practical/written exam #1 - immunohematology lab (cls 545)
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
98 Terms
reading for agglutaintion
use jiggle and tilt motion
read when the button fully dislodges
*pay attention how the button dislogdes
grade from 0-4+
interpretation of agglutination
negative (0): cells fail to show visible agglutination after button dislodges
1+: hazy red background with many small agglutinates available
2+: clear bkg w medium sized agglutinates
3: clear bkg w fewer than 5 larger clumps
4+: one large clump or one large and one smaller clump w clear bkg (no free cells)
considerations for reading agglutinations
if pt tube is negative, it does not mean that they are pro- or post-zoning
most often means either the antigen or antibody is not in the tube
positive reactions occur when BOTH antigen and antibody are in the reaction tube
direct antiglobulin test (DAT)
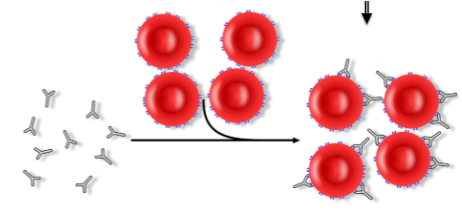
aka direct coomb’s test; stand-alone test on CELLS to demonstrate IN VIVO attachment (sensitization) of rbcs by antibody/complement using a modified indrect antiglobulin procedure
if IgG or complement is bound to a pt’s rbcs, the antiglobulin will crosslink the sensitized cells to create a visible agglutination reaction
first performed w polyspecifc AHG and followed up with separate monospecific reagents (anti-IgG/C3d) to confirm
**note: newborns are tested against anti-IgG ONLY!!!!
purpose of DAT test
investigate antibody or complement mediated hemolytic anemia (HDFN, HTR, AIHA, DIHA)
negative control: saline + washed rbcs; ensures that positive reactions are actually due to IgG or complement coating the cells
indirect antiglobulin test (IAT)
technique applied to many tests either using patient cells or plasma; can be performed on plasma or serum in detection of unexpected antibodies or on cells to type for antigens (weak D, Kell, Kidd)
antibodies from patient plasma or antibodies from commerical reagents will attach to designated cells under optimal conditions (IN VIVO sensitization)
uses monospecific AHG (anti-IgG only) to crosslink antibody coated cells (if present) to form visible agglutination
purpose of IAT
discover and identify blood group antibodies of class IgG prior to transfuion by “antibody screening” and “compatibility testing”
antibody screening: cells are commerical preparations containing selected blood group antigens
compatibility testing: cells are the intended donor cells
autocontrol: tests if pt’s plasma is agglutinating the cells of that same patient (autoantibody)
if autocontrol positive, DAT is likely to be positive
explain the procedural difference between an autocontrol for an IAT and a DAT
anti-IgG is added to the autocontrol for the IAT autocontrol whereas the the control tube for DAT is just pt cells + saline (?)
difference between an autocontrol used in IAT testing and control used in a DAT?
IAT: tests to see if the patient has an autoantibodies causing nonspecific agglutination
DAT: control ensures that the positive reactions are actually due to IgG or complement coating the cells
why is the 5 minute RT incubation needed for a DAT?
enhance the detection of weakly reactive anti-complement antibodies—a weak reaction may not be evident without additional time for the complement antibodies to bind and form a stable lattice
what are Coombs control cells?
O positive cells coated with IgG
checks for false negative results
ensures reagent was added
why aren’t Coombs control cells added to the control for DAT?
control tube has no reagent added (pt cells + saline) & it is supposed to be a negative control so there is no need to confirm it with check cells
difference between polyspecific and monospecific AHG globulin and for what is each used?
polyspecific: has anti-IgG AND anti-C3d; used in the DAT procedure as an initial screen
monospecific: antibody with specificity to only IgG OR complement; used in follow up testing after polyspecific AHG in DAT
IAT testing only uses anti-IgG
sources of error for DAT procedure
mislabeling
incorrect serum:cell ratio
incorrect times and/or temps during incubation
over/under centrifugation
interrupted/delayed washing and addition of AHG (antibody dissociates = false negative
loss of reaction when dispersing a cell button (overzealous shaking)
applications for IAT testing
antibody screen
antibody identification
weak D
RBC antigen typing
full crossmatching
IAT interpretation notes
any cells w a pos DAT will also be positive in tests using an indirect antiglobulin technique (antibody is already coating cells)
thus, valid IATs cannot be obtained on DAT+ cells, i.e. weak D antigen testing
test will result in false positive and the specificity of the antibody may not be what you are looking for
antibody screen & crossmatch are not affected by a pos DAT
identify 3 purposes for which anti-A,B could be used
when there is a missing reaction with anti-A and/or anti-B
investigating possible weak Type A or B subgroups
confirmation of type O blood
at what age can ABO reverse typing be reliably performed? why?
