Acids, Bases and Salts (Salt Preparation)
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
Solubility Rules
So, Po, Ammo, Ni are soluble
For Chloride → lead (II) and silver
For Sulphate → lead (II), calcium, barium
Carbonates, Oxides, Hydroxides → INSOLUBLE
Reactivity Series
K
Na
Ca
Mg
Al
Zn
Fe
Pb
H
Cu
Ag
Au
Please Stop Cooking My AppleZ! For Like Hundred Cost SGD !
Formation of Salt
Acid + Metal → Salt + Hydrogen Gas
Acid + Carbonate → Salt + Water + Carbon Dioxide Gas
Acid + Base → Salt + Water
Ammonium Salt + Base → Salt + Water + Ammonia Gas
2 soluble (aqueous) reagents → Insoluble salt
Experimental Procedure for Reaction of acid + excess metal/insoluble base and carbonate (8)
↣ This procedure is used when preparing a soluble salt with insoluble reactants.
↣ It is not recommended to use excess metal due to the extreme reactivity or lack thereof of some metals.
↣ Saturation can be tested by dipping a cold, dry glass rod into the filtrate. If the filtrate is saturated, crystals will form on the glass rod.
Using the measuring cylinder, measure out 10 cm3 of (acid) into a 100 ml beaker.
Add excess (metal) to the acid in the beaker. Stir constantly using a glass rod until no more (metal) dissolves in the acid.
Filter the mixture into a 100ml beaker using a filter funnel and filter paper to separate the excess (metal) from the aqueous solution of the (salt). The salt is collected as the filtrate and the (metal) is collected as the residue.
With a Bunsen burner and evaporating dish, heat the filtrate to evaporate the water and concentrate the salt solution to obtain a saturated solution of (salt).
On cooling, (salt) crystals will form.
Filter the mixture with a filter funnel and filter paper to collect the (salt) crystals as residue.
Wash the crystals with 1–2 drops of cold distilled water to remove impurities.
Dry the crystals by pressing them between layers of filter paper.
Titration Experimental procedure (13)
↣ This procedure is used when preparing a soluble salt with soluble reactants.
Fill a burette with (base). Record the initial burette reading as V1.
Using a pipette, transfer 25.0cm3 of (acid) into a 250cm3 conical flask.
Add 1 to 2 drops of methyl orange indicator to the (acid). The solution appears red.
Add (base) slowly from the burette, dropwise near the end point, until the end point colour change from red to orange is observed.
Record the final burette reading as V2.
Record the volume of (base) required for complete neutralisation as V2 - V1.
Using a pipette, transfer 25.0cm3 of (acid) into a 250cm3 beaker.
Add V2 - V1 cm3 of (base) from the burette to the (acid).
Heat the mixture with a Bunsen burner to evaporate off water to prepare a saturated solution.
Allow the saturated solution to cool down slowly. Crystals will start forming. Use an ice bath if necessary.
Filter off the crystals using a filter funnel and filter paper.
Wash the crystals with 1–2 drops of cold distilled water to remove impurities.
Dry the crystals by pressing them between layers of filter paper.
Why PbO(s) cannot be added directly to HI(aq) to prepare PbI2(s)?
Equation: PbO + 2HI → PbI2 + H2O
Reaction of PbO with HI forms insoluble PBI2 which coats the surface of PbO solid. This protective layer prevents PbO from further reaction with HI, leading to low yield of PbI2.
Experimental Procedure for Ionic Precipitation (with dissolving) (9)
↣ This procedure is used when preparing an insoluble salt.
↣ The reactants must be two suitable ionic compounds. It is compulsory to convert any insoluble starting material to a soluble salt by dissolving.
The starting material, (insoluble salt) is insoluble.
Add 2g of (insoluble salt) to 10cm3 of (suitable acid) in a 250ml beaker.
Stir with a glass rod until no more (insoluble salt) dissolves.
Filter the mixture with a filter funnel and filter paper to obtain (soluble salt) as the filtrate.
Using a 50 ml measuring cylinder, transfer 2cm3 of (soluble salt) into a clean 100 ml beaker.
Add (salt 2) solution in excess using a measuring cylinder and stir until no more precipitate forms.
Filter the mixture with a filter funnel and filter paper to obtain (salt) as the residue.
