Pathology of the Male Reproductive system
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
Define Benign Nodular Hyperplasia of the Prostate (BPH).
BPH is a non-neoplastic enlargement of the prostate gland, primarily affecting the transitional zone, leading to urethral compression and urinary obstruction.
Which zone of the prostate is most commonly affected in BPH?
The transitional zone (around the urethra).
List two main aetiological factors associated with BPH.
Aging
Hormonal imbalance (altered androgen–estrogen ratio stimulating stromal and epithelial proliferation)
Explain the initial pathophysiological change that occurs due to BPH.
Urethral obstruction occurs due to nodular expansion of periurethral tissue, increasing resistance to bladder emptying.
Name two complications that can arise from urinary stasis in BPH.
Recurrent urinary tract infections (UTIs)
Bladder stone formation
What compensatory change occurs in the bladder in response to urethral obstruction in BPH?
Detrusor muscle hypertrophy develops to overcome increased resistance to urine flow.
Describe how BPH can eventually lead to renal damage.
Chronic obstruction elevates intravesical pressure, causing retrograde pressure transmission → hydroureter, hydronephrosis, and pressure atrophy of renal parenchyma → possible renal failure.
Discuss the aetiology, pathophysiology, and complications of Benign Nodular Hyperplasia of the Prostate (BPH).
Aetiology:
Strongly associated with aging.
Hormonal imbalance: altered androgen–estrogen ratio → stimulates proliferation of stromal and epithelial cells.
Pathophysiology:
Nodular expansion of periurethral and stromal tissue → compression of prostatic urethra → urethral obstruction.
Increased bladder resistance → detrusor hypertrophy (compensatory).
Elevated intravesical pressure → retrograde pressure transmission → hydroureter, hydronephrosis, and pressure atrophy of renal parenchyma.
Complications:
Urinary stasis → recurrent UTIs, bladder stones.
Incomplete bladder emptying → worsening obstruction and potential renal failure.
Explain the mechanism by which hormonal changes contribute to the development of BPH.
With aging, the androgen–estrogen ratio alters.
Increased estrogen levels enhance the proliferative response of stromal and epithelial cells in the prostate.
This leads to nodular hyperplasia, particularly in the periurethral/transitional zone.
Resulting urethral compression causes obstructive urinary symptoms.
Describe the sequence of pathophysiological events from urethral obstruction in BPH to potential renal failure.
Nodular growth compresses prostatic urethra → urethral obstruction.
Bladder must exert more force → detrusor hypertrophy develops.
Persistent obstruction elevates intravesical pressure → retrograde transmission to ureters (hydroureter) and kidneys (hydronephrosis).
Chronic pressure causes renal parenchymal atrophy → can result in renal failure.
Define corpora amylacea.
Corpora amylacea are rounded, lamellated, eosinophilic structures composed mainly of polysaccharide-rich material (sulphated glycosaminoglycans) mixed with proteins, sometimes with an amyloid-like nature.
What does the term “bodies of starch” indicate about corpora amylacea?
It refers to their PAS (Periodic Acid-Schiff) positivity; they do not actually contain starch.
Where are corpora amylacea most commonly found?
The prostate gland. They can also be found in the brain (perivascular spaces), lung, and uterus.
Describe the genesis of corpora amylacea in the prostate.
They arise from inspissated (thickened) secretions, progressively condense, may undergo mineralization (hydroxyapatite), and chronic inflammation (e.g., bacterial prostatitis) contributes via epithelial injury and altered secretory dynamics.
What can calcified corpora amylacea lead to in the prostate?
Formation of prostatic calculi (small stones).
List two potential factors that contribute to the formation of corpora amylacea.
Chronic inflammation (e.g., bacterial prostatitis)
Age-related degenerative changes
Are corpora amylacea generally harmful?
No, they are usually innocuous, incidental findings, and do not cause disease by themselves.
Discuss the composition, staining characteristics, and clinical significance of corpora amylacea.
Composition: Mainly polysaccharide-rich material (sulphated glycosaminoglycans) with protein components; sometimes described as amyloid-like.
Staining: PAS-positive (“bodies of starch”), eosinophilic on H&E; do not contain actual starch.
Clinical significance: Usually harmless and incidental; may represent age-related degenerative change or response to chronic inflammation; sometimes considered localized amyloidosis; may calcify to form prostatic stones.