on patients >6 mo; RBC antibodies are not developed until 3-6 months after birth, meaning that reverse typing on newborns is unreliable
what does a mixed field (mf) reaction in the reverse type of tube agglutination indicate?
presence of two distinct populations of RBCs are present due to recent transfusion or BMT
can also indicate chimerism/mosaicism
explain why weak D testing is often invalid in the DAT is positive
if the DAT is positive, it indicates that the cells were already coated with an ABY that wasn’t the anti-D that was added to detect the D antigen
how does a positive Rh control affect the results of typing for D? what should be done to attempt to get a valid test?
a positive result in the Rh control means that the testing is invalid/indicates an invalid reaction
report according to the last valid D status obtained during testing
can redo test using different tube for control
who is weak D testing for? (3)
newborns <4 months ; cord bloods
pregnant mothers w/o a history
bone marrow transplant pts who are Rh neg @ IS
check for mixed fields !
cord blood testing
cord blood samples are collected from the umbilical cord at the time of delivery & may be used to determine the ABO type of the newborn as well as the sensitization status of the newborn rbcs (DAT)
perform a FRONT TYPE and anti-IgG/saline ONLY DAT (!!)
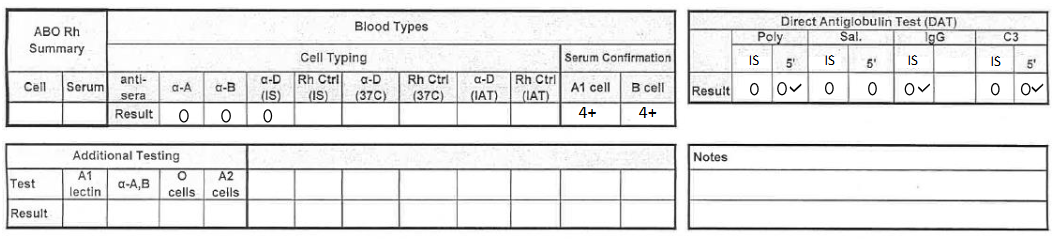
what would this result be reported as? (see pic)
O negative, DAT negative
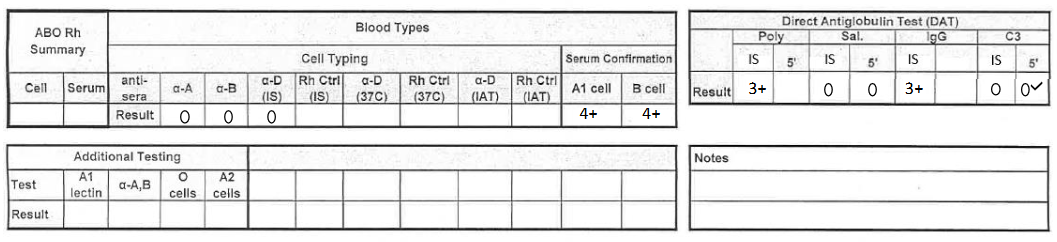
what would this result be reported as? (see pic)
O negative, DAT positive (IgG positive, C3 negative)
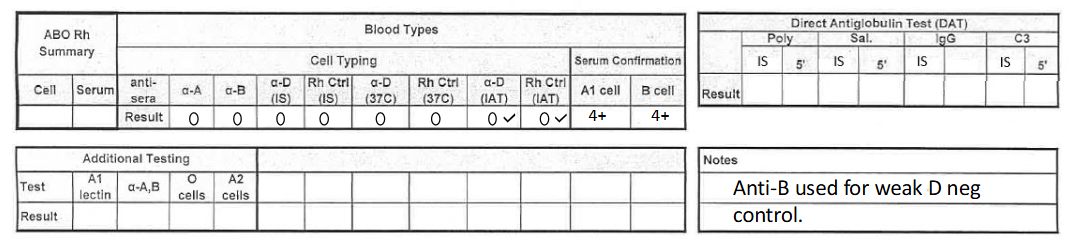
what would this result be reported as? (cord blood)
