Changes of state and internal energy: Matter: Physics: GCSE (9:1)
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
State of matter
A physical property that describes matter as a solid, liquid, or gas
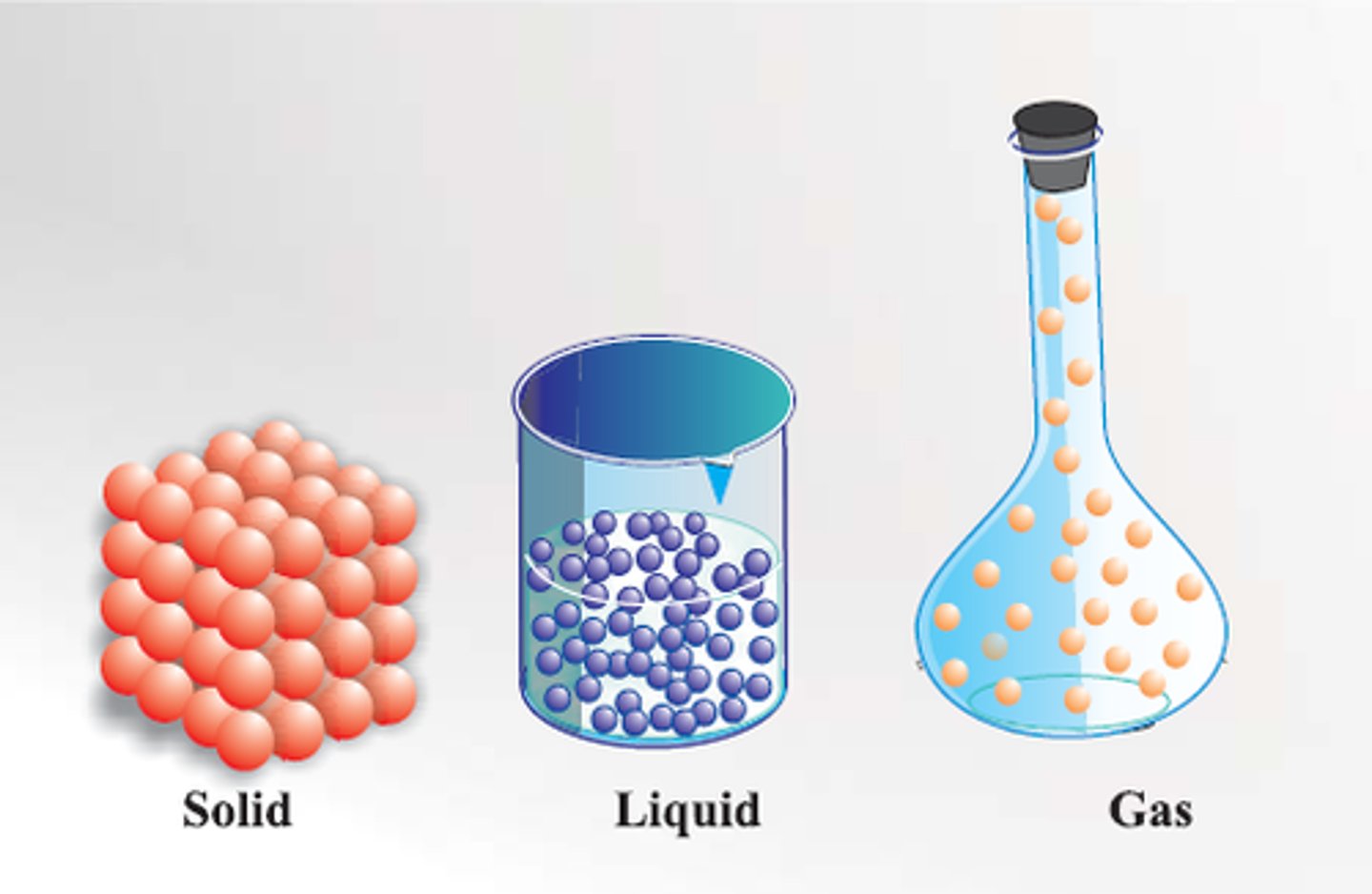
Change of state