Explain the pathogenesis of corpora amylacea formation in the prostate.
Originates from inspissated secretions in the prostate.
Chronic inflammation (e.g., bacterial prostatitis) induces epithelial injury and altered secretory dynamics.
Progressive condensation of secretions leads to formation of lamellated eosinophilic bodies.
Some may undergo mineralization with hydroxyapatite → can form prostatic calculi.
Describe the distribution of corpora amylacea in the human body and their possible biological role.
Distribution: Most common in the prostate; also found in brain (perivascular spaces), lung, and uterus.
Biological role: Uncertain; may represent age-related degeneration or a response to chronic stress/inflammation; sometimes interpreted as localized amyloidosis.
What is the most common type of testicular germ cell tumour?
Seminoma, accounting for approximately 50% of testicular germ cell tumours.
At what age do seminomas most commonly occur?
In males in their 30s.
Describe the lymphatic spread of seminomas.
Seminomas initially spread via lymphatics to the iliac and para-aortic lymph nodes.
Describe the gross appearance of a seminoma.
Well-demarcated, pale creamy-white, homogenous mass with subtle lobulation; lacks haemorrhage and necrosis.
What are the key microscopic features of seminoma?
Sheets/nests of uniform germ cells with distinct borders
Large round/polygonal cells with clear glycogen-rich cytoplasm
Large central nuclei with 1–2 prominent nucleoli
Fibrous septa separating nests with lymphocytic/plasmacytic infiltrates
Granulomatous inflammation in ~1/3 of cases
Discuss the epidemiology, gross features, and microscopic characteristics of seminoma.
Epidemiology: Most common testicular germ cell tumour (~50%); occurs in males in their 30s.
Gross Features: Well-demarcated, creamy-white, homogenous mass; subtle lobulation; lacks haemorrhage and necrosis.
Microscopic Features: Sheets or nests of uniform germ cells; clear glycogen-rich cytoplasm; large central nuclei with prominent nucleoli; fibrous septa with lymphocytic/plasmacytic infiltrates; granulomatous inflammation in ~1/3 of cases.
Explain the clinical significance of lymphatic dissemination in seminoma.
Seminomas primarily spread via lymphatics to iliac and para-aortic nodes.
Knowledge of this pattern is crucial for accurate staging and planning treatment (surgery, radiotherapy, chemotherapy).
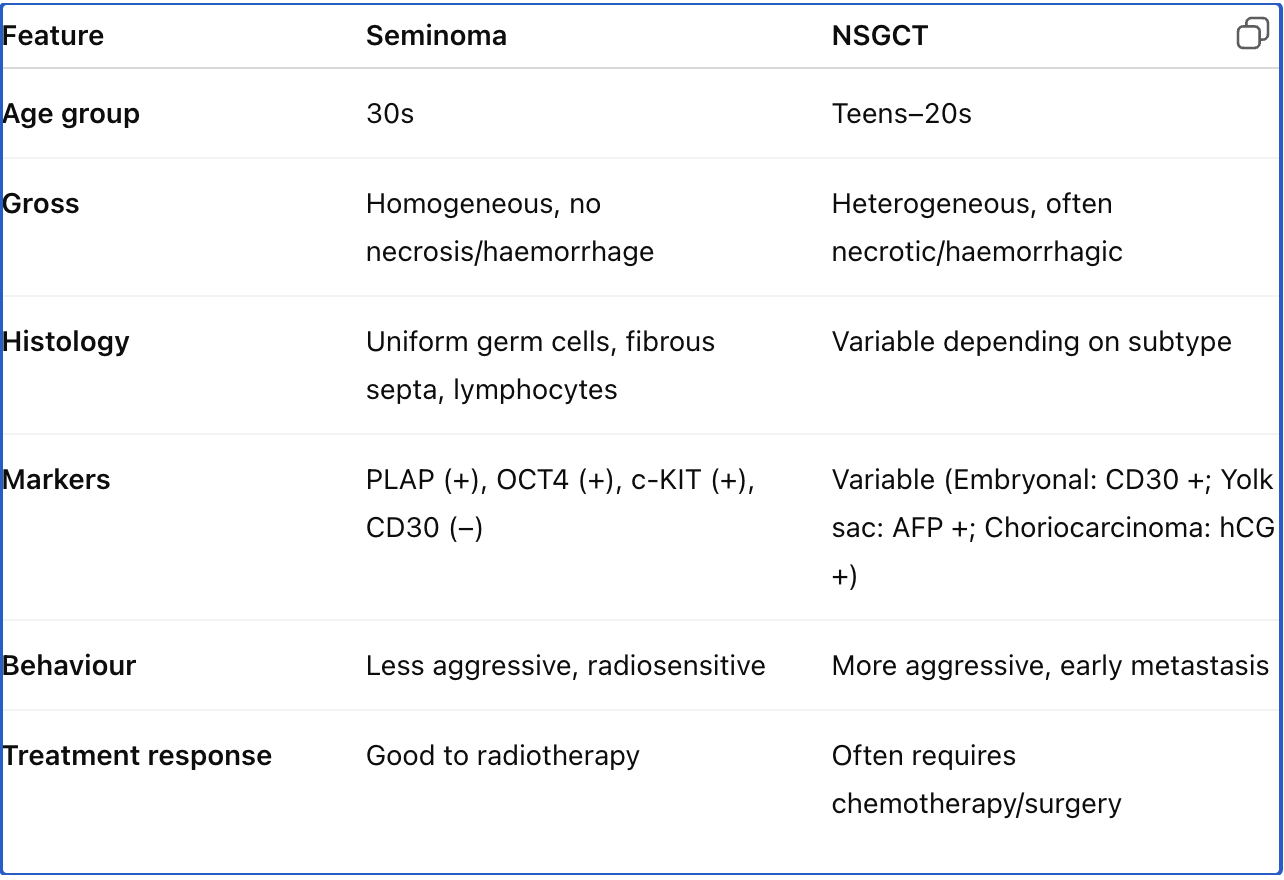
Differentiate seminoma from non-seminomatous germ cell tumours in terms of pathology and clinical presentation.
Seminoma: Uniform germ cells, clear cytoplasm, fibrous septa, often lymphocytic infiltrates, lacks haemorrhage/necrosis; occurs in 30s.
