TOPIC 1 - STOICHIOMETRIC RELATIONSHIPS
0.0(0)
0.0(0)
Card Sorting
1/46
Earn XP
Description and Tags
Last updated 3:11 PM on 4/28/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
1
New cards
What is an element
substances made from one kind of atom
2
New cards
What is a compound
two or more elements chemically combined
3
New cards
Elements chemical reactions
* Elements take part in chemical reactions in which **new substances are made**
* These processes most often involve an **energy change**
* In these reactions, atoms combine together in **fixed ratios** that will give them **full outer shells** of electrons, **producing compounds**
* These processes most often involve an **energy change**
* In these reactions, atoms combine together in **fixed ratios** that will give them **full outer shells** of electrons, **producing compounds**
4
New cards
What happens to elements / compounds in mixtures
* In a mixture, elements and compounds are mixed with each other, but are **not** chemically combined
* this means that the **components of a mixture** (the original elements / compounds) retain the **same characteristic properties** as when they are in their **pure form**
* this means that the **components of a mixture** (the original elements / compounds) retain the **same characteristic properties** as when they are in their **pure form**

5
New cards
Homogenous mixture
* A **homogeneous** mixture has **uniform** **composition** and **properties** throughout
* Therefore, seperate components are not able to be seen
* Therefore, seperate components are not able to be seen
6
New cards
Heterogenous mixture
* A **heterogeneous** mixture has **non-uniform** **composition**, so its **properties** are not the same throughout
* It is often possible to see the seperate components (e.g bubbles in a soda)
* It is often possible to see the seperate components (e.g bubbles in a soda)
7
New cards
Separating mixtures
* Since the original components of mixtures retain their individual properties - we can often separate them pretty easily
* Image below: mixtures & seperation techniques
* Image below: mixtures & seperation techniques
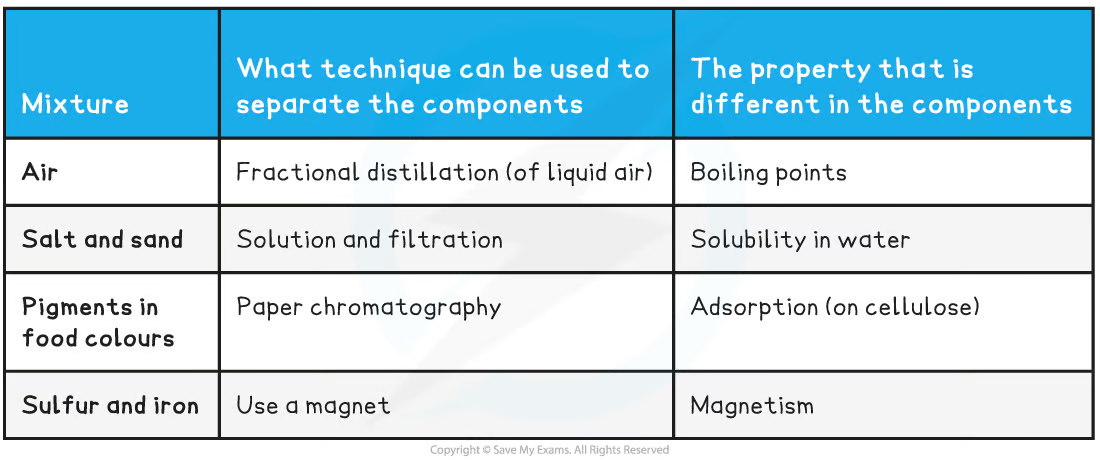
