Structure of Prokaryotic and Eukaryotic cells
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
plasma membrane
made up van a bilayer of phospholipids and proteins
separates the cell van its surroundings and allows selective transport in and out of the cell.
Maximum magnification reached by light microscope
100 x.
Euchromatin
chromatin fibers met transcriptionally active genes, met wider spaces between the nucleosomes (DNA + histone protein = repeating units of chromatin structure);
Euchromatin constitutes the chromatin structure associated met the active transcription of genes.
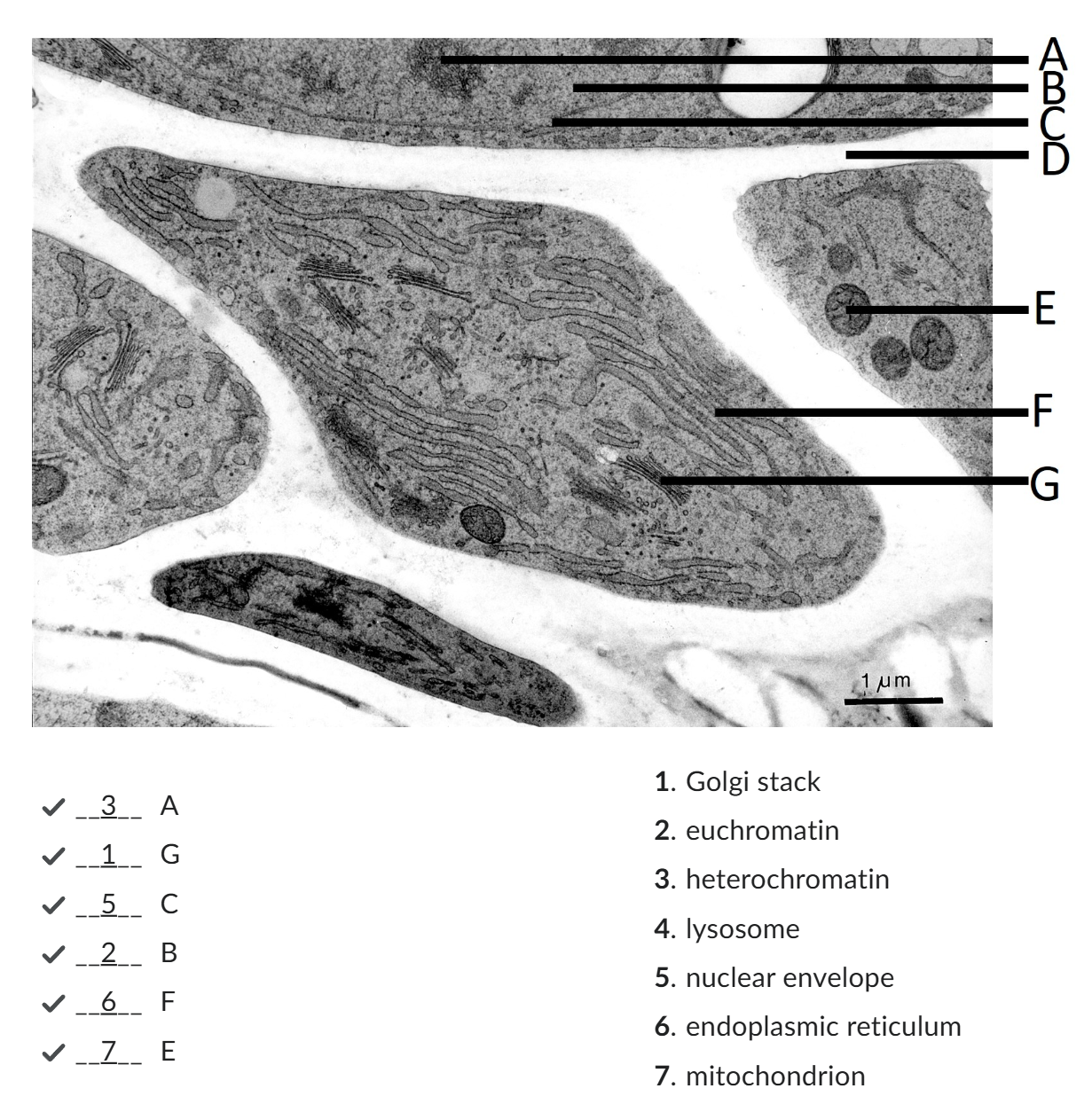
Heterochromatin
Heterochromatin makes up less accessible chromatin fibers that are associated met silencing
darker
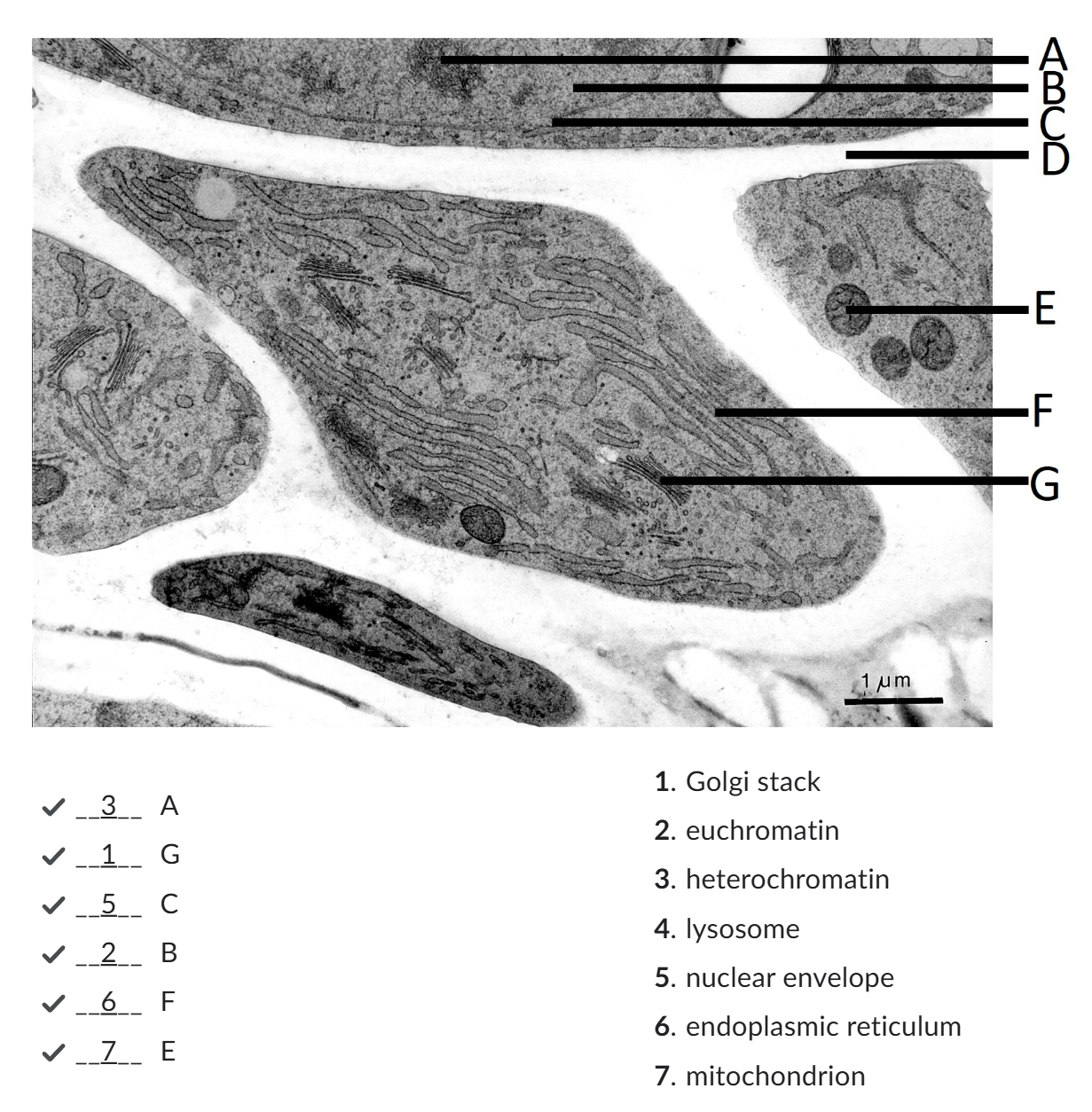
cell wall
surrounds the plasma membrane.
The cell wall components found in prokaryotes differ van those found in eukaryotes.
Cytoplasm
located on the inside of the plasma membrane and consists of liquid cytosol containing cellular components, such as ribosomes.
Prokaryotes lack membrane-bound organelles.
DNA
holds the genetic information.
located in the nucleoid which is not surrounded by the membrane (mostly circular)
Ribosomes
dispersed in the cytoplasm.
site of protien synthesis
nucleus
DNA is located. The nucleus is surrounded by a double membrane that has many openings: nuclear pores.