NSGCT: Heterogeneous histology (embryonal carcinoma, yolk sac tumour, choriocarcinoma, teratoma), often presents with haemorrhage and necrosis; occurs in younger males; more aggressive behaviour and earlier metastasis.
Name three key immunohistochemical markers used in the diagnosis of seminoma.
→ PLAP, OCT4, and c-KIT (CD117).
What is the typical immunohistochemical profile of seminoma?
→ PLAP (+), OCT4 (+), c-KIT (CD117) (+), CD30 (–).
Describe the staining pattern of PLAP in seminoma.
→ Strong membranous and cytoplasmic staining.
What is the staining pattern of OCT4 in seminoma?
→ Intense, diffuse nuclear positivity.
What is the diagnostic utility of OCT4 in germ cell tumours?
→ Helps distinguish seminomas and embryonal carcinomas (OCT4+) from yolk sac tumours and teratomas (OCT4–); also detects germ cell neoplasia in situ.
How does c-KIT (CD117) expression help differentiate seminoma from embryonal carcinoma?
→ Seminoma: c-KIT positive, CD30 negative; Embryonal carcinoma: c-KIT negative, CD30 positive.
Why is PLAP not considered a specific marker for seminoma?
→ Because it can also be expressed in other malignancies and can be elevated in smokers.
Which immunomarker is negative in seminoma but positive in embryonal carcinoma?
→ CD30.
Discuss the role of immunohistochemistry in the diagnosis of seminoma. Include the major markers, their staining patterns, and their diagnostic relevance.
Introduction:
Seminoma is a malignant germ cell tumour of the testis, typically affecting males in their 30s. Morphologically, it shows uniform germ cells with clear cytoplasm and fibrous septa infiltrated by lymphocytes.Role of IHC:
Confirms diagnosis and differentiates seminoma from non-seminomatous germ cell tumours.Key markers:
Marker
Type / Location
Staining Pattern
Diagnostic Role
PLAP
Membranous + cytoplasmic
Strong positivity
Sensitive but not specific; supports germ cell origin
OCT4
Nuclear transcription factor
Intense nuclear positivity
Distinguishes seminoma and embryonal carcinoma (positive) from yolk sac tumour & teratoma (negative); identifies germ cell neoplasia in situ
c-KIT (CD117)
Membrane receptor tyrosine kinase
Strong membranous positivity
Distinguishes seminoma (positive) from embryonal carcinoma (negative)
CD30
Cell surface glycoprotein
Negative in seminoma
Positive in embryonal carcinoma; useful for differential diagnosis
IHC profile summary:
Seminoma → PLAP (+), OCT4 (+), c-KIT (+), CD30 (–)Conclusion:
IHC is essential in confirming seminoma diagnosis and excluding NSGCT components, ensuring accurate classification and appropriate management.