A physical change of a substance from one state of matter to another
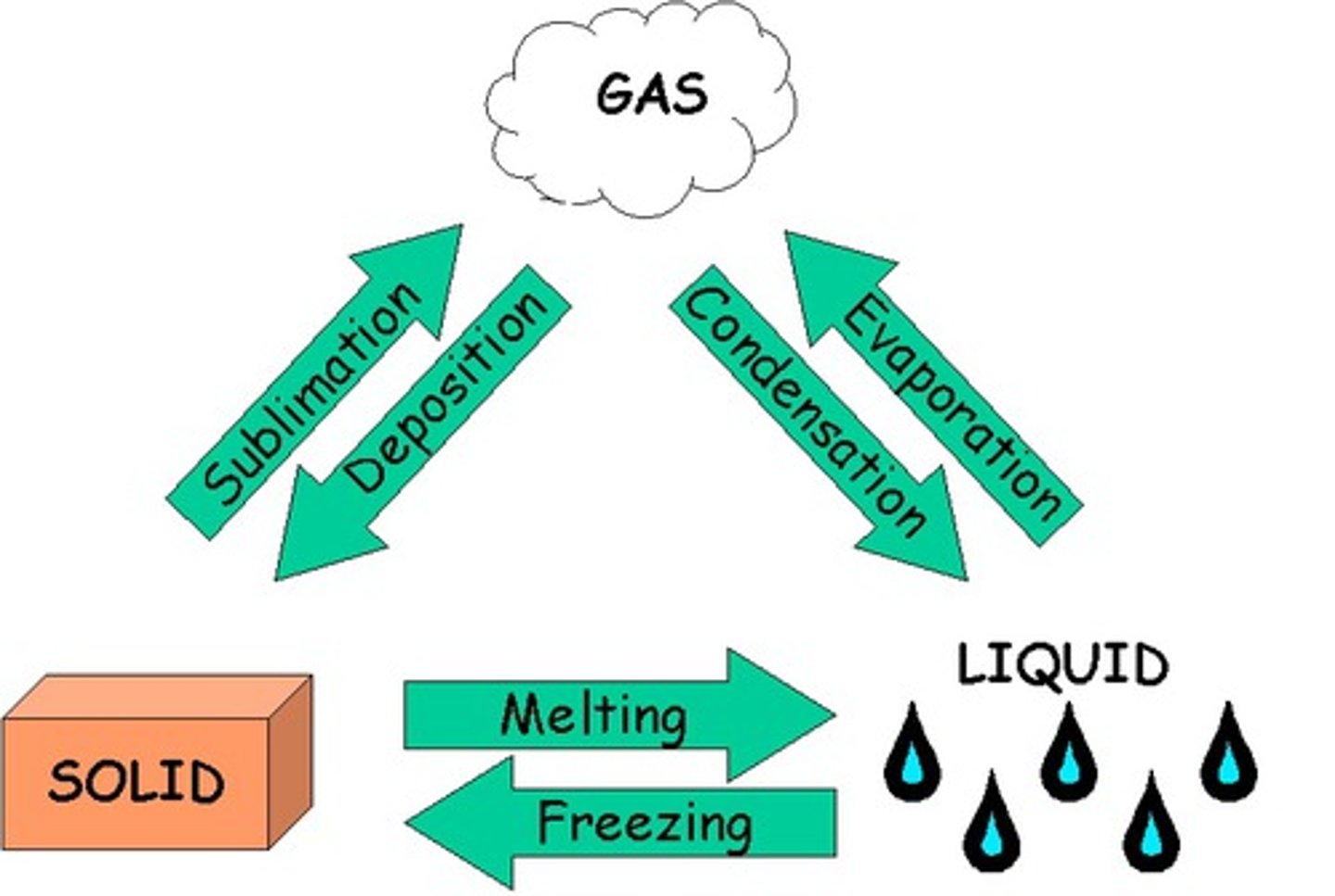
Melting
The change of state from a solid to a liquid

Boiling
Change of state from a liquid to a gas (occurs at a fixed temperature known as the boiling point)

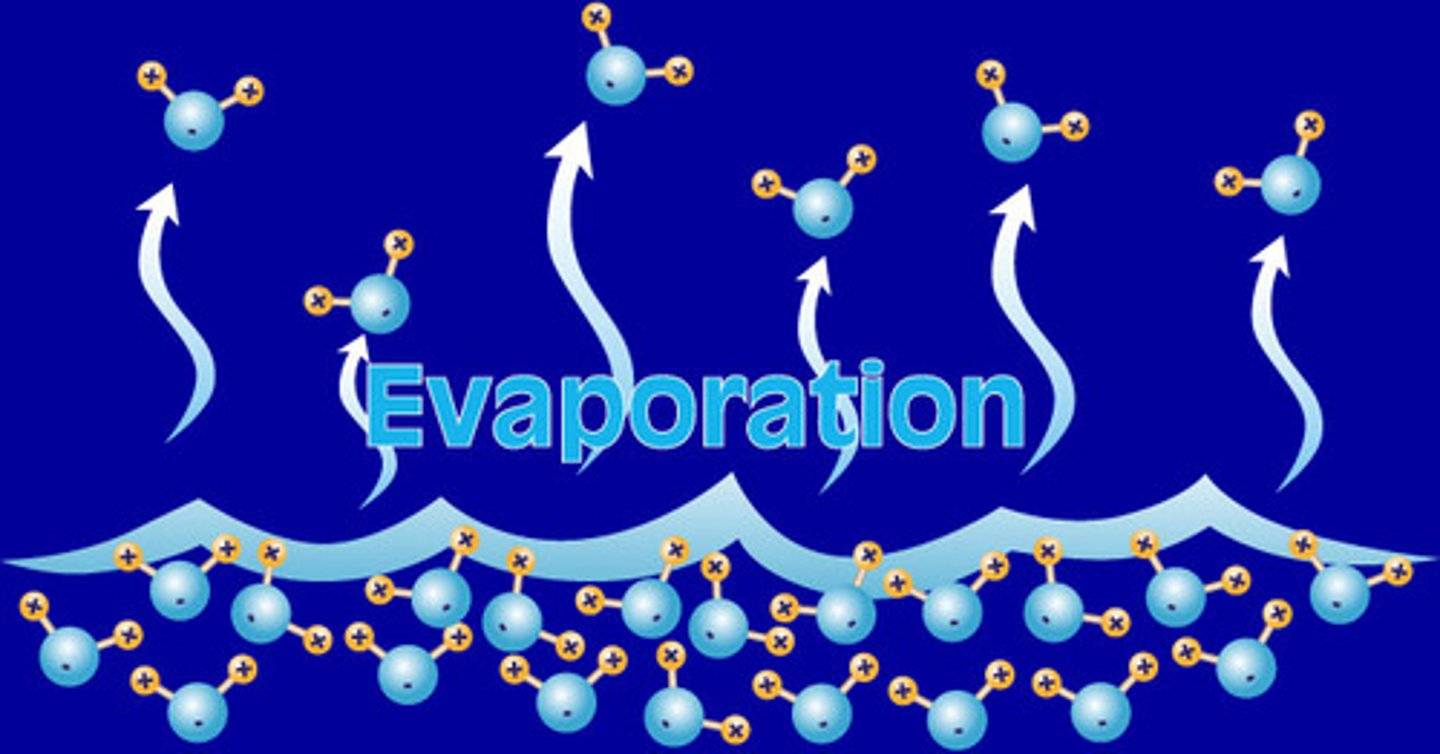
Evaporating