Peroxisomes
degradation of lipids and neutralisation of toxins, such as alcohol. During degradation of molecules, such as alcohol, hydrogen peroxide is produced
Hydrogen peroxide itself is toxic, but peroxisomes do contain enzymes that degrade hydrogen peroxide.
Lysosomes and its replacement in plants
contain hydrolytic enzymes to break down cellular components.
In plant cells the breakdown of macromolecules takes place in the central vacuole.
Endosomes
Contain molecules that arrive in endocytic vesicles.
Early endosomes develop into late endosomes and they act as a sorting station for molecules that have been taken up van the extracellular space. Molecules that need to be broken down are transported van late endosomes to lysosomes.
endoplasmic reticulum
plays a major role in protein synthesis, protein modification and lipid synthesis.
Golgi apparatus,
roteins are packed into membrane bound vesicles before they are sent to their final destination. The Golgi apparatus consists of the cis Golgi network, met cisternae, and the trans Golgi network.
Formula for resolving power

Why can electron microscopes reach magnifications of upto 1000000x ?
electron beam is very short in comparison to visible light
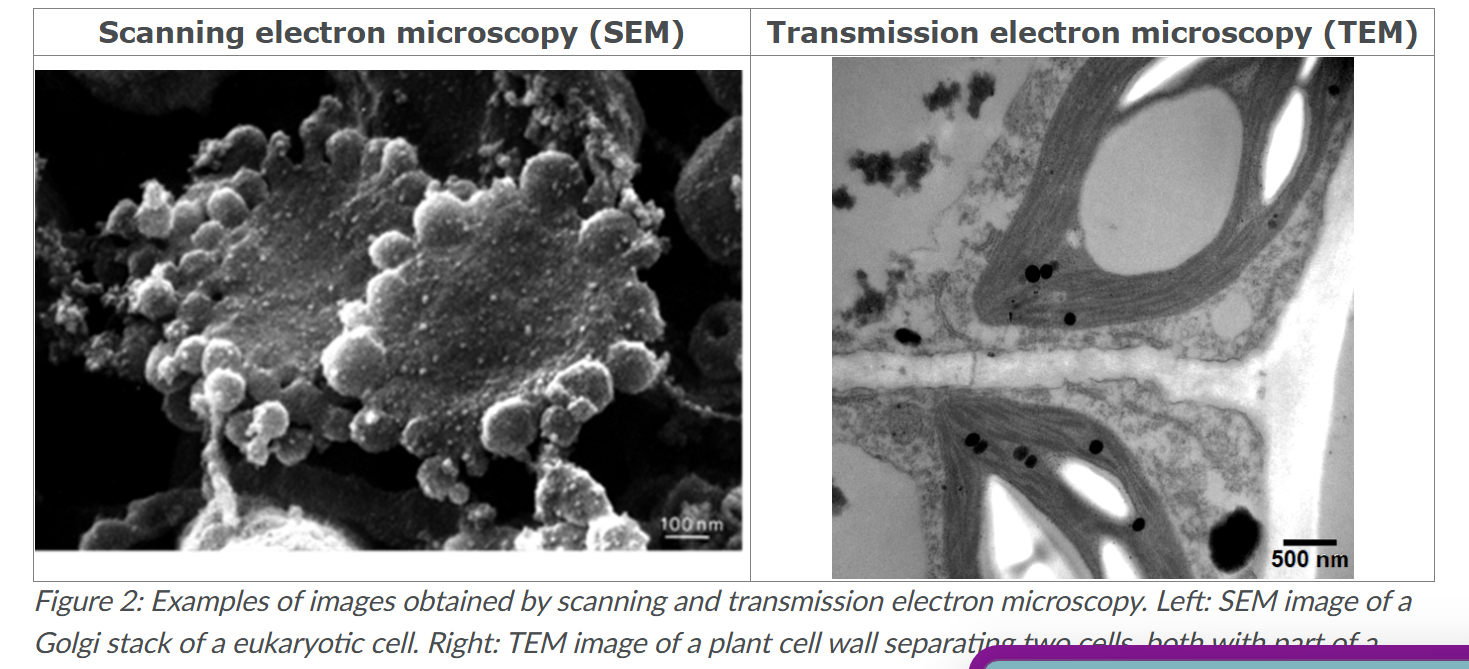
What’s SEM
Scanning electron microscope.