O negative
**make sure to add note!
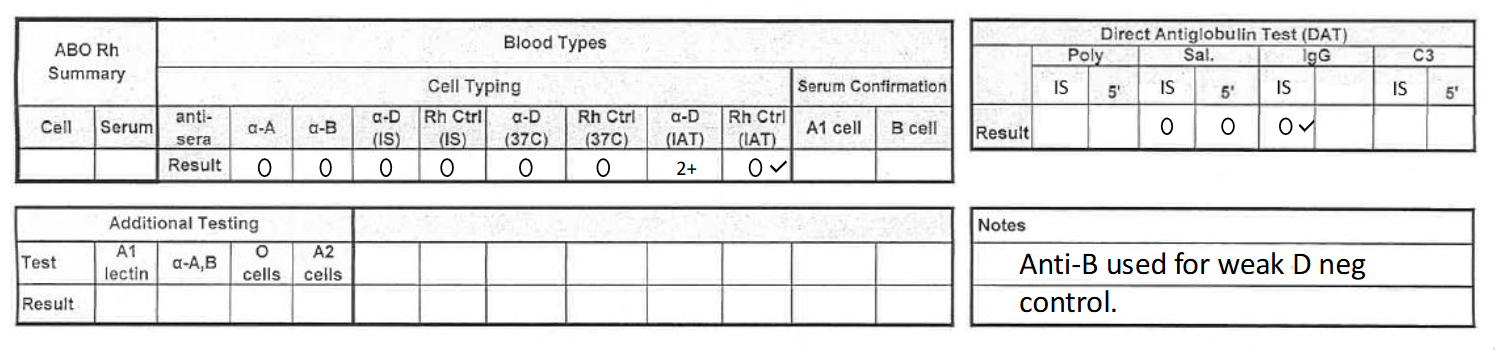
what would this result be reported as? (cord blood 2)
O positive, DAT negative
**make sure to add note!
***cord bloods only need a-IgG and saline tubes for DAT testing
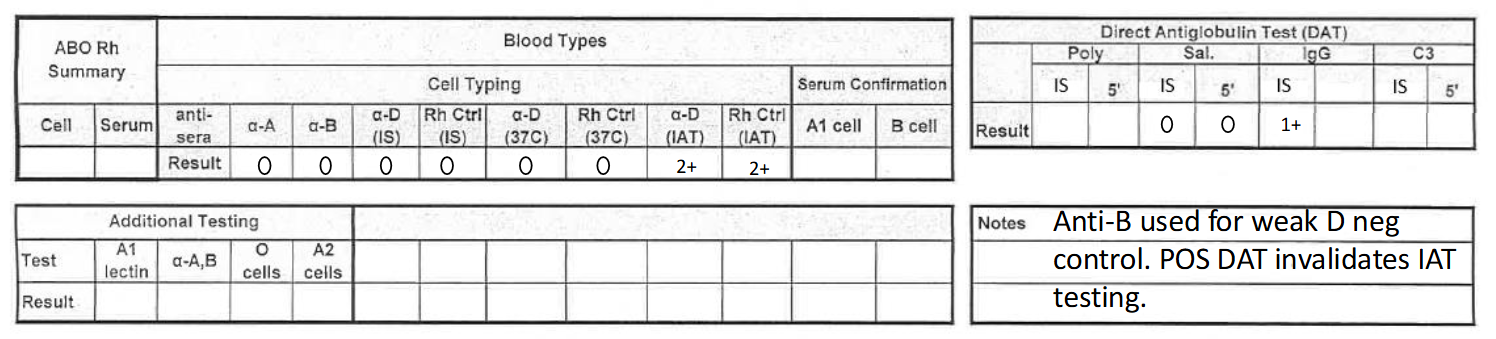
what would this result be reported as? (cord blood 3)
O negative, DAT positive (IgG positive)
**make sure to add note + DAT pos result invalidates IAT (weak D) testing
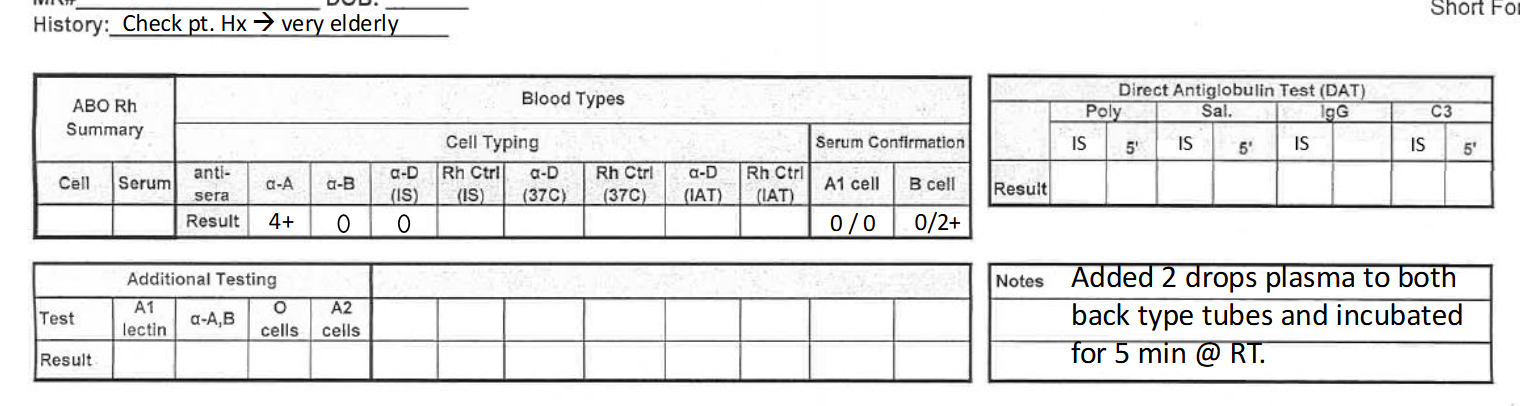
what would this result be reported as? (see pic)
A negative
**elderly patients may need 5 min incubation for valid result in back type—see note
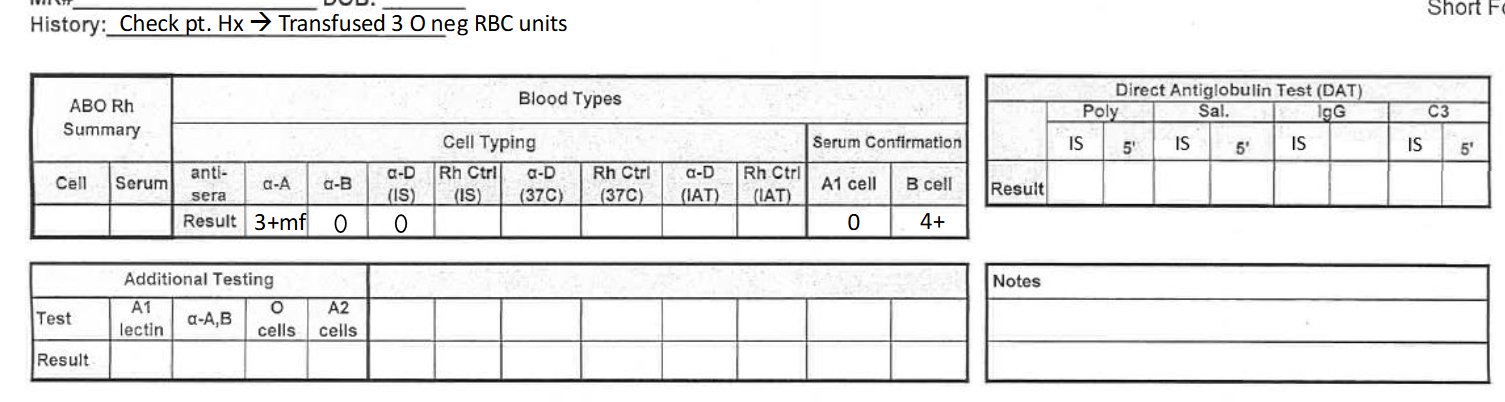
what would this result be reported as? (see pic)
NTD, give O RBCs
transfusion word origin
trans = across
fundere = to pour
what clinically significant antibody do type O individuals make that pose a danger to pregnant mothers & their babies?
O individuals will make IgG antibodies more readily and also make anti-A,B antibodies that are extremely sensitive to A and B antigens
IgG can cross the placenta which is a problem for pregnant mothers
blood types (general)
8 common blood types
A-, A+, B-, B+, O-, O+, AB-, AB+
Blood types are determined by the presence or absence of antigens found on the surface of the red blood cells
blood type is an inherited trait
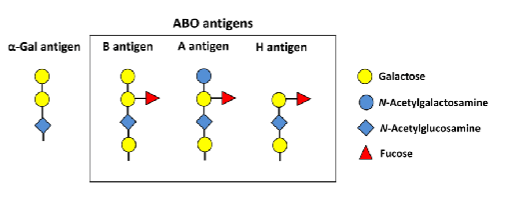
blood type antigens biochemistry
Genes code for the glycosyltransferases NOT the actual antigen
A antigen = N-acetylgalactosamine
B antigen = galactose
O antigen = fucose
forward (front) typing
Patient RBCs tested with commercially produced anti-sera
Testing for the presence (or absence) of antigens on the RBCs
reverse (back) typing
Patient's serum/plasma tested with commercially produced RBCs
Testing for the presence (or absence) of antibodies in the patient's serum/plasma
routine reagents used for ABO/Rh testing
Anti-A, Anti-B, Anti-D, and Rh control for forward typing
A1 and B cells for reverse typing
A2 cells are also available but not routinely used
importance of RhD typing
Determining the presence or absence of RhD is critical in pretransfusion testing
The principle antigen is D (the MOST immunogenic)
Second most clinically important antigen
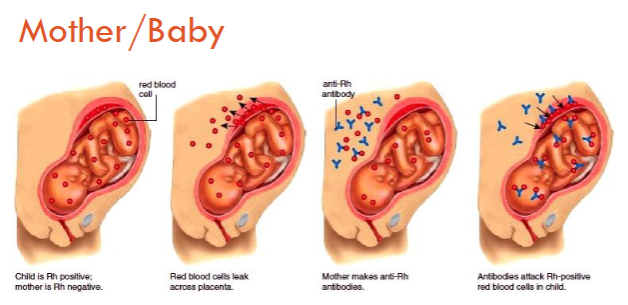
HDFN (Rh negative mom / Rh positive fetus)
Hemolytic transfusion reaction
