Fuel cells and Batteries
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
primary cell
non re chargeable - due to the products slowly migrating away from the electrodes or are consumed by side reactions occurring in cell
alkaline cells
they contain no fluids
simple and light
cheap
short lasting
lower self discharge
higher energy density
Properties of secondary cells
Rechargeable- done by reversing reaction through attaching cell to an electrical charge which has a potential difference a little greater than that of the cell. Positive electrode of the charger to positive electrode of cell and negative electrode of the charger to negative electrode.
electrical energy is converted to chemical in cell, in order for it to work products formed must stay in contact with electrodes
These are made up of wet cells (flooded and liquid cells) and molten salt (liquid cells with different composition)
complex and heavy
expensive
long lasting
can withstand higher electrical currents
energy transformation in secondary cells
when cell discharges it acts as a galvanic cell converting chemical energy into electrical
when cell is recharged it acts as an electrolytic cell converting electrical to chemical energy
properties of fuel cells
cells that are constructed with a continuous flow of reactants allowing for a constant production of chemical energy
typically use hydrogen and oxygen gases as fuel and produce water
transform chemical energy into electrical energy
continuous electricity
efficient
a fuel cell using hydrogen produces electricity, water, heat and small amounts of nitrogen dioxide
anode of a leclanche cell
zinc case: Zn → Zn2+ + 2e
cathode of leclanche cell
carbon rod surrounded by paste: 2MnO2 + 2NH4+ + 2e → Mn2O3 + 2NH3 +H2O
lead-acid cell anode and cathode
anode: Made of lead and surrounded by sulfuric acid Pb + SO42- → PbSO4 +2e
Cathode: made of solid lead oxide: PbO2 + 4H+ + SO42- +2e → PbSO4 + 2H2O + 2e
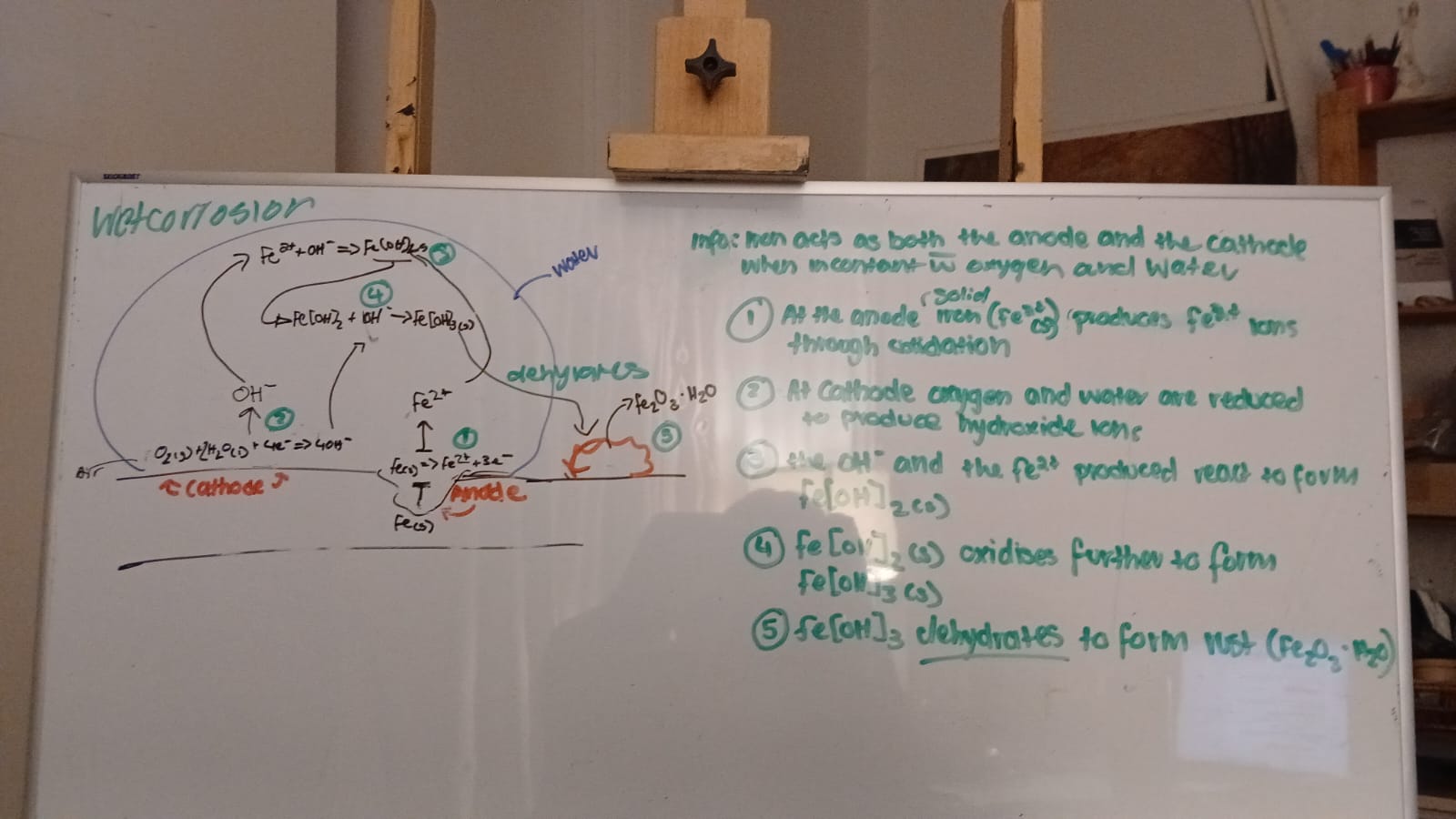
wet corrosion
at anodic site, electrons are released when iron atoms are oxidised to iron (II) ions
at cathodic site the electrons reduce oxygen gas and water to produce hydroxide ions
these combine to form insoluble iron (II) hyrdoxide
iron (II) hydroxide is readily oxidised by more oxygen in the air to form iron (III) hydroxide
Iron (III) hydroxide dehydrates to form rust, Fe2O3*H2O
types of methods to prevent corrosion
surface protection, galvanising, sacrificial anode and cathodic protection
Surface protection
prevents iron from coming into contact with oxygen and water
oil, grease, paint, plastic, other metals
advantages: cheap and easy, can be flexible, effective for as long as coat remains in tact
Disadvantages: coating must be reaplied when it deteriorates any scratch will leave metal susceptible
For less reactive metal coating: any scratch will mean the more reactive iron will act as the anode and will corrode faster
