a characteristic spectral pattern.
Analyzing the wavelengths and colors in an emission spectrum identifies the specific elements or compounds in the material.
What is the relationship between energy sublevels and the block nature of the periodic table?
The periodic table is divided into blocks, including s-block, p-block, d-block, and f-block.
The s-block elements (Groups 1 and 2)
The p-block elements (Groups 13 to 18)
The d-block elements (transition metals)
The f-block elements (lanthanides and actinides)
s>p>d>f
describes an emission line spectrum in the visible region
A series of coloured lines on a black background that converge at high energy
The lines converge at high energy and frequency which corresponds to short wavelength.
explains the formation of an emission line spectrum
Electrons emit energy as they transition from higher to lower energy levels in the form of photons.
Each emission line corresponds to a specific energy transition.
How can isotope traces provide evidence for a reaction mechanism?
Radioactive tracers are radioisotopes that can be added to compounds to determine reaction mechanisms.
By tracking the movement
What are radioisotopes?
Radioactive isotopes that are unstable due to the number of subatomic particles contained in the nucleus (more neutrons compared to protons) hence contains excess energy.
What’s shown on the x and y axis for a mass spectrum
The percent abundance (or relative intensity) on a mass spectrum is shown on the y-axis
The m/z ratio on the x-axis.
Aufbau’s principle
When adding electrons to an atom, the lower energy orbitals must be filled first.
Hund’s rule
when we have degenerate orbitals (orbitals of the same energy) then each orbital is filled with a single electron before being doubly occupied.
Pauli exculsion princple
an atomic orbital can only hold two electrons and they must have opposite spin
Electron configaration of Cr
It is not: Cr = [Ar] 4s2 3d4
It’s : Cr = [Ar] 4s1 3d5
Electron configuration of Cu
Its not: Cu = [Ar] 4s2 3d9
Its: Cu = [Ar] 4s1 3d10
What are the 3 sub shells and how many orbitals and electrons do they have?
Each shell can be divided further into subshells, labelled s, p, d and f
Each subshell can hold a specific number of orbitals:
s subshell : 1 orbital
p subshell : 3 orbitals labelled px, py and pz
d subshell : 5 orbitals
f subshell : 7 orbitals
Each orbital can hold a maximum number of 2 electrons so the maximum number of electrons in each subshell is as follows:
s : 1 x 2 = total of 2 electrons
p : 3 x 2 = total of 6 electrons
d : 5 x 2 = total of 10 electrons
f : 7 x 2 = total of 14 electrons
Describe the emission spectrum of the hydrogen atom, including the relationship between the lines and energy transitions to the first, second and third energy levels
A series of lines;
Electrons transition between higher energy levels to lower energy levels
Electrons transitions into first energy level cause the UV series
Electron transitions into second energy level cause the visible series
Electron transitions into third energy level cause the infrared series;
There is convergence at higher frequency/energy/short wavelength;
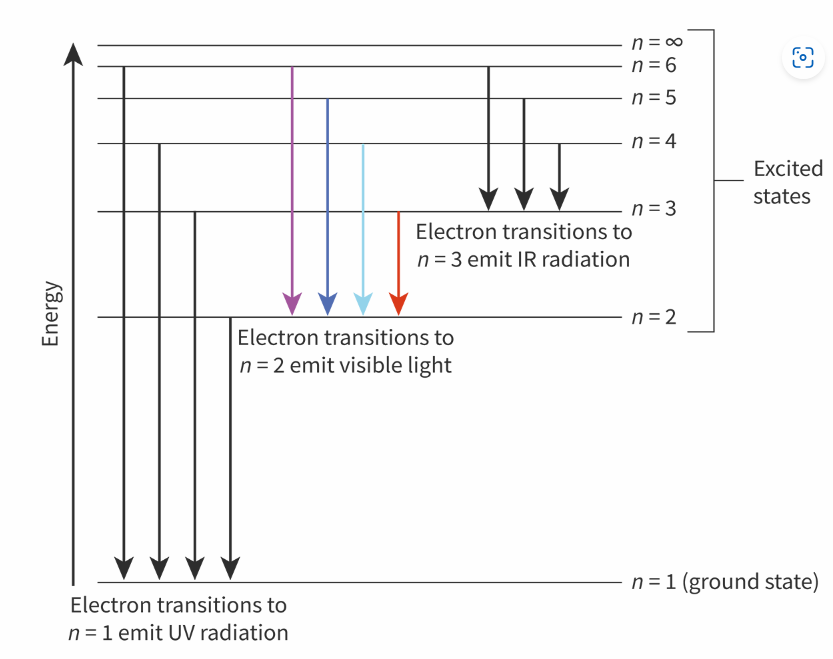
what happens when electrons move from higher to lower energy levels?
they emit energy
Which energy level represents the ionisation of a hydrogen atom? and why?
n = ∞ energy level
As it is said to be completely removed from the attraction of the nucleus and the atom has been ionised.