microscope scans the surface of the sample using a focussed electron beam.
A detector detects secondary electrons that are generated at the surface.
To image cells, they need to be deeply frozen and/or dehydrated.
When such frozen samples are fractured, organelles become visible and proteins in their membranes can for example be discerned
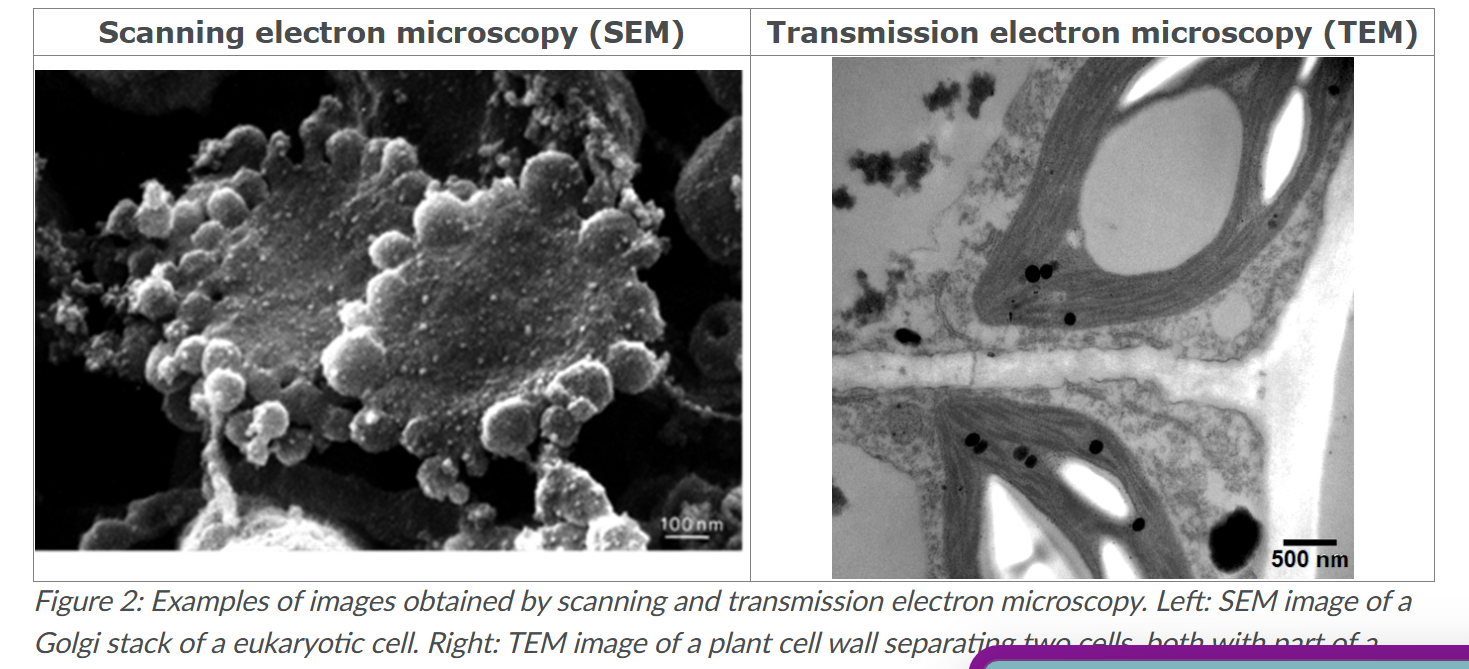
Whats TEM
Transmission electron microscopy
uses very thin slices of a specimen, onto which an electron beam falls. Whether electrons are transmitted onward to the observer by the sample generates the final image.
When preparing samples for TEM, often contrasting agents are added. This is necessary because there is hardly any appreciable difference in scattering or absorption of electrons present in biological specimens; Without this, only images met very low contrast between structures would be obtained. Such contrasting agents are often based on heavy metals like lead and uranium, because they strongly interact met the electrons.
What are macromolecules and the four main groups of them?
large molecules build of a chain of small repeating subunits.