Individuals who lack RhD are deemed "Rh negative"
Lack the D antigen on their red blood cells
Rh HDFN
Does not happen during the first pregnancy--happens to Rh neg women
Type O moms have the highest risk of HDFN regardless of Rh neg/pos status
rhogam is used to treat—”hides” the baby’s D antigen from the mother
in what clinical scenarios would we order a DAT? (3)
Hemolytic Disease of the Newborn (HDN)
Mom's antibodies are binding to the baby's red cell antigens
Hemolytic Transfusion Reaction (HTR)
Recipient antibody coating donor RBCs
Autoimmune Hemolytic Anemia (AIHA)
Warm autoantibody and/or cold autoantibody
The auto-control will be positive
Drug induced hemolysis
*all have to do with antibodies that target RBCs
direct antiglobulin test (lecture notes)
Standalone test that can be ordered by clinician
Detects IN VIVO sensitization by:
IgG
Complement (C3)
Can be positive in "normal" people
1 in 13,000
3% in hospital inpatients
DAT procedure (simplified)
Take patient red cells
Wash
Obtain dry button
Add anti-IgG and anti-C3 to detect IgG and/or complement binding to the patient's red cells
DAT reagents (lecture notes)
Polyspecific reagent
Contains anti-IgG and anti-C3bC3d
Monospecific reagents
Contains only anti-IgG
Contains only Anti-C3b and C3d
Check cells (if DAT is negative)
AKA Coombs Control cells
how are the tubes labeled for DAT testing?
1st tube: "POLY" + "last name, first initial"
2nd tube: "SALINE" + "last name, first initial"
3rd tube: "IgG" + "last name, first initial"
4th tube: "C3" + "last name, first initial"
After we label our tubes we will add the patient's 3% cell suspension first!
(DAT) purpose of washing the rbcs
Will remove any free antibody that might be surrounding the red cells
Red cells are always in plasma & plasma potentially contains antibodies that would interfere with our reaction
We want to make sure we detect antibody that is bonded to the red cell (not just floating in the plasma)
check cells (coombs control cells; lecture notes)
Are O positive red cells coated in IgG
Used to double check that we added our anti-IgG reagent
Checks if anti-IgG is working
(DAT) out of all the components in the complement cascade, why do we test for C3b and C3d?
C3 is the only component shared by all pathways of the complement cascade
list the two main parts (phases) to a blood type and describe what you are testing for in each part