8
New cards
Neutralisation reaction
* acid + base → salt + water
* 1 hydrogen from the acid, 1 hydrogen + 1 oxygen from the base to form water
* the other elements form the salt
* 1 hydrogen from the acid, 1 hydrogen + 1 oxygen from the base to form water
* the other elements form the salt
9
New cards
Complete + incomplete combustion reaction
* Incomplete → **substance + O2 → products**
* Complete → **substance + O2 → CO2 + H2O**
* Complete → **substance + O2 → CO2 + H2O**
10
New cards
What are state changes
* **physical changes** that are reversible
* these changes do not change the chemical properties or chemical makeup of the substances involved
* these changes do not change the chemical properties or chemical makeup of the substances involved
11
New cards
What does vaporization include
**evaporation** and **boiling**
12
New cards
What does evaporation include
* the change of **liquid to gas**
* unlike boiling, **evaporation** occurs **only at the surface** and takes place at temperatures **below** the **boiling point**
* unlike boiling, **evaporation** occurs **only at the surface** and takes place at temperatures **below** the **boiling point**
13
New cards
Solid → Gas name
sublimation
14
New cards
Gas → Solid name
deposition
15
New cards
Overview of state changes drawing
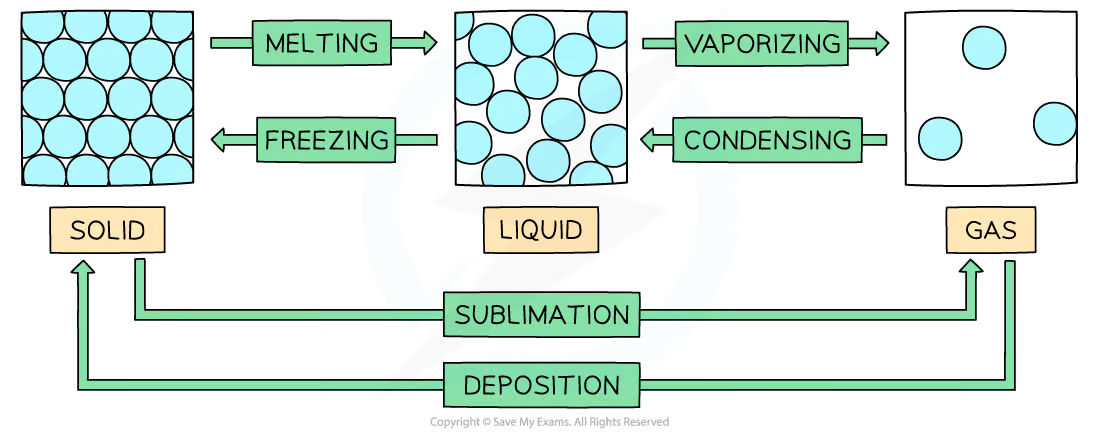
16
New cards
Draw and explain a graph of the relationship between temperature and energy during state changes
* Between 1 & 2, the particles are vibrating and gaining **kinetic energy** and the temperature rises
* Between 2 & 3, all the energy goes into breaking bonds – there is **no** increase in **kinetic energy** or **temperature**
* Between 3 & 4, the particles are moving around and gaining in **kinetic energy**
* Between 4 & 5, the substance is boiling, so bonds are breaking and there is **no** increase in **kinetic energy** or **temperature**
* From 5 & 6, the particles are moving around rapidly and increasing in **kinetic energy**
* Between 2 & 3, all the energy goes into breaking bonds – there is **no** increase in **kinetic energy** or **temperature**
* Between 3 & 4, the particles are moving around and gaining in **kinetic energy**