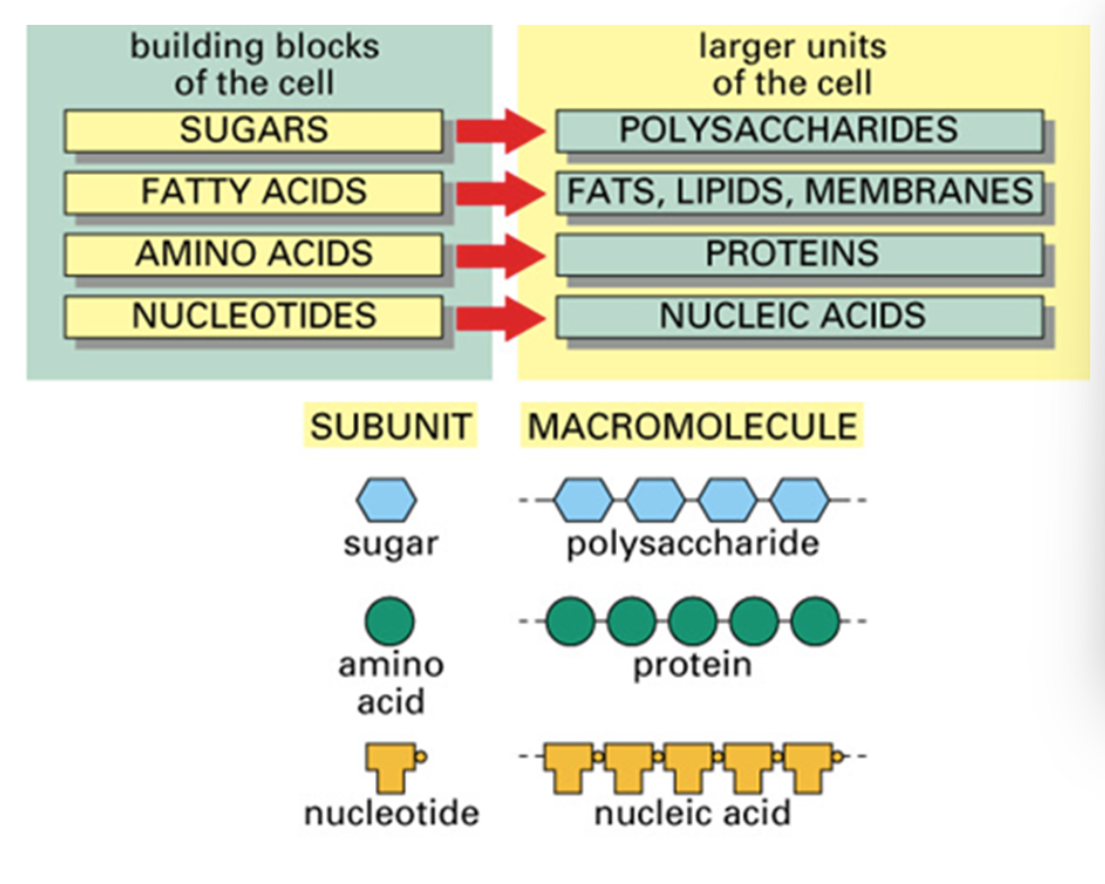
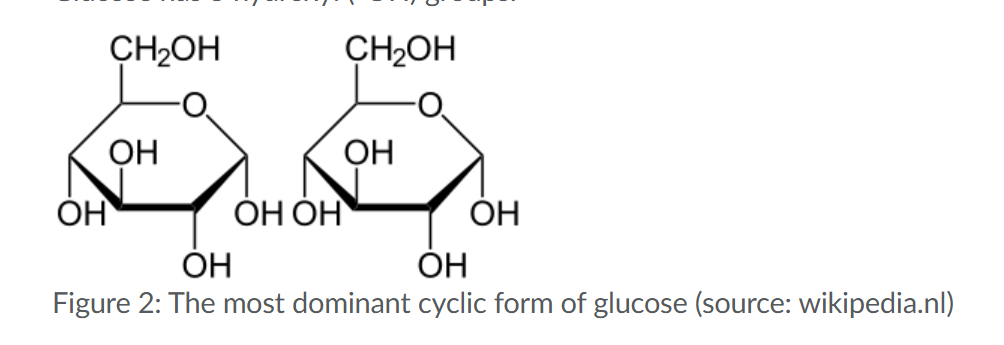
Sugar (dominant form and the monosasachires/polysaccharides_
General formula of monosaccharide:(CH2O)n
C5 and C6 sugars can exist in linear and cyclic forms, of which the cyclic form is dominant.
Hydroxyl groups react during condensation releasing water and forming disaccharide/polysaccharide.
opposite in hydrolysis which requires water.
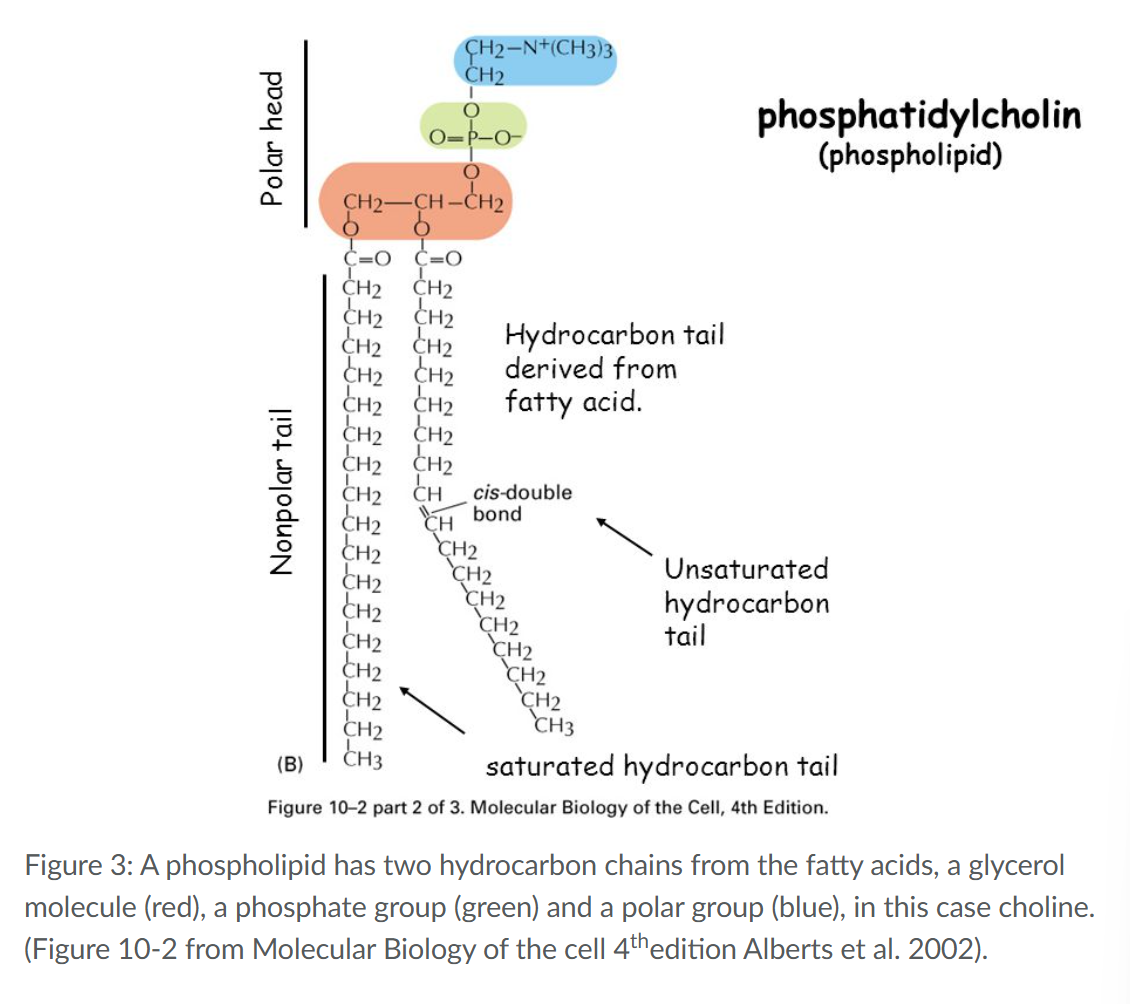
Saturated vs Unsaturated along met the composition of a fatty acid
Storage of fatty acids in cells occurs mainly in the form of Triacylglycerol (one glycerol coupled to 3 fatty acids
hydrophilic (polar head) and a hydrophobic (tail) part.