Galvanising
more reactive metal e.g. zinc is used as a coating
more reactive metal will corrode quickly and form a thin layer of zinc oxide which protects the iron
will protect the iron even if scratched since zinc is more reactive
Sacrificial anode
force iron to become the cathode by electrically connecting it to a more reactive metal which will act as the anode
Sacrificial anode will need to be replaced
Cathodic protection
Use of sacrificial anode may be improved by use of a DC electrical current
The (-) terminal of the DC power supply is electrically connected to iron making it the cathode of a galvanic cell
the (+) terminal is connected to the sacrificial anode, which could be made of iron in this scenario but typically a more reactive metal
Electrolysis
Forcing a non-spontaneous to occur reaction by using electricity
Rechargeable batteries act as a voltaic cell when discharging and a electrolytic cell when recharging
under what conditions are standard reduction potentials measured
solution concentration of 1.00 mol/L
temp of 298 K (25C)
pressure of 100 kPa
Why is the salt bridge needed?
it completes the circuit by allowing ions to move between the half cells
cell notation
anode on the left, cathode on the right
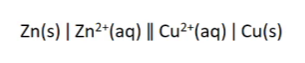
rules of standard reduction potentials
reduction half equation must be above oxidation half on the table
reduction is in the forward and oxidation in the reverse for reaction to occur
voltage (E^0) must be positive for it to be spontaneous
Hydrogen half cell
How are the standard equation found
by constructing a hydrogen half cell with that element
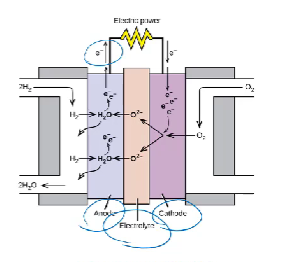
electrolysis of water
water can be split into hydrogen and oxygen gas
small amount of sulphuric acid is added to increase the number of ions in the solution, since pure water is a poor conductor
amount of Hydrogen gas produced is 2x the amount of Oxygen gas produced
H2 is produced at the cathode and O2 is produced at the anode
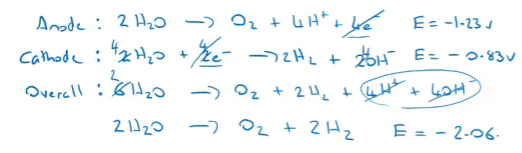
what makes electrolysis of Aqueous solutions more complicated than molten
water can be oxidised or reduced instead of the ions of the salt
electroplating
electrolysis can be used to coat a thin layer of a metal over another metal
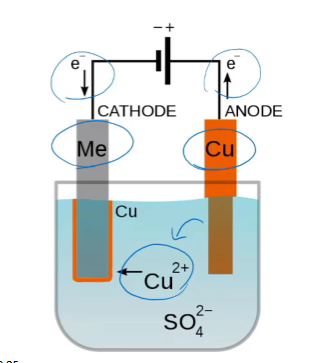
electrorefining of copper
electrolysis of aqueous solutions rules
the oxidation reaction is above the reduction
the oxidation reaction goes in reverse
reduction reaction goes forward
types of voltaic cells
primary and secondary cells
dry cell (zinc-carbon cell or leclanche cell)
primary cell
the zinc case acts as the anode
the cathode is a rod made from carbon surrounded by a paste of MnO2, ZnCl2, NH4Cl and powdered carbon
initial overall voltage is 1.5 volts
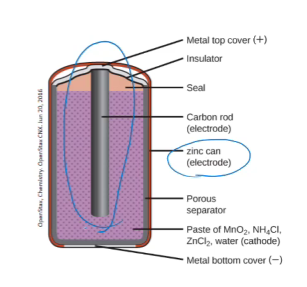
Disadvantage of the dry cell
since the zinc case takes part in the reaction, it will deteriorate and eventually leak