Wash the precipitate with 1–2 drops of cold distilled water to remove impurities.
Dry the precipitate by pressing them between layers of filter paper.
Salt preparation Mind Map
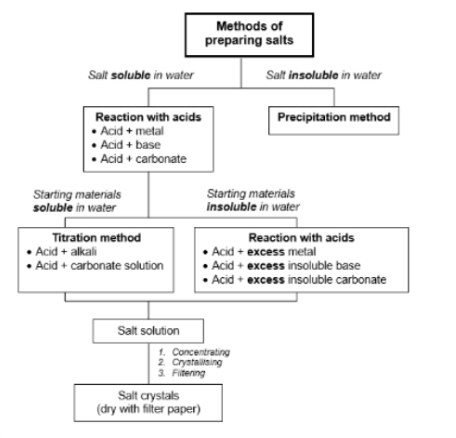
Which starting material should you NOT choose if you have a choice for reaction with acid and why?
If you have a choice of starting material, don’t choose the metal reaction (either lack of reactivity or violently react)
Solubility Rules for Salts
TYPE | SOLUBLE | INSOLUBLE |
Salts with Group 1 Cations Li+, Na+, K+, Rb+, Cs+, Fr+ So, Po | All | - |
Ammonium Salts NH4+ Ammo | All | - |
Nitrate Salts NO3- Ni | All | - |
Chloride Salts | Almost All | Lead (II)) Silver PbCl2 AgCl |
Sulphate Salts | Almost All | Calcium Lead Barium > everything under calcium |
Carbonate Salts | Group 1 | Almost All |
Oxides/Hydroxides | Group 1 | Almost All |
Why do water of crystallisation happen?
Regular ionic lattice → form regularly shaped crystals
Many combine with water molecules to form crystals
Water molecules are chemically bonded to the ions within crystals
Water trapped inside due to extra space → space between anion and cation, water occupies it
Water of Crystallisation
water bonded chemically within the crystal
Anhydrous Salts
Salts that do not contain water of crystallisation
Hydrated Salts
Salts that contain water of crystallisation
Hydrated sodium carbonate = Na2CO3 . 10H2O
Salts
ionic compounds – NaCl, CaSO4
Contain a metal cation or ammonium ion and a nonmetal anion
Usu. formed by replacement of hydrogen ions of an acid by a metallic ion or an ammonium ion
Come from Acid-Base reaction
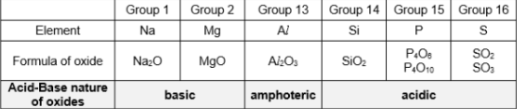
What are some neutral oxides?
Carbon monoxide (CO)
Water
Nitric oxide (NO)
Nitrous oxide (N2O)
Neutral Oxides
Do not react with acids or bases
Amphoteric oxide reacting with acid: Al2O3 + 6HCl →
Al2O3 + 6HCl → 2AlCl3 + 3H2O
What does Al2O3 not react with?
water
Which oxides are amphoteric?
Aluminium, Lead (II), Zinc
> Al2O3’
> PbO (only 2+, 4+ cannot)
> ZnO
IMPORTANT!! Aluminium Oxide Reaction with base to form sodium aluminate (amphoteric)
Al2O3 + 2NaOH + 3H2O → 2NaAl(OH)4 [sodium aluminate]
Amphoteric Oxide
Reacts with both acids and bases
Silicon Oxide reaction (acidic) with NaOH
SiO2 is not soluble
SiO2 reacts with concentrated hot NaOH or molten NaOH at high temperature to form sodium silicate
SiO2 (s) + 2NaOH (l) → Na2SiO3 (l) + H2O (g)
molten sodium silicate
acidic reacting with base
acidic oxide + base → salt + water
P4O10 (s) + 12NaOH → 4Na3PO4(aq) + 6H2O (l)
sodium phosphateSO2 (g) + 2NaOH (aq) → Na2SO3 (aq) + H2O (l)
sodium sulfiteSO3 (g) + 2NaOH (aq) → Na2SO4 (aq) + H2O (l)
sodium sulfate
axidic oxide reacting with water
When acidic nature is mixed with water, it turns into an acid without any by-products.
These reactions are non-redox. Oxidation states do not change.