Pyramidine
ring structures of cytosine, thymine and uracil have 4 carbon and 2 nitrogen atoms
Purine
Adenine and guanine have a 6-ring pyrimidine bound to an extra 5-ring,
How ae amino acids encoded in DNA?
in the form three successive bases called a triplet.
How to distinguish between the 5 bv 3 prime end?
One side of a single strand DNA molecule contains a free phosphate group (-PO32-) attached to carbon-5, and is referred to as the ‘five prime end’, whereas the other side contains a free hydroxyl group (-OH) attached to carbon-3, known as the ‘three prime end’.
What’s a peptide bond? mention its structure and how its formed
bond between the amino and carboxyl groups formed during a condensation reaction
hydroxyl group (-OH) of the carboxyl (-COOH) will bind the H+of the amino group (NH2) forming H2O (blue water molecule in Fig 7).
made by ribosomes, in which translation of mRNA into proteins occurs.
what determines the order of amino acids in the proteins?
the order of triplets in the mRNA
what is the end of a polypeptide met the amino group called?
amino terminus (N-terminus)
what is the end of a polypeptide met the Carboxylic group called?
Carboxylic terminus C terminus
What’s part of the polypeptide backbone vs the side chain?
Backbone: repeating core of nitrogen and carbon atoms -NH-CH-C=O-
Side chains: R groups