Front type—commercial antisera + patient cells; looks for antigens on pt cells
Back type—patient plasma + commercial cells; looks for antibodies in pt plasma
how can you assure consistency of volume dispensed when using controlled drop pipettes?
hold the pipette at a consistent angle throughout the dispension of a given substance
holding the pipette vertically will result in smaller drops as opposed to holding the pipette at a 45 degree angle,
err on the side of "big drops" at 45 degree angle
what technique can you use when centrifuging in blood bank immunofuges to increase technologist speed and avoid mix-ups?
place tubes in the centrifuge in the same order they were in the rack
helps to avoid mixups
don't mix and match patients in the centrifuge—try to only spin one patient at a time
what is the centrifugation time (at 3400 rpm) for washing cell suspensions ______________ and reaction tubes _______________?
washing: 3400 rpm for 60 seconds
reaction tubes: 3400 rpm for 20 seconds
what general information do you NEED to label on your test tubes used in blood bank (not the tube used to draw the blood). Mother tubes should include______. Reaction tubes should include___________.
Mother tubes: Patient name and one other identifier (DOB/MRN) + contents of tube
ex: 3% cell suspensions need pt full first & last name + MRN + “3%"
Reaction tubes: patient last name first initial and what reaction/reagent is in tube (anti-A, etc)
how do you judge a 3-5% cells suspension (without measuring precisely)?
compare it to the turbidity of commerical reagent cells such as screening cells
aka use reagent cells to compare suspensions
why is it important to have a specific strategy (that is used consistently) to organize a rack for reaction tubes in blood bank?
reduces error that could adversely affect patient outcomes
ensures that reactions are performed the same way every time between all techs
also if you drop dead in the middle of setting up a reaction, someone else could hop right in and know where to continue
what are 3 rules for handling bottled reagents (antisera or cells)?
Never leave uncapped bottles on the table or bench. When adding reagent to tubes, it is important to hold the bottles to prevent spillage and contamination.
Never have more than one bottle open at once.
