Chemistry 1.2 + 1.7- Amount of Substance + Redox
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What is a mole?
The amount of substance that has the same number of particles as there are in 12g of carbon-12 (6.022×10²³)
What is the relative atomic/molecular mass?
The average mass of one atom/molecule relative to 1/12 of the mass of one atom of carbon-12
Concentration formula
Concentration = moles/volume (dm³)
What is the ideal gas equation?
PV = nRT → pressure (kPa) x volume (dm³) = moles x gas constant (8.314) x temperature (K)
How do you convert Celsius to Kelvin? I
Add 273
What are oxidation and reduction?
Oxidation is the loss of electrons (oxidation number increases)
Reduction is the gain of electrons (oxidation number reduces)
What are oxidising agents and reducing agents?
Oxidising agents oxidise other species and are themselves reduced- they are electron acceptors
Reducing agents reduce other species and are themselves oxidised- they are electron donors
What are the oxidation state rules?
Any uncombined element is zero eg. Al or Cl2
For an ion it is the same as its charge eg. 2+ in Fe²+
Fluorine is always -1
Hydrogen is +1, except for when -1 in metal hydrides eg. NaH
Oxygen is -2, except for when -1 in H2O2 and +2 in F2O
Group 1, 2 + 3 metals all have the same charge as their group number
In compounds they add up to the overall charge eg. add up to 2- in SO4²-
What is a disproportionation reaction?
A reaction where the species is both oxidised and reduced in separate products
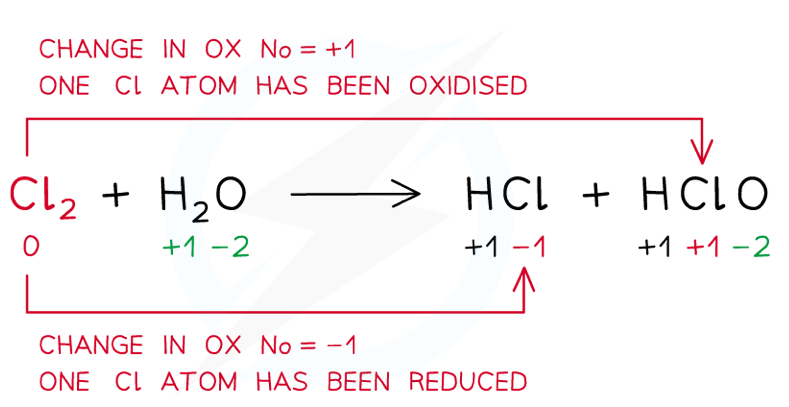
What is a comproportionation reaction?
A reaction where two species of the same element reduce and oxidise to the same oxidation state