* Between 4 & 5, the substance is boiling, so bonds are breaking and there is **no** increase in **kinetic energy** or **temperature**
* From 5 & 6, the particles are moving around rapidly and increasing in **kinetic energy**
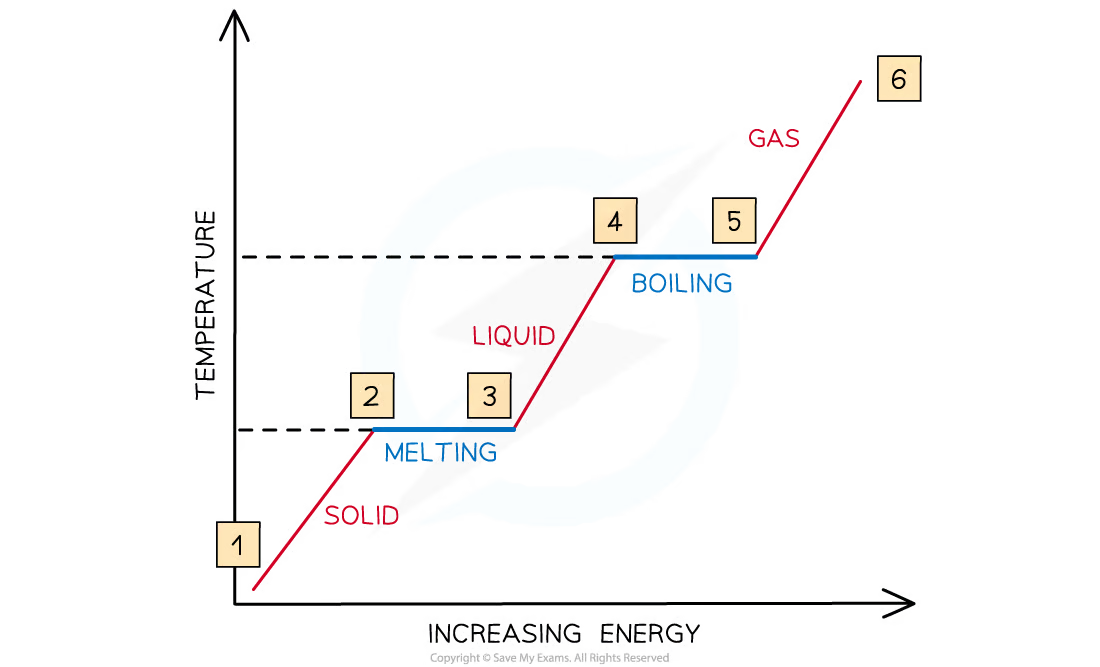
17
New cards
Define the avogadro constant
* the number of particles in one mole of a substance
* applies to atoms, molecules and ions
* the value of the Avogadro constant is **6.02 x 1023 g mol-**
* applies to atoms, molecules and ions
* the value of the Avogadro constant is **6.02 x 1023 g mol-**
18
New cards
What is the relative atomic mass / molar mass
* The **relative molecular mass** (*Mr*) is the weighted average mass of a molecule compared to one twelfth the mass of a carbon-12 atom
* Has no units
* Has no units
19
New cards
Draw the moles and particles formula triangle
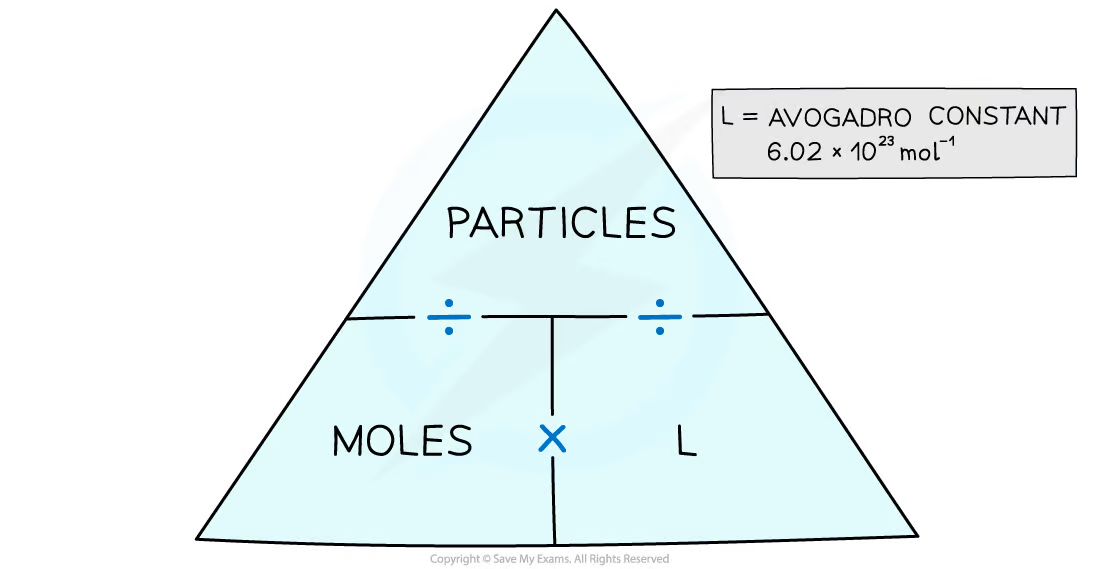
20
New cards
Draw the moles, mass and molar mass, formula triangle
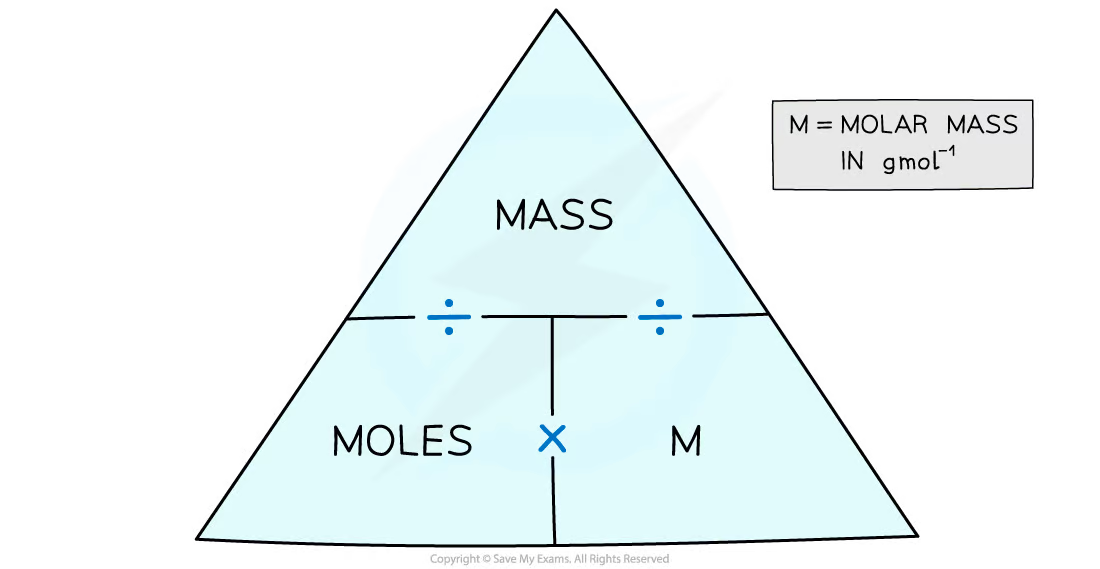
21
New cards
What is the molecular formula
* the formula that shows the **number** and **type** of each atom in a molecule
* e.g: ethanoic acid: C2H4O2
* e.g: ethanoic acid: C2H4O2
22
New cards
What is the empirical formula
* the simplest whole number ratio of the atoms of each element present in one molecule or formula unit of the compound
* e.g: ethanoic acid: CH2O
* e.g: ethanoic acid: CH2O
23
New cards
How to calculate empirical formula - example: find the empirical formula of a compound that contains 10 g of hydrogen and 80 g of oxygen
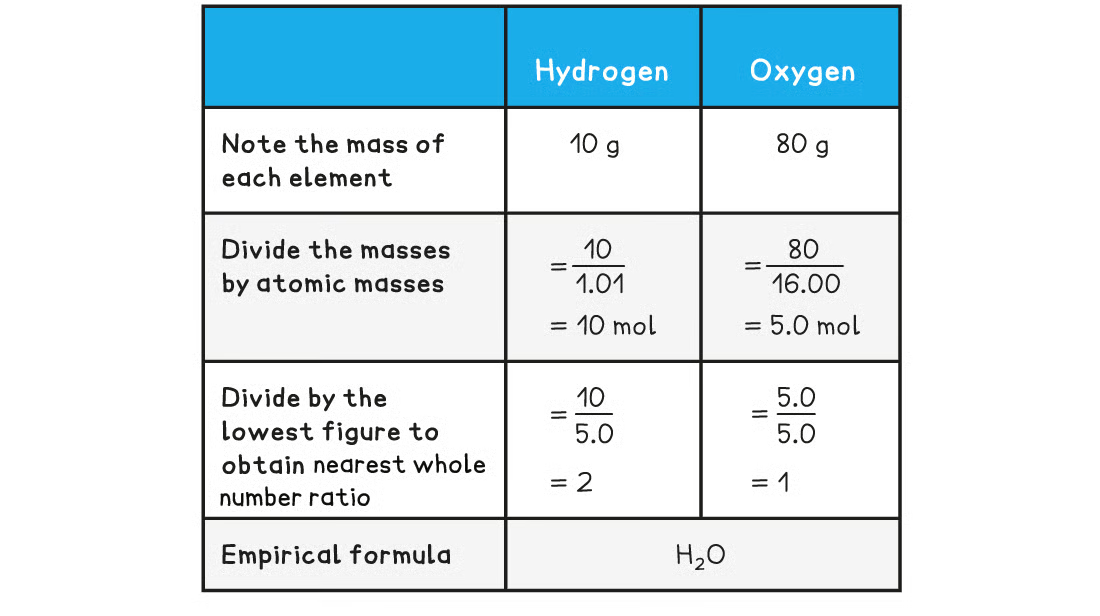
24
New cards
How to calculate molecular formula from empirical formula - example: The empirical formula of X is C4H10S and the relative molecular mass of X is 180.42. What is the molecular formula of X? **Relative Atomic Mass** Carbon: 12.01 Hydrogen: 1.01 Sulfur: 32.07
1. Calculate the total molar mass of the empirical formula
2. Divide the given molecular molar mass of the compound by the molar mass calculated for the empirical formula
3. Multiply each subscript by the whole number that resulted from step 2 - This is now the molecular formula.
\