Do not touch dropper tips to reaction tubes or lay them down on work surfaces. Doing so leads to contamination of the entire reagent bottle, which are often costly to replace and are preventable errors
in what order should reagents be added to reaction tubes and why?
clear reagents (antisera/plasma/serum) should be added first followed by cellular products (patient/commercial RBCs)
ensures that all of the reagents make it into the tube
easier to visualize that a clear substance was added to a tube if it is put before reagents that are opaque
describe Landsteiner’s Law
states that individuals do not make antibodies to antigens that are present on the surface of their cells as their immune system registers these antigens as "self" antigens
For example, if an antigen is present on a patient's red blood cell (ex: "A" antigen), the corresponding antibody (ex: anti-A) will be absent in the plasma of that individual
An exemption to this law is the presence autoimmune antibodies to RBCs
what volume of anti-sera and patient's cells do you add in the Front Type? how much patient plasma and commercial cells do you add in the back type?
front type: 1 drop of pt’s 3% RBC suspension + 1 drop of anti-sera
back type: 1 drop of commerical cells + 2 drops of pt plasma
which of the following is not a correct way to grade a reaction?
a. 0
b. 1+
c. mf
d. (-)
d. (-)
explain what is meant by clinically significant. are ABO antibodies clinically significant?
antibodies that demonstrate reactivity at 37 degrees Celsius (body temperature)
ABO antibodies are clinically significant as they maintain their reactivity at 37 degress Celsius and may cause severe harm to a patient
why do we use a 3% suspension of patient cells. what do you think could happen if our suspension is too light? too heavy?
3% suspension of patient cells used to achieve correct serum to cell ratio that allows testing to be run within the zone of equivalence
suspension too light: the test runs the risk of falling into the prozone where there is excess antibody causing false negative results to occur
suspension too heavy: the test will be performed in the postzone where there is an abundance of antigen in the test system leading to false negative outcomes as well.
what are the 3 situations where a DAT would be appropriate to order? what would a positive result tell you?
A DAT would be appropriate to order if there's a possibility of:
Hemolytic disease of the fetus and newborn (HDFN)
Hemolytic transfusion reactions (HTR)
Autoimmune (or drug induced) hemolytic anemias (AIHA)
A positive result would indicate that the patient's RBCs are coated in vivo with IgG antigens, complement, or both
what are Coombs Control Cells ("check" cells)? why are these cells added to the control for DAT?
O POSITIVE cells coated in IgG antibodies (anti-D); use them to confirm that we added our anti-IgG and that it is working properly
Coombs Control Cells are IgG coated cells and are DIFFERENT from complement check cells which are coated in C3b/d
Coombs check cells = O POSITIVE RBCs coated in IgG
Complement check cells = O POSITIVE RBCs coated in complement
how would you perform donor reconfirmation on a Bneg unit? What results would you expect?
make 3% suspension with the unit of blood, and confirm label by testing it with anti-A, anti-B, and anti-D
If the label is correct, the results with the anti-A and anti-D reagents should be negative, while the result with the anti-B reagent should be positive
For donor reconfirmation, an Rh control or weak D test is not needed as this testing has been done previously by donor services
After confirming that the results match, the blood can be determined as "Inventory." In the event that the blood type reconfirmation did not match the label, the unit would be marked as "Quarantine."
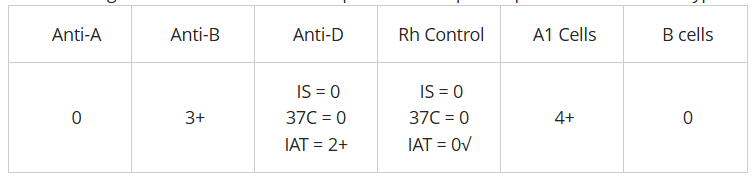
the following results were obtained on a patient workup. interpret the ABO and Rh type (see pic)
B pos
what type of error is caused by insufficient washing of cells in a reaction tube? Provide a reason why.
false negatives occur when there is insufficient washing of cells in a reaction tube
Washing the red blood cells in tests like the DAT helps remove any unbound antibodies that may interfere within the reaction tube
If unbound antibodies are not washed away, they remain in the reaction tube and can bind anti-IgG that should be binding to antibody of the cells. You will get reagent neutralization (of anti-IgG), no agglutination, and a false negative result
(T/F) coombs check cells are IgG coated type O RBCs used for checking the validity of anti-human globulin in a test system
true
what test should be run on a patient that tests positive for Weak D at the IAT phase only?
a. IAT
b. DAT
c. repeat weak D
d. monoclonal control
b. DAT
What must be run on a patient with an AB-positive front type?
a. High protein anti-D
b. Low protein anti-D
c. Monoclonal control
d. DAT
c. monoclonal control
in the DAT gel procedure, what is the percent cell suspension used? what is the source of cells? how does the procedure differ from the Antibody Screen done in gel?