List the main subtypes of NSGCTs.
→ Embryonal carcinoma, yolk sac tumour, choriocarcinoma, teratoma, and mixed germ cell tumour.
Which NSGCT subtype is most common in children?
→ Yolk sac tumour.
What is the characteristic histologic feature of a yolk sac tumour?
→ Schiller-Duval bodies (glomeruloid structures).
Which tumour marker is elevated in yolk sac tumours?
Alpha-fetoprotein (AFP).
Which NSGCT subtype is associated with increased hCG levels?
Choriocarcinoma.
Name two immunohistochemical markers of embryonal carcinoma.
→ OCT4 positive, CD30 positive, c-KIT negative.
What is the biological behaviour of choriocarcinoma?
→ Highly malignant, with extensive vascular invasion and early hematogenous spread
Which germ cell tumour subtype is resistant to radiotherapy in adults?
→ Teratoma (adult type).
What is a mixed germ cell tumour?
→ A tumour containing components of seminoma and/or various NSGCT subtypes.
Compare seminoma and embryonal carcinoma immunoprofiles.
Marker | Seminoma | Embryonal Carcinoma |
|---|
OCT4 | + | + |
c-KIT (CD117) | + | – |
CD30 | – | + |
PLAP | + | + (variable) |
What is the most common malignant tumour of the prostate?
→ Prostate adenocarcinoma.
From which part of the prostate does adenocarcinoma most often arise?
→ The peripheral zone.
Which serum marker is used for screening and monitoring prostate cancer?
→ Prostate-specific antigen (PSA).
What are the microscopic features of prostate adenocarcinoma?
→ Small, crowded, irregular glands infiltrating the stroma, absence of basal cell layer, prominent nucleoli.
Name two tissue markers used in the diagnosis of prostate adenocarcinoma.
→ PSA and α-methylacyl-CoA racemase (AMACR).
What are the routes of spread for prostate adenocarcinoma?
→ Local extension beyond capsule and seminal vesicles; hematogenous spread to bone (osteoblastic lesions).
Why is PSA not specific for prostate cancer?
→ It can also be elevated in BPH, prostatitis, or after prostatic manipulation.
Discuss the types, histological features, and tumour markers of non-seminomatous germ cell tumours (NSGCTs).
Definition: NSGCTs are malignant germ cell tumours showing differentiation along embryonic or extra-embryonic lineages.
Types and features:
Embryonal carcinoma: Aggressive, anaplastic; OCT4 (+), CD30 (+), c-KIT (–).
Yolk sac tumour: Common in children; Schiller-Duval bodies; ↑ AFP.
Choriocarcinoma: Highly malignant; syncytiotrophoblasts → ↑ hCG.
Teratoma: Contains tissues from all 3 germ layers; malignant potential in adults.
Mixed GCTs: Combination of seminoma and NSGCTs.
Clinical relevance: Aggressive behaviour, early metastasis; require multimodal therapy.
Compare seminoma and non-seminomatous germ cell tumours in terms of morphology, immunoprofile, and clinical features.
Describe the pathology, markers, and clinical utility of PSA in prostate adenocarcinoma.
Origin: Glandular epithelium (secretory cells) of prostate; usually in peripheral zone.
Histology: Small, irregular infiltrating glands; loss of basal cells; enlarged nuclei with prominent nucleoli.
Markers: PSA and AMACR (positive in carcinoma).
Spread: Local (capsule, seminal vesicles) and distant (bone — osteoblastic).
PSA:
Role: Screening, diagnosis, monitoring, therapy response, recurrence detection.
Limitations: Not cancer-specific — increased in BPH, prostatitis, and after manipulation.
Interpretation: Should always be correlated with DRE, imaging, and biopsy.