**ANSWER:** The molecular formula of **X** is **C8H20S2**
25
New cards
What are the masses of reactants useful for
They are useful to determine exactly how much of the reactants react with each other to prevent waste
26
New cards
Reasons for reactants not forming products
* Other reactions take place simultaneously
* The reaction does not go to **completion**
* Products are **lost** during separation and purification
* The reaction does not go to **completion**
* Products are **lost** during separation and purification
27
New cards
What does the percentage yield show / what is the equation
how much of a particular product you get from the reactants (actual yield) compared to the maximum theoretical amount (theoretical) that you can get
Av
* The **actual yield** is the number of moles or mass of product obtained **experimentally**
* The **theoretical yield** is the number of moles or mass obtained by a reacting mass calculation
Av
* The **actual yield** is the number of moles or mass of product obtained **experimentally**
* The **theoretical yield** is the number of moles or mass obtained by a reacting mass calculation
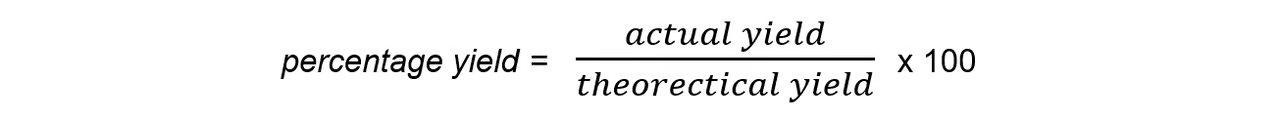
28
New cards
Avogadro’s law and gases
* **Avogadro’s law** allows the mole ratio of reacting gases to be determined from volumes of the gases
* He deduced that equal volumes of gases must contain the same number of molecules
* At standard temperature and pressure(**STP**) **one mole** of any gas has a volume of **22.7 dm3**
* The units are normally written as **dm3 mol-1**(since it is 'per mole')
* He deduced that equal volumes of gases must contain the same number of molecules
* At standard temperature and pressure(**STP**) **one mole** of any gas has a volume of **22.7 dm3**
* The units are normally written as **dm3 mol-1**(since it is 'per mole')
29
New cards
What are the conditions of STP
* a temperature of **0° C** (**273 K**)
* pressure of **100 kPa**
* pressure of **100 kPa**
30
New cards
Worked example of gases and avogadro’s law - Example:
\
What is the **total volume of gases remaining** when **70 cm3** of **ammonia** is combusted completely with **50 cm3** of **oxygen** according to the equation shown?