0.8% suspension of the patient's packed red cells are used for testing
no incubation period is needed for DAT gel (antibody screen requires a 15-40 minute incubation step before centrifuging to read the results)
only cells are loaded into the gel cards that contain anti-IgG
what's generally the "first rule" when beginning to troubleshoot a discrepancy? is this always the best approach?
trust the strongest reactions first and then use them as a guide to troubleshoot the weak/missing reactions
not always the best approach as "strong" reactions do not always equate to a value of 4+ or 3+ as one would typically assume
there are ABO discrepancies in which the "strong" reactions are indicative of a different underlying issue (i.e. a reaction with a cold autoantibody may appear strong)
when resolving ABO discrepancies, how would O cells and an autocontrol help you? when would you use them?
used to resolve missing/weak reactions in the back type or unexpected positive back type rxns
O cells: used as form of screening cell that would serve as a method to differentiate ABO antibodies and other blood group antibodies (alloantibodies)
positive = presence of possible alloantibodies that can react w reagent cells or block anti-A or anti-B from reaction w reagent cells
autocontrol: serve as a method to detect if the patient has produced any autoantibodies
when resolving ABO discrepancies, when would you use Anti-A,B?
Anti-A,B is used when resolving ABO discrepancies that involve a missing reaction in the front type
one reason that the forward type may be missing an expected reaction is because of weak antigen expression; thus, Anti-A,B is used because this reagent antibody is more sensitive in picking up weak A and/or B antigen expression
Anti-A,B is also used to identify weak A/B subgroups and can help distinguish type O from types A or B
would using Anti-A,B help you if you have strong reactions with Anti-A and/or Anti-B? explain your answer
NO—anti-A,B only helps when there are missing reactions in the front type; if there is already a strong reaction w anti-A and/or anti-B, anti-A/B is no help bc those reactions are already diagnostic of a blood type for the patient
using Anti-A,B in a discrepancy helps distinguish between Type A/B or O
use Anti-A,B to see if the patient is truly O or if they could be a weak reacting A or B subgroup—If it's already positive in the front-type, anti-A,B won't help
what is the purpose of anti-A1 lectin? explain when you would choose to use it and what results you would expect in your scenario. how would it help you in solving a discrepancy?
Anti-A1 differentiates blood group A1 from ALL other blood groups. It ONLY agglutinates A1 blood types, therefore, it is basically saying “this is A1 or this is NOT A1”
use anti-A1 when we suspect another subgroup of A (like A2 for example). It can help solve a discrepancy when we have an individual who’s front-type looks like blood group A, BUT we have an unexpected positive reaction with the A1 cells in the reverse type
We would expect a negative result with anti-A1 if the patient was in fact a subgroup of A (likely A2) with anti-A1 antibodies
describe QC for anti-A, anti-B, anti-D; A1 cells, and B cells
anti-A: 1 drop anti-A + 1 drop A1 cells, read @ IS
anti-B: 1 drop anti-B + 1 drop B cells, read @ IS
anti-D: 1 drop anti-D+ 1 drop SC1/SC2 cells, read @ IS
A1 cells: 1 drop anti-A1 lectin + 1 drop A1 cells, read @ IS
neg control tube: use O cells
same idea for B cell QC
describe QC for anti-A,B and A2 cells
anti-A,B (two tubes):
1 drop anti-A,B + 1 drop A1 cells, read @ IS
1 drop anti-A,B + 1 drop SC1 or SC2 cells, read @ IS
A2 cells (two tubes):
1 drop anti-A,B + 1 drop A2 cells, read @ IS = should be pos
1 drop anti-B + 1 drop A2 cells, read @ IS = should be neg
describe QC for DAT testing (IgG + complement)
coomb’s check cells (3 tubes)
1 drop check cells + 1 drop saline, read @ IS
1 drop check cells + 1 drop POLY, read @ IS
1 drop check cells + 1 drop anti-IgG, read @ IS
complement check cells
1 drop comp check cells + 1 drop saline, read @ IS
1 drop comp check cells + 1 drop POLY, read @ IS
1 drop comp check cells + 1 drop anti-C3, read @ IS
difference between Coomb’s control cells and complement control cells?
Coombs: O pos RBCs coated with IgG (anti-D IgG antibodies)
Complement: RBCs sensitized w C3d/C3b
why is anti-IgG is added to a testing system?