What is the final structure of the protein determined by?
the noncovalent bonds between atoms of the polypeptide backbone as well as atoms of the side chains of amino acids.
hydrogen bonds, electrostatic interaction, van der Waals interaction and the hydrophobic force.
Minimum resolvable power of an electron microscope
200nm
Histological stains (dyes)
Improves contrast however is often incompatible with live cells
What causes low image contrast?
Limited absorption and light scattering by cells other than dense objects like cell walls and chloroplasts
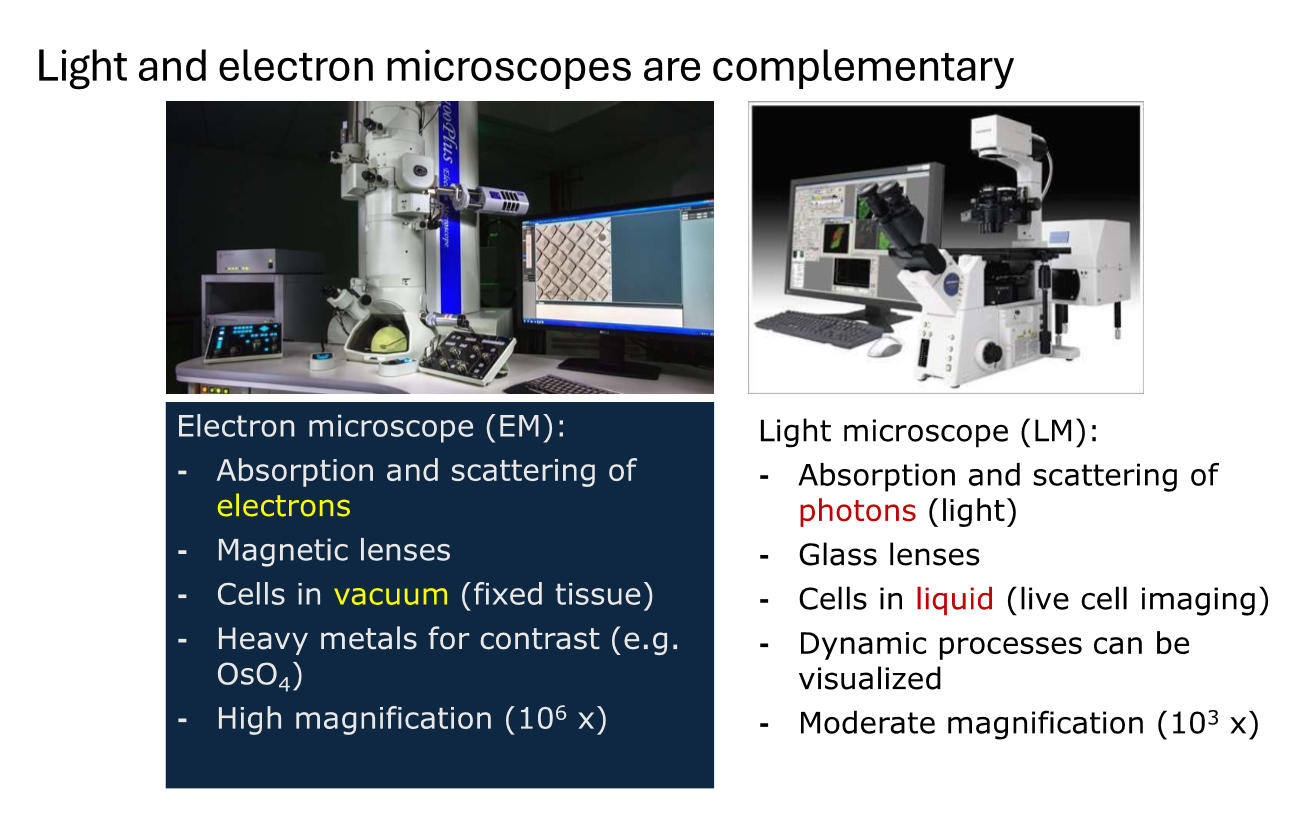
Compare light vs electron microscpes
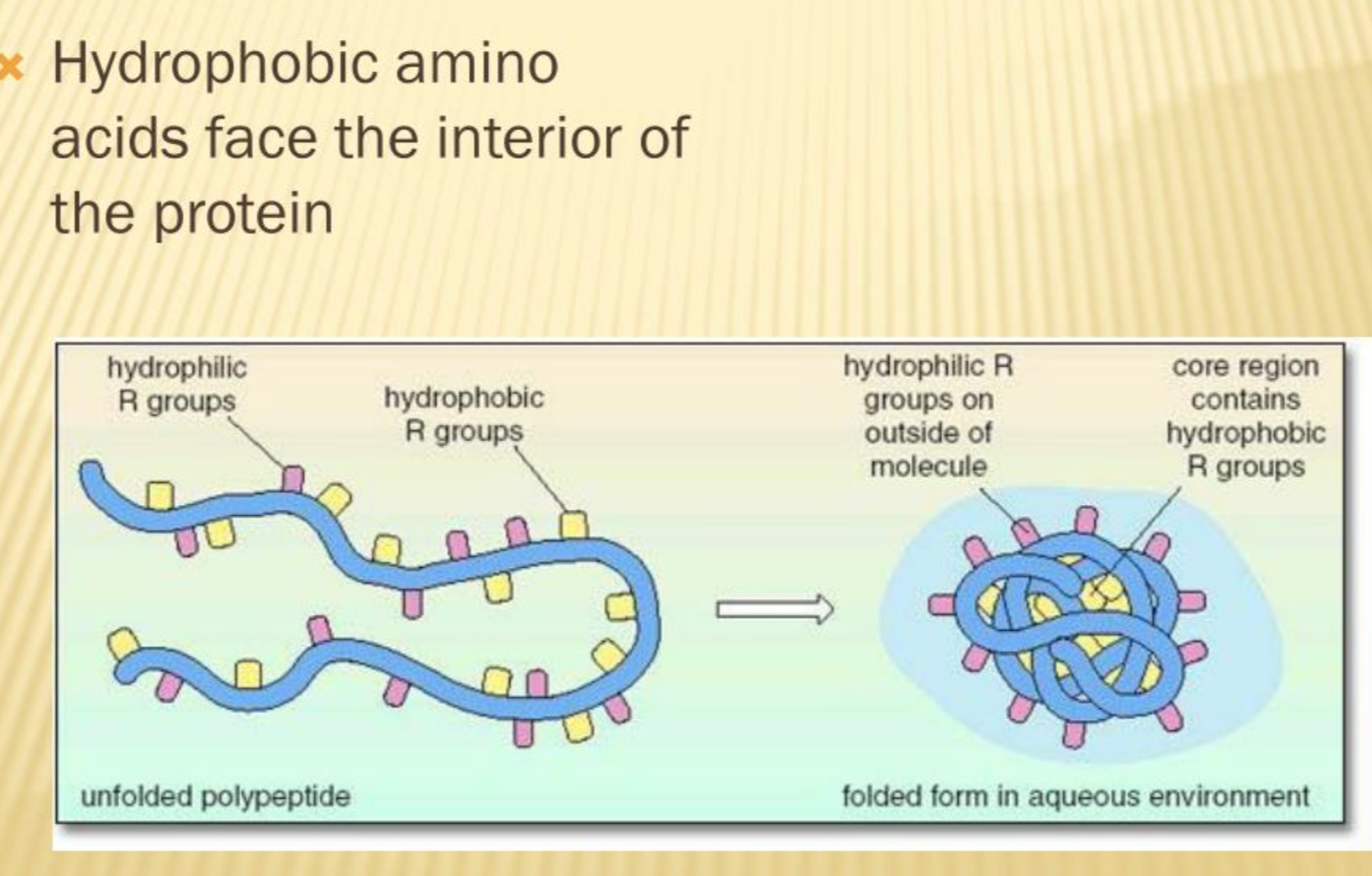
What is the hydrophobic effect in protein folding?
they cluster together to avoid interaction with water molecules so the nonpolar hydrophobic side chains of amino acids cluster together at the interior of a folded protein.
This causes the polar hydrophilic and charged side chains sticking out of the protein towards the aqueous environment.
What are the available forms in the secondary structure?
α-helices, β-sheets and β-turns.
When does an α (alpha)-helices form?
formed during the folding of for example the protein RNase.
The side chains of the amino acids are all directed to the exterior of the helix.
The interior of the helix is completely filled met atoms of the polypeptide backbone.