also ammonium ions are weakly acidic which causes further deterioration of the zinc
can’t be reversed because running the current through it in reverse will cause potentially dangerous side reactions which can produce gases (producing gases in a confined space will cause an explosion
alkaline battery
primary cell
Anode is a zinc rod: Zn + 2OH- → ZnO +H2O + 2e
Cathode is the steel casing: 2MnO2 + H2O + 2e → Mn2O3 + 2OH
similar voltage to dry cells but can sustain higher currents
small and inexpensive
cannot be recharged as products of the discharge reaction can move away from the electrodes and attempts to recharge can produce gases or cause the case to rupture
Lead - acid battery (car battery)
secondary cell (can be recharged)
made up of 6 cells, able to produce 2 volts each therefor the total volatge of the battery = 12 volts
Anode is made up of lead surrounded by sulphuric acid: Pb + SO4²→ PbSO4 + 2 e
Cathode is made of solid lead oxide: PbO2 + 4H+ + SO4² + 2 d → PbSO4 + 2 H2O
because both reactions produce solid PbSO4 which remains on the electrodes, the battery can be effectively recharged by applying a voltage of at least 12v
impractical due to their size and weight
contains large amounts of lead and sulphuric acid, both of which are quite toxic, so they must be handled with care and disposed of properly
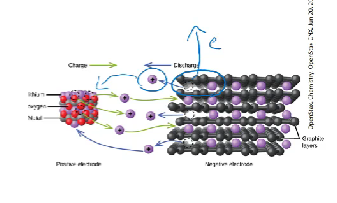
Lithium Ion Battery
secondary cell (can be recharged)
Anode is made from lithium ions attached to a thin layer of graphite. this is called intercalation
lithium ions (having a positive charge) are attached to the delocalised electrons in the graphite
combination of lithium ions with atoms of carbon makes them behave like a lithium metal (a strong reducing agent) with a high tendency to donate electrons and become cations
takes at least 6 carbon atoms per lithium ion for this to work perfectly
electrolyte is an organic solvent containing dissolved lithium ions
function: as battery discharges the lithium ions from the graphite anode move through the electrolyte to the cathode, releasing electrons through the external circuit and producing a voltage of about 3.7 v
Anode: LixC6 → X li+ + X e- + C6
Cathode: Li1-xCoO2 + XLi+ Xe- → LiCoO2
Recharge: exact opposite process occurs with electrons moving to graphite from the external circuit which attracts lithium ions back to graphite
Disadvantage:
expensive due to complexity and need for purity
solid oxide fuel cell
Anode: 2 H2 + 2 O2- → 2 H2O + 4 e
Cathode: O2 + 4e- → 2O²
disadvantage of fuel cells
poor reliability over long periods of time
expensive
producing hydrogen gas requires a lot of electricity
about 30% efficient
IPHE

Battery definition
two or more cells together is the traditional definition
dry corrosion
example: the patina that forms on copper, copper reacts with the air to form copper oxide
2Cu(s) + O2(g) → 2CuO(s)