to detect IgG antibodies that have attached (sensitized) to red blood cells (RBCs) in vivo or in vitro
used in DAT in AHG reagent
used in IAT testing to bind to antibodies that are sensitized to pt RBCs in vitro
rules for solving ABO discrepancies (2)
weak reactions are usually discrepant
antibody problems are MUCH more common than AGN problems
most common cause of ABO discrepancies are missing rxns in the back type
troubleshooting methods for ABO discrepancies (3)
clerical errors
check patient history
repeat testing
list of technical errors leading to ABO discrepancies
under/over centrifugation
failure to add reagents
RBC suspension issues
specimen mix-up
tech inexperience
list of antigen issues leading to ABO discrepancies
missing reactions: subgroups, leukemia, transfusions, soluble Ag
extra reactions: acquired B*, cold autoantibody, cord sample, poly-agg
mixed field: A3 subgroup, chimera, transfusion, HPC transplant
list of antibody issues leading to ABO discrepancies
missing reactions: babies/elderly, hypogammglobulinemia, transfusion, HPC transplant
extra reactions: anti-A1, cold auto/alloantibody, transfusion, HPC transplant, rouleaux
evaluate cause of discrepancy & determine follow up steps:
anti-A: 4+
anti-B: 4+
anti-D: 3+
monoclonal control: 3+
A cells: 0
B cells: 4+
pos monoclonal control invalidates front type
check patient history
if cord blood, wash RBCs due to possible spontaenous agg & redo front type only
if not cord blood, wash RBCs & run DAT
note: negative reactions are also considered as “strong” rxns
evaluate cause of discrepancy & determine follow up steps:
anti-A: 4+
anti-B: 2+
anti-D: 3+
A cells: 0
B cells: 4+
acquired B phenomenon
occurs in type A individuals w gram neg sepsis, colon cancer, or stomach cancer
bacteria have D-acetylase that converts the A antigen (N-acetylgalactosamine) into substance that is recognized as B antigen
**report: Probable Type A w/ acquired B phenotype, Give O RBCs
evaluate cause of discrepancy & determine follow up steps:
trauma pt is typed as A pos on arrival to ER
3 days later & after 3 units of O pos are transfused:
anti-A: 3+mf
anti-B: 0
anti-D: 3+
A cells: 0 / B cells: 4+
report as “No Type Determined (NTD), GIve O RBCs”
women greater than 45 yo and men get O pos RBCs
women less than 45 yo get O neg to protect against Rh HDFN
evaluate cause of discrepancy & determine follow up steps:
anti-A: 4+
anti-B: 0
anti-D: 3+
A cells: 0 / B cells: 0
patient is immunodeficient, on chemo, elderly, or a baby
troubleshoot: add 2 drops of plasma to back type, incubate @ RT for 5 mins and re-centrifuge
evaluate cause of discrepancy & determine follow up steps:
anti-A: 0
anti-B: 4+
anti-D: 3+
A cells: 4+
B cells: 2+
look for rouleaux under the scope
if yes → saline replacement
spin down rxn tube
take off plasma & replace w saline
spin
if no agg in back type, then the 2+ was due to rouleaux (not true agg)
if no → run autocontrol (2 drops pt plasma + 1 drop 3%)
pos agg = presence of autoantibodies
negative → check alloantibodies
run back type w O cells to find non-ABO ABYs
if pos, then the 2+ was from a cold-reacting alloantibody (not clinically significant if pos result @ RT)
evaluate cause of discrepancy & determine follow up steps:
anti-A: 4+
anti-B: 4+
anti-D: 3+
A cells: 2+ / B cells: 2+
run a monocontrol
negative → check pt hx → pt has multiple myeloma
check for rouleaux (almost always affects back type)
yes → saline replacement
evaluate cause of discrepancy & determine follow up steps:
anti-A1: 4+
anti-B: 0
anti-D: 3+
A1 cells: 2+
B cells: 4+
check for rouleaux → none
suspect A subgroup & check pt hx
test with anti-A1 lectin & A2 cells
patient hx ended up being: hx of transfusion a A pos RBCs (A1)
pt has created antibodies to A1 cells
anti-A1 lectin testing = 0
A2 cell test = 0
report: subgroup of A, likely A2
evaluate cause of discrepancy & determine follow up steps:
anti-A1: 4+
anti-B: 4+
anti-D: 3+
A1 cells: 2+
B cells: 0
run monocontrol
run anti-A1 & A2
anti-A1 = 0
A2 = 0
suspect: subgroup of A; likely A2 w anti-A1 antibodies
pt hx should have hx of transfusion
type is most likely A2B
what does anti-A reagent react with? anti-A1?
anti-A: has anti-A and anti-A1
agglutinates type A1 AND A2 blood
anti-A1 lectin (Dolichos biflorus)
antibody to anti-A1 ONLY
those with type A1 have what antigens on their RBCs? what about type A2 individuals?
Type A1: A1 + A antigen
Type A2: A antigen only
determine the possible blood type(s) given the following reactions:
anti-A1: 4+
A2 cells: 0
type A1
determine the possible blood type(s) given the following reactions:
anti-A1: 0
A2 cells: 4+
Type B or O
pos reaction w A2 cells means pt has anti-A antibodies
determine the possible blood type(s) given the following reactions:
anti-A1: 0
A2 cells: 0
subgroup of A
cannot be A1, and patient does not have any anti-A antibodies