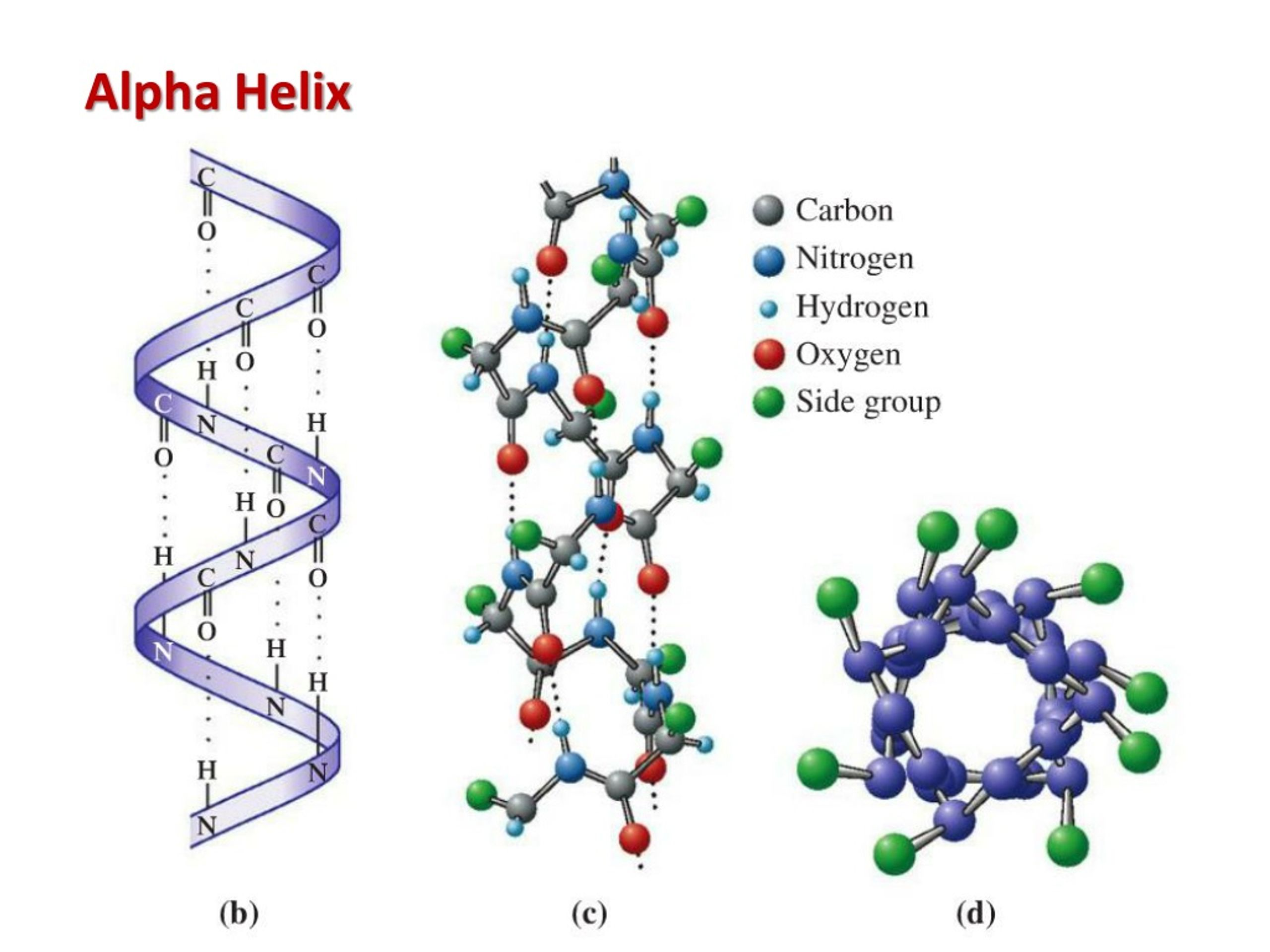
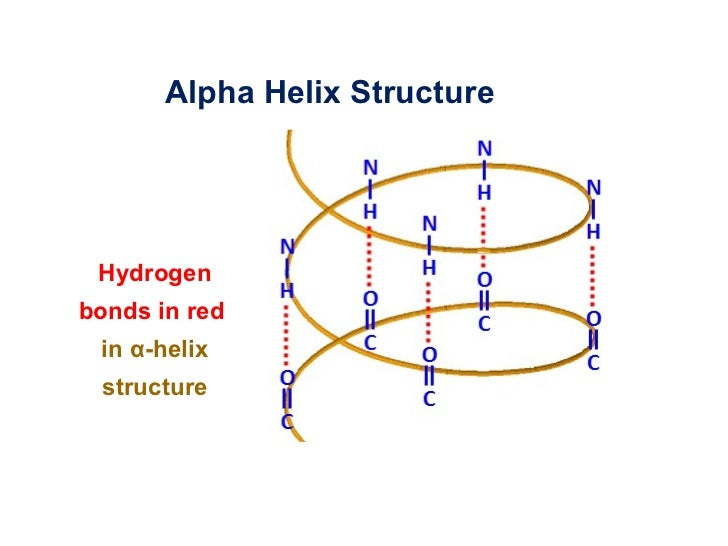
Composition of an α (alpha)-helices
hydrogen bonds are formed between the -C=O group of one amino acid and the –N-H group of an amino acid 4 amino acids further up along the polypeptide chain.
These hydrogen bonds stabilize and are a driving force for the formation of α-helices
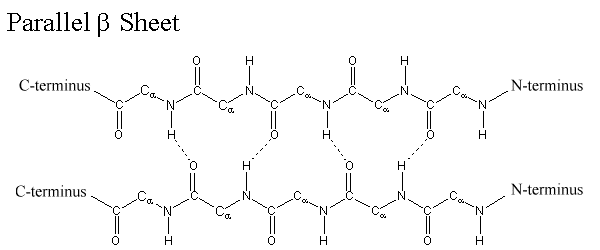
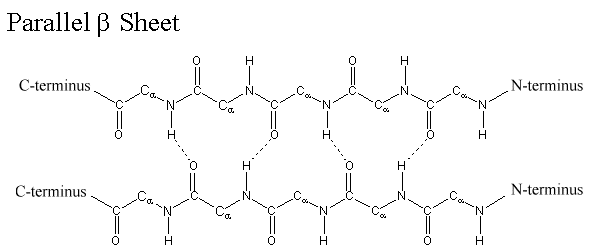
Composition of β (beta)-sheets
The side chains of amino acids are projected alternatively above and below the plane of the β-sheet. Hydrogen bonds are formed between -C=O groups and –N-H groups of the polypeptide backbone
Composition of β (beta)-turns
connects two β-sheets and ensures that proteins maintain a compact structure
The hydrogen bond is between the –C=O group of one amino acid and the –N-H group of an amino acid 3 residues further up along the polypeptide chain.
Tertiary structure
he three-dimensional structure of a protein
Quaternary structure
when multiple polypeptide chains interact and form a protein complex.
What type of bonds stabilize the tertiary structure?
Hydrogen bonds and hydrophobic effect
Disulfide bonds composition
Joins part of a longer polypeptide chain via a covalent bond between two Sulphur atoms, in which the atoms share an electron.
The oxidation of two –S-H (sulfhydryl) groups of cysteine residues results in the formation of a S-S bond: 2 (-S-H) + O2→ -S-S- + H2O2.