\
**4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (l)**
\
What is the **total volume of gases remaining** when **70 cm3** of **ammonia** is combusted completely with **50 cm3** of **oxygen** according to the equation shown?
\
**4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (l)**
\
**Step 1**: From the equation deduce the molar ratio of the gases, which is NH3 :O2 :NO or 4:5:4 (water is not included as it is in the liquid state)
\
**Step 2**: We can see that oxygen will run out first (the **limiting reactant**) and so 50 cm3 of O2 requires 4/5 x 50 cm3 of NH3 to react = 40 cm3
\
**Step 3**: Using Avogadro's Law, we can say 40 cm3 of NO will be produced
\
**Step 4**: There will be of 70-40 = 30 cm3 of NH3 left over
\
Therefore **the total remaining volume will be 40 + 30 = 70 cm3 of gases**
**Step 1**: From the equation deduce the molar ratio of the gases, which is NH3 :O2 :NO or 4:5:4 (water is not included as it is in the liquid state)
\
**Step 2**: We can see that oxygen will run out first (the **limiting reactant**) and so 50 cm3 of O2 requires 4/5 x 50 cm3 of NH3 to react = 40 cm3
\
**Step 3**: Using Avogadro's Law, we can say 40 cm3 of NO will be produced
\
**Step 4**: There will be of 70-40 = 30 cm3 of NH3 left over
\
Therefore **the total remaining volume will be 40 + 30 = 70 cm3 of gases**
31
New cards
Molar gas volume, volume of gas, and moles formula triangle
\
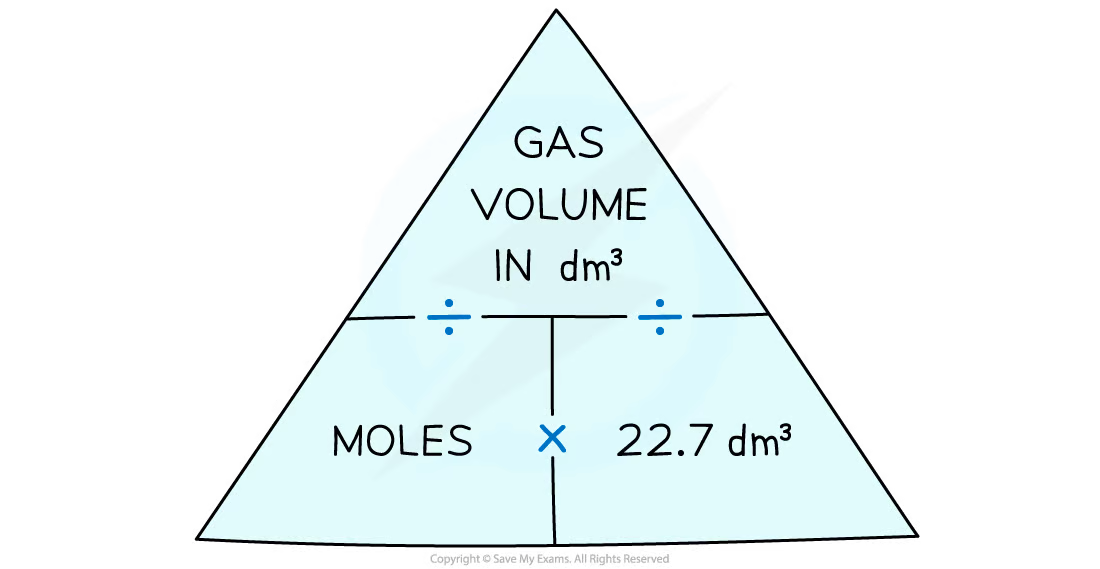
32
New cards
What does the kinetic theory of gases state
that molecules in gases are constantly moving
33
New cards
What are the 5 assumptions that the kinetic theory of gases is making
1. The gas molecules are moving **very fast** and **randomly**
2. The molecules **hardly have any volume**
3. The gas molecules **do not attract** or **repel** each other (**no** **intermolecular forces**)
4. **No kinetic energy is lost** when the gas molecules **collide** with each other (**elastic collisions**)
5. The temperature of the gas is directly proportional to the average kinetic energy of the molecules - as temperature increases so does kinetic energy
34
New cards
What is the name of gases that follow the kinetic theory
Ideal gases
35
New cards
What does the volume that a gas occupies depend on (2)
* pressure
* temperature
* temperature
36
New cards
Ideal gas equation
### **PV = nRT**
37
New cards
Why do gases in a container exert a pressure?