Forms pH < 7
SO3 + H2O → H2SO4
sulfuric acidSO2 + H2O → H2SO3
sulphurous acid
Which oxides are acidic in nature?
Group 14, Group 15, Group 16
basic oxide reacting with acid
acid + metal base → salt + water
Na2O + 2HCl → 2NaCl + H2O (sodium chloride)
K2O → Potassium chloride
MgO → Magnesium chloride
CaO → Calcium chloride
basic oxide reacting with water
Na2O + H2O → 2NaOH (sodium hydroxide)
K2O + H2O → 2KOH (potassium hydroxide)
Na2O and K2O are soluble in water → give alkaline solution with pH > 7
Basic oxides are usually ___ in water
insoluble
Which oxides are basic in nature?
Group 1, Group 2
Metal oxides are ____
either basic or amphoteric
Non-metal oxides are ____
acidic
How does nature of oxide change across Period?
Nature of oxides change from basic (metal) to amphoteric to acidic (non-metal) across Period
basic (metal) → amphoteric → acidic (non-metal)
What is the haber process?
Industrial method to manufacturer ammonia
Reversible reaction
N2(g) + 3H2(g) → ← 2NH3(g)
High pressure of 250 atm
Moderate temperature of 450 degrees Celsius
Iron catalyst
Molar ratio of N2 : H2 = 1 : 3
What is synthesised in the haber process?
Ammonia
Neutralisation Reaction
Base + acid → salt + water
KOH(aq) + HCl(aq) → KCl(aq) + H2O(l)
Why can [acid] conduct electricity in water but not in organic solvent?: Answering Format (6)
In water, [acid] ionises to form free mobile ions, H+ and [B]; [formula].
Since H+ > [B] and pH < 7, the blue litmus paper turns red. The free mobile ions, H+ and [B] are able to act as mobile charge carriers to conduct electricity.
In organic solvent, [acid] exists as a simple molecular compound.
It does not ionise to form H+ and [B] but remains as a molecule, [acid].
Hence, there is no change in the colour of litmus.
There is no electrical conductivity as there are no free mobile ions to act as mobile charge carriers.
Properties of Alkalis (5)
Bitter taste and feel soapy
Turn damp red litmus paper blue
Turn Universal Indicator blue or violet
pH greater than 7
Conduct electricity; can act as charge carriers as they exist as free mobile ions in aqueous solutions
NaOH → Na+ + OH-
Properties of Acids (6)
Sour taste
pH less than 7
Turns damp blue litmus paper red
Turn Universal Indicator orange or red
Must be dissolved in water before they can act as acids
Conduct electricity; can act as charge carriers as they exist as free mobile ions in aqueous solutions
H2SO4 → 2H+ + SO42-
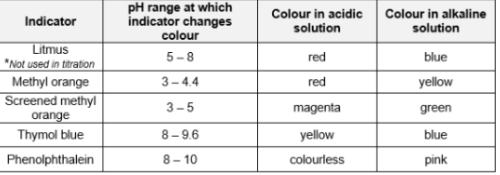
Give me some indicators and their color changes.
— is a measure of the concentration of H+ in a solution. A strong acid of the same concentration will — to give a higher H+. A weak acid of the same concentration will — only to give a lower H+. Hence, the pH of the strong acid will be — than the weak acid of the same concentration.
pH is a measure of the concentration of H+ in a solution. A strong acid of the same concentration will dissociate fully to give a higher H+. A weak acid of the same concentration will dissociate partially only to give a lower H+. Hence, the pH of the strong acid will be lower than the weak acid of the same concentration.
Indicators
Dyes/Mixture of dyes which change colour when added to acids or alkalis
Universal indicator
mixture of dyes that can be used to estimate the pH of a solution by tallying the colour of the solution with the Universal colour chart
What must be kept constant when using pH values to compare strength of 2 acids?
concentration
temperature
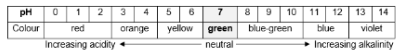
Alkaline on pH scale
Alkaline
pH > 7
H+ < OH-
Acidic on pH scale
Acidic
pH < 7
H+ > OH-
Neutral on pH scale:
Neutral
pH = 7
H+ = OH-
pH scale
pH scale – used to indicate whether a solution is acidic, neutral or alkaline; 25 degrees Celsius
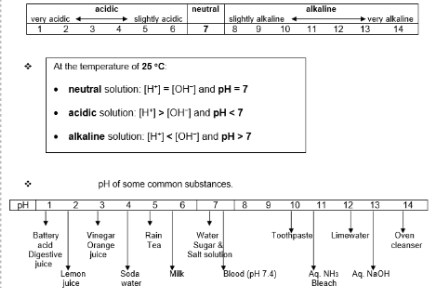
Examples of finding pH value of acids
The pH value of 0.001 mol dm-3 hydrochloric acid is ________
> Is it strong?