Stabilization of both tertiary and quaternary structures of proteins.
Intracellular proteins = no disulfide bond
extracellular proteins = multiple disulfide bonds.
Effect of a disulfide bond
The energy required for folding most proteins is 15-20% of the energy of a covalent bond, so adding covalent disulfide bonds greatly increases protein stability
retains structure at high temperatures
Water soluble protiens
They have nonpolar side chains in the interior of a protein. Nonpolar groups are also present at the exterior but in-between the polar and charged groups
Immunoglobins
antibodies.
consists of four polypeptide chains that are kept together by disulfide bonds.
Enzyme
proteins met catalytic function
increases the reaction rate by lowering the activation energy required for the reaction
-can only catalyse reactions that can also occur without the presence of the enzyme.
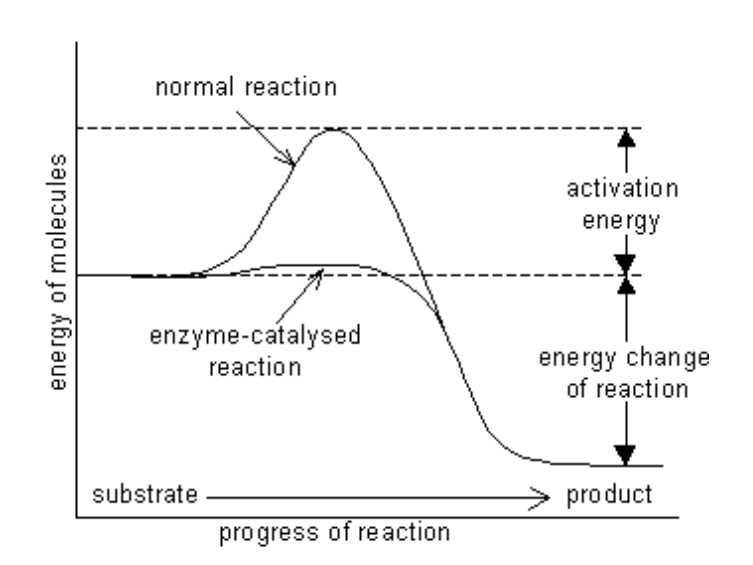
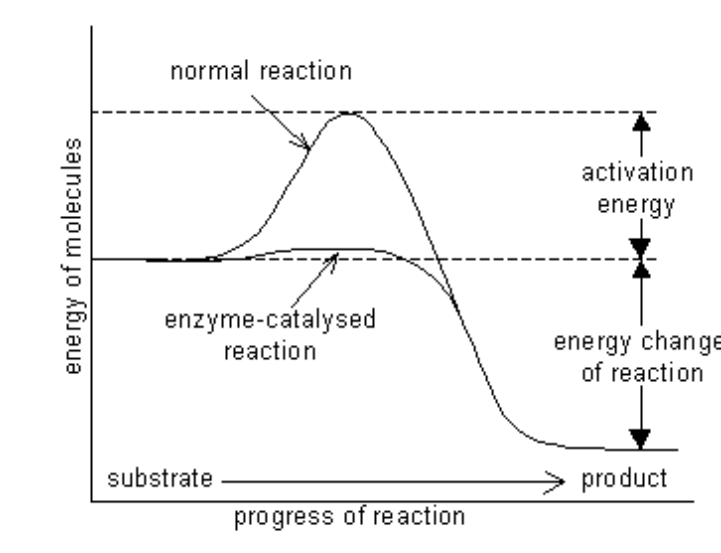
How to determine the direction of reaction?
difference in energy between substrate (S) and the product (P)
In this example the reaction occurs van substrate to product because the free energy of the product is lower than the substrate. Energy is released via this reaction.
What is induced fit?
The binding energy is often used to cause a conformation
This conformation change repositions the side chains of amino acids of the enzyme in such a way to enable the reaction the enzyme catalyses
change of the active site.
How does pH influence an enzyme?
influences the ionization state of the side chains of the amino acids (depending on if it is charged or not)
A pH change can result in a side chain to become charged and thereby repelling side chains met a similar charge causing a conformation change.
ionization state also affects interaction met other molecules/proteins.
What happens to an enzyme if the temperature is too high?
hydrophobic groups of an enzyme become exposed to the aqueous environment and the enzyme molecules aggregate (clump together). Aggregation keeps the enzyme in an unfolded conformation causing denaturation.
What’s X-ray crystallography? and what is its purpose?
The positions of individual atoms in a protein are determined via x-ray scattering.
(only possible if the proteins are in a well ordered three-dimensional array/protein crystal)
provides information about one specific conformation of a polypeptide chain.
Not memorizing needed but more for breadth:
Proteins can undergo conformational changes, for example when a protein binds a ligand. These conformational changes can be visualized by making multiple crystal structures, both of the protein met and without its ligand. Conformational changes are especially interesting because visualizing these dynamics helps to understand how a protein functions.
what is the covalent distance between atoms?
0.1 to 0.22nm (1nm = 10-9).
What is a plastid?
a membrane bound organelle found exclusively in algal and plant cells where it is the site of manufacture and storage of important chemical compounds.
Amyloplast
the location of the storage and synthesis of starch
chromoplast
the location of the storage and synthesis of pigments
Chloroplast
surrounded by two membranes: the outer chloroplast membrane and the inner chloroplast membrane, which surrounds the chloroplast matrix (the stroma).
contain a third membrane: the thylakoid membrane, which is a highly folded membrane system containing the green chlorophyll pigment
Chloroplasts may transform into chloro-amyloplasts: plastids that contain both starch and chlorophyll.
Endosymbiotic theory
as mitochondria and chloroplast have their own:
ciruclar dna
70s ribosome
binary fission
same size
Because they resemble bacteria in many ways, both organelles are thought to have once been free-living bacteria, which were engulfed by eukaryotic cells. Both chloroplasts and mitochondria are surrounded by two membranes, which are both thought to be of bacterial origin.
The third membrane, derived van the plasma membrane during the engulfment, is thought to have disappeared.
Nucleoplasm
a highly viscous liquid, which contains the genetic
material, organized as multiple long DNA molecules that are wound around a
variety of proteins.