as the gas molecules are constantly **colliding** with the **walls** of the container
38
New cards
Describe Boyle’s law
* As volume decreases, pressure increases (at constant temperature)
* this is because it causes the molecules to be **squashed** together which results in more **frequent** **collisions** with the container wall
* this is because it causes the molecules to be **squashed** together which results in more **frequent** **collisions** with the container wall

39
New cards
Describe Charle’s Law
* as volume increases, so does temperature (at constant pressure)
* when a gas is **heated -** the particles gain more **kinetic energy** and undergo more **frequent collisions** with the container walls
* To keep the **pressure constant**, the molecules must get further apart and therefore the **volume increases**
* when a gas is **heated -** the particles gain more **kinetic energy** and undergo more **frequent collisions** with the container walls
* To keep the **pressure constant**, the molecules must get further apart and therefore the **volume increases**
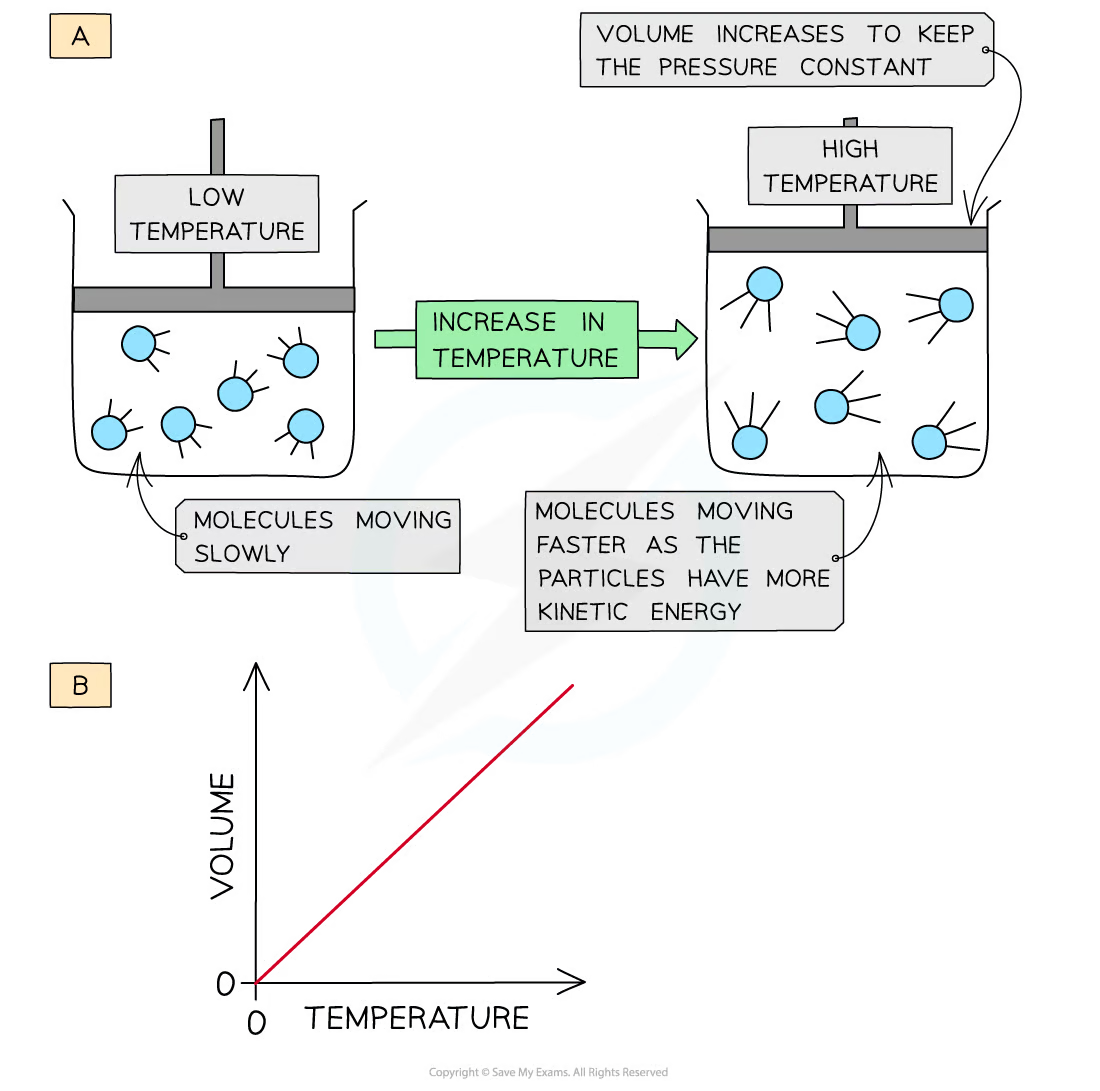
40
New cards
Describe Gay-Lussac’s law
* As temperature increases, so does pressure (at constant volume)
* **Increasing** the **temperature** of the gas causes the molecules to gain more **kinetic energy**
* This means that the particles will move **faster** and **collide** with the container walls more **frequently -** increasing the pressure
* **Increasing** the **temperature** of the gas causes the molecules to gain more **kinetic energy**
* This means that the particles will move **faster** and **collide** with the container walls more **frequently -** increasing the pressure
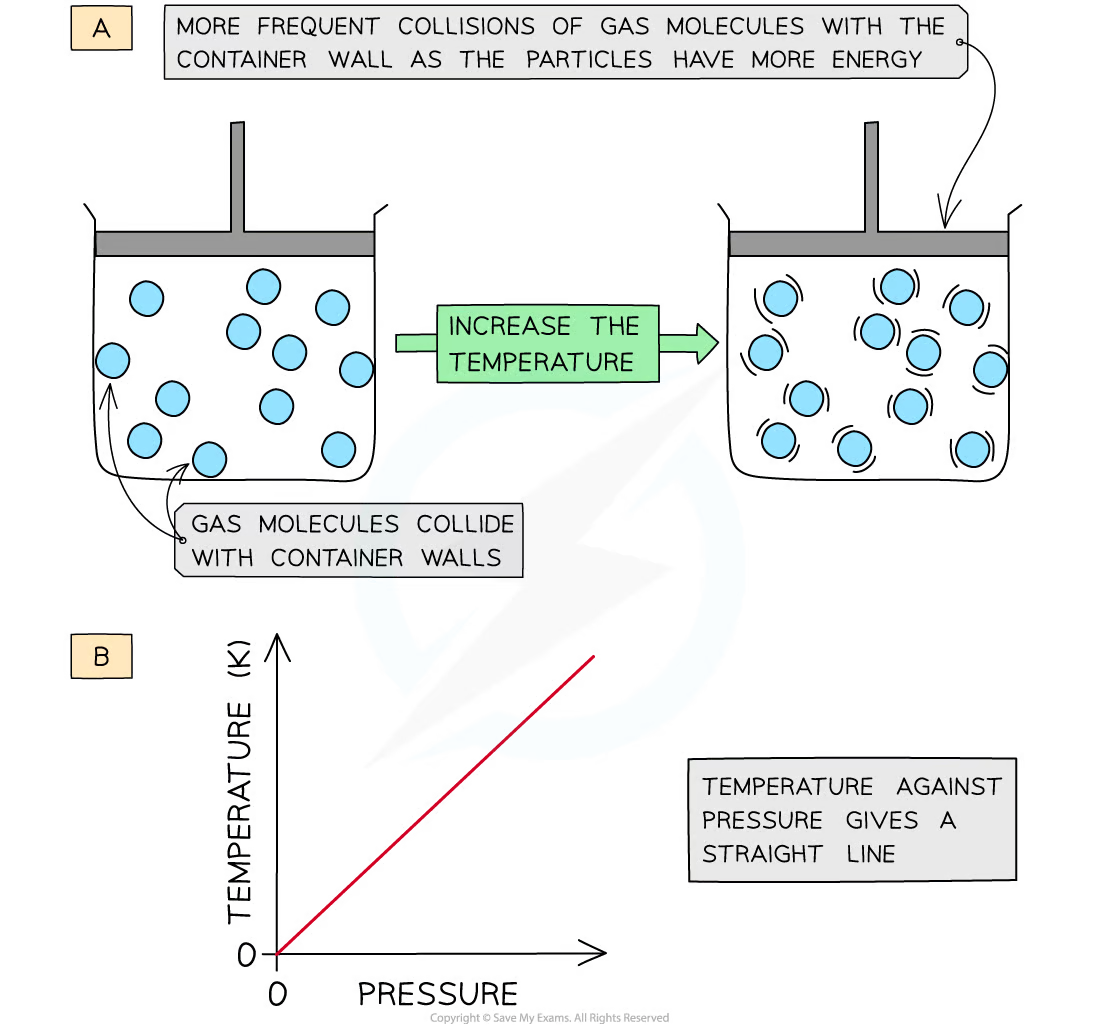
41
New cards
What is the equation when you change the conditions of a fixed amount of gas and why?