> If yes, all of it becomes H+
> Input into equation
> Thus, the answer will be 3The pH value of 0.3 mol dm-3 H2SO4 is ________
> Is it strong?
> If yes, all of it becomes H+
> Since there are 2 H, multiply with 2 to become 0.6
> Input into equation
> Thus, the answer will be 0.222
How to find pH value of an acid?
Determine whether if it is strong or not
If it is strong, all parts of the acid will get turned into H+
If there are more than 1 H+ in equation, multiply with suitable number
Input into the equation: pH = –log10[H+]
Find answer
Larger pH → ____ [H+]
larger pH → smaller [H+]
What does H+ mean?
[H+] means concentration in mol dm-3
[H+] = 10–pH
pH = –log10[H+]
pH of solution
pH of solution – negative logarithm to base 10 of the concentration of hydrogen ions in solution in mol dm-3
pH = –log10[H+]
[H+] = 10–pH
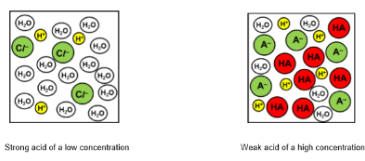
Strong acid + Low concentration → everything dissociated
Strong acid + High concentration → everything dissociated
Weak acid + Low concentration → left
Weak acid + High concentration → left
Strength is ________ on concentration!
independent
Strength of an acid measures:
Strength of an acid – measure of the extent of dissociation of an acid in solution
A weak base:
Weak Base | |
Definition | Dissociates partially in solution to give hydroxide ions, OH- |
Degree of dissociation | < 100% |
Type of arrow in dissociation equation | Double arrow |
Example | NH3 + H2O → ← NH4+ + OH- |
A weak acid:
Weak Acid | |
Definition | Dissociates partially in solution to give protons, H+ |
Degree of dissociation | < 100% |
Type of arrow in dissociation equation | Double arrow |
E.g. of organic acid | HCOOH→ ← H+ + HCOO- |
E.g of monobasic acid | HCN → ← H+ + CN- |
A strong base:
Strong Base | |
Definition | Dissociates fully in solution to give hydroxide ions, OH- |
Degree of dissociation | Approximately 100% |
Type of arrow in dissociation equation | → single arrow |
E.g. of monoacidic base | KOH → K+ + OH- |
E.g of diacidic base | Ca(OH)2 → Ca2+ +2OH- |
A strong acid:
Strong Acid | |
Definition | Dissociates fully in solution to give protons, H+ |
Degree of dissociation | Approximately 100% |
Type of arrow in dissociation equation | → single arrow |
E.g. of monobasic acid | HNO3 → H+ + NO3- |
E.g of dibasic acid | H2SO4 → 2H+ + SO42- |
How are the strength of Acids and Bases classified?
Classified based on their extent of dissociation to give ions in solution
Must be 100% to be considered strong
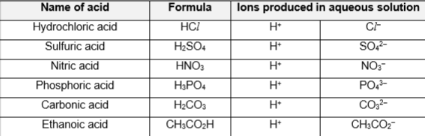
Common acids (6)
Common alkalis (4)
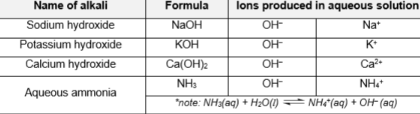
Alkali
bases that are soluble in water and produces OH-
[NaOH, KOH, Ca(OH)2]
Base
a species that can accept a proton, H+ and donates OH-
metal oxides, metal hydroxides [Na2O, ZnO, Al(OH)3]
Acid
a species that can donate a proton, H+
Bronsted-Lowry reaction
involves the transfer of a proton from the acid (proton donor) to the base (proton acceptor) in water