How do electrons absorb and emit energy?
in the form of photons.
What is the ‘excited’ state?
Unstable, relative to the ground state
What happens when an electron absorbs energy?
It transitions from a lower energy level to a higher energy level. (n=1 to n=2)
(The electron is now said to be in an excited state. The excited state is unstable relative to the ground state)
The unstable electron then emits the same amount of energy that it absorbed and it tranitions back down to n=1
The amount of energy emitted by the elctron in the transition from n=2 to n=1 correponds to the wavelengths of visible light.
how does the size of the energy transition affect the emitted radiation in electron transitions?
larger energy transitions result in the emission of radiation with shorter wavelengths and higher energy.
Characteristics of energy levels in a hydrogen atom
The energy levels in a hydrogen atom converge at high frequencies, high energy and short wavelengths.
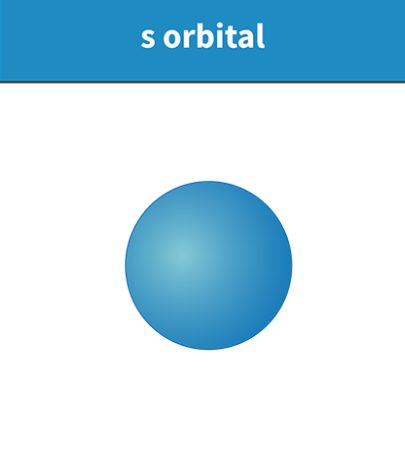
Draw a shape of the S atomic particle
its Spherical
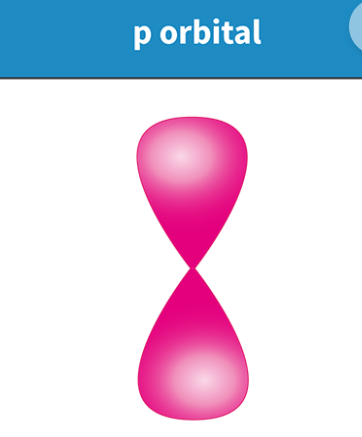
Draw the shape of a P orbital
Dumb-bell shaped
Maximum number of electrons an orbital can hold
2
How does the fragmentation pattern of a compound in the mass spectrometer help in the determination of its structure?
Mass spectrometry breaks compounds into fragments. ( When a compound is introduced into a mass spectrometer, it is bombarded with high-energy electrons, which can cause the compound to fragment.)
Each fragment has a specific mass-to-charge ratio.
A mass spectrum shows the intensity of these fragments.
The unique pattern helps identify a compound's structure.\
Patterns provide information on functional groups, connectivity, and molecular weight.
What is a mass spectrometer?
An instrument that is used to determine the abundance of the various isotopes of an element based on the m/z ratio of ions within a sample of the element,
According to spec:
Used to determine the relative atomic masses of elements from their ionic composition.
What does heisenberg’s uncertainty principle state
it is not possible to know both the location and velocity of an electron at the same time.
How to determine which electron transmission radiates the longest wavelength?
Electrons emit energy when they jump from higher to lower energy levels
Jumps from higher levels to the first level, n = 1, emit the largest amount of energy
Energy and wavelength are inversely proportional, the lower the energy the longer the wavelength
E = hc/wavelength
H is the Planck constant and c is the speed of light
What is an orbital?
A region in space with a high probability of finding an electron
How does the arrangement of electrons into energy levels provide an explanation for the production of a line spectrum rather than a continuous spectrum?
Lines show that there are jumps/gaps between energy levels
If an electron could have any amount o energy, then a continuous membrane would be produced.
Why do the lines in an emission spectrum series get closer together at shorter wavelengths?
As the gaps between the different energy levels is not equal
What name is given to the point where the emission spectrum merges and what does it repsent?
Convergence; the point beyond which the electron can have any energy and so is free from the influence of the nucleus.
Why is 4s1 always lost before 3d1
4s sublevel is in a lower energy level than 3d