* For a fixed amount of gas, **n** and **R** will be constant, so if you change the conditions of a gas we can ignore **n** and **R** in the **ideal gas equation**
* So the new equation is:
* (p1v1/t1 = p2v2/t2)
* So the new equation is:
* (p1v1/t1 = p2v2/t2)
42
New cards
What are standard solutions
solutions which are used for analysis whose concentrations are known precisely
* made as accurately and precisely as possible
* made as accurately and precisely as possible
43
New cards
Formula triangle for moles, concentration, and volume
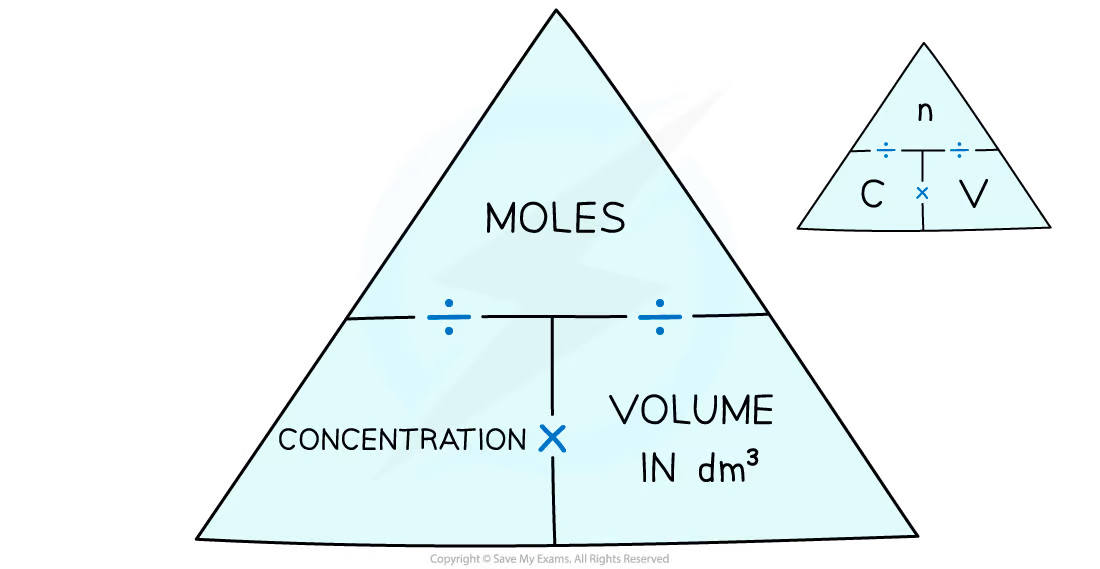
44
New cards
Worked example of concentration calculation:
Calculate the volume of hydrochloric acid of concentration 1.0 mol dm-3 that is required to react completely with 2.5 g of calcium carbonate.
\
Calculate the volume of hydrochloric acid of concentration 1.0 mol dm-3 that is required to react completely with 2.5 g of calcium carbonate.
\
**Answer:**
\
**Step 1:** Write the balanced equation for the reaction
\
**CaCO3 + 2HCl → CaCl2 + H2O + CO2**
\
**Step 2:** Determine the moles of the known substance, calcium carbonate
\
\
\
**Step 3:** Use the balanced equation to deduce the mole ratio of calcium carbonate to hydrochloric acid:
\
1 mol of CaCO3 requires 2 mol of HCl
\
So 0.025 mol of CaCO3 requires 0.050 mol of HCl
\
**Step 4:** Calculate the volume of HCl required
\
\
**Step 1:** Write the balanced equation for the reaction
\
**CaCO3 + 2HCl → CaCl2 + H2O + CO2**
\
**Step 2:** Determine the moles of the known substance, calcium carbonate
\
\
\
**Step 3:** Use the balanced equation to deduce the mole ratio of calcium carbonate to hydrochloric acid:
\
1 mol of CaCO3 requires 2 mol of HCl
\
So 0.025 mol of CaCO3 requires 0.050 mol of HCl
\
**Step 4:** Calculate the volume of HCl required
\
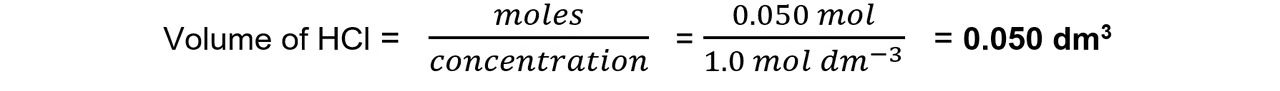
45
New cards
What is volumetric analysis (titration)
a process that uses the volume and concentration of one chemical reactant (**a standard solution**) to determine the concentration of another unknown solution
* The technique most commonly used is a **titration**
\
* The technique most commonly used is a **titration**
\
46
New cards
What is the end / equivalence point in a titration
\
The **end point** or **equivalence point** occurs when the two solutions have reacted completely and is shown with the use of an **indicator.**
The **end point** or **equivalence point** occurs when the two solutions have reacted completely and is shown with the use of an **indicator.**
47
New cards
What are the steps of a titration
* Measure a known volume of solution 1 (with unknown concentration)
* The other solution (solution) (with a known concentration) is placed in the burette
* A few drops of the **indicator** are added to solution 1
* The tap on the **burette** is carefully opened and the solution added, portion by portion, to solution 1 until the **indicator** just changes colour
* Multiple trials are carried out until **concordant** results are obtained
* The other solution (solution) (with a known concentration) is placed in the burette
* A few drops of the **indicator** are added to solution 1
* The tap on the **burette** is carefully opened and the solution added, portion by portion, to solution 1 until the **indicator** just changes colour
* Multiple trials are carried out until **concordant** results are obtained