Change of state from a liquid to a gas (that occurs at at the surface of a liquid below it's boiling point)
Condensing
Change of state from a gas to a liquid

Sublimation
The change of state from a solid to a gas (or a gas to a solid)

Freezing
The change of state from a liquid to a solid

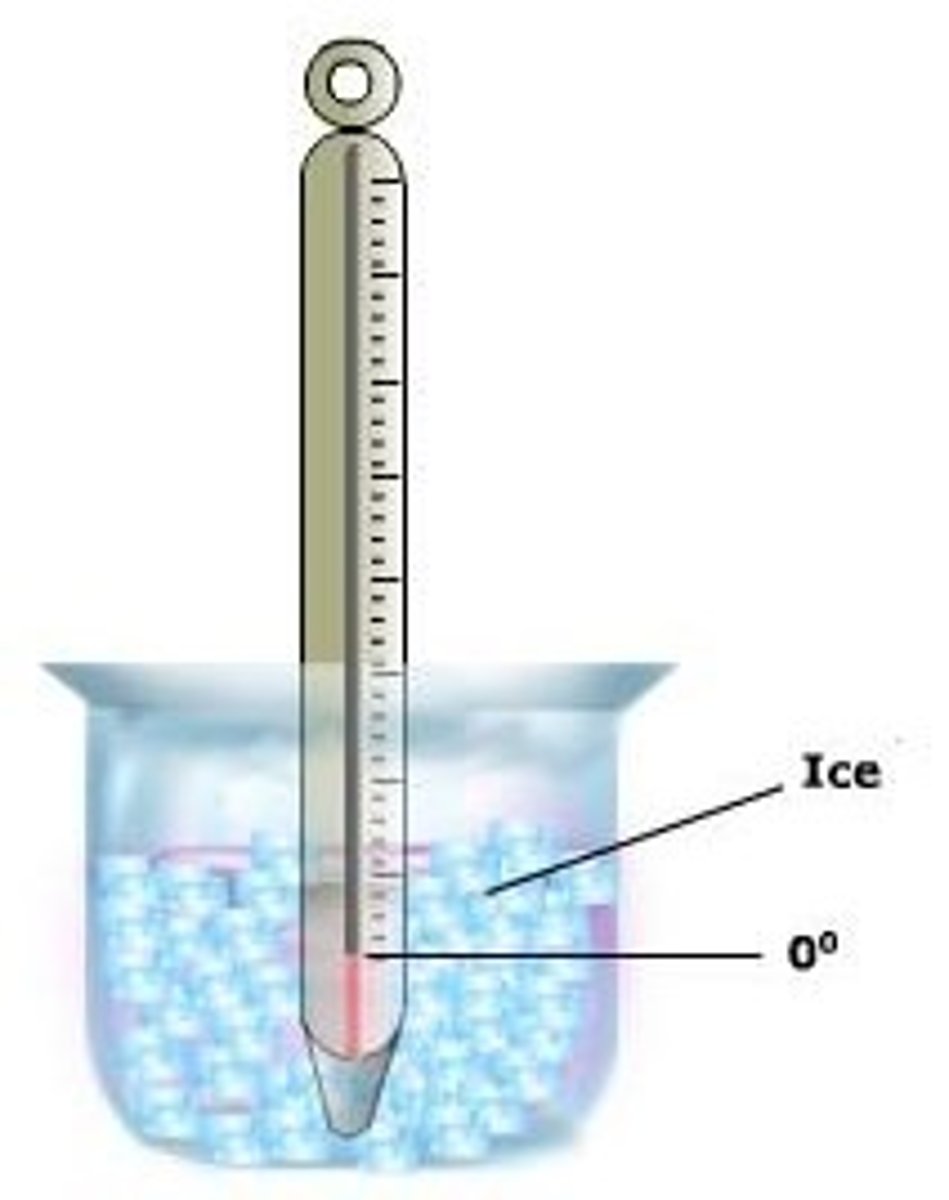
Melting point
The temperature at which a solid becomes a liquid (when being heated) or a liquid becomes a solid (when being cooled)
Boiling point
The temperature at which a liquid becomes a gas (when being heated) or a gas becomes a liquid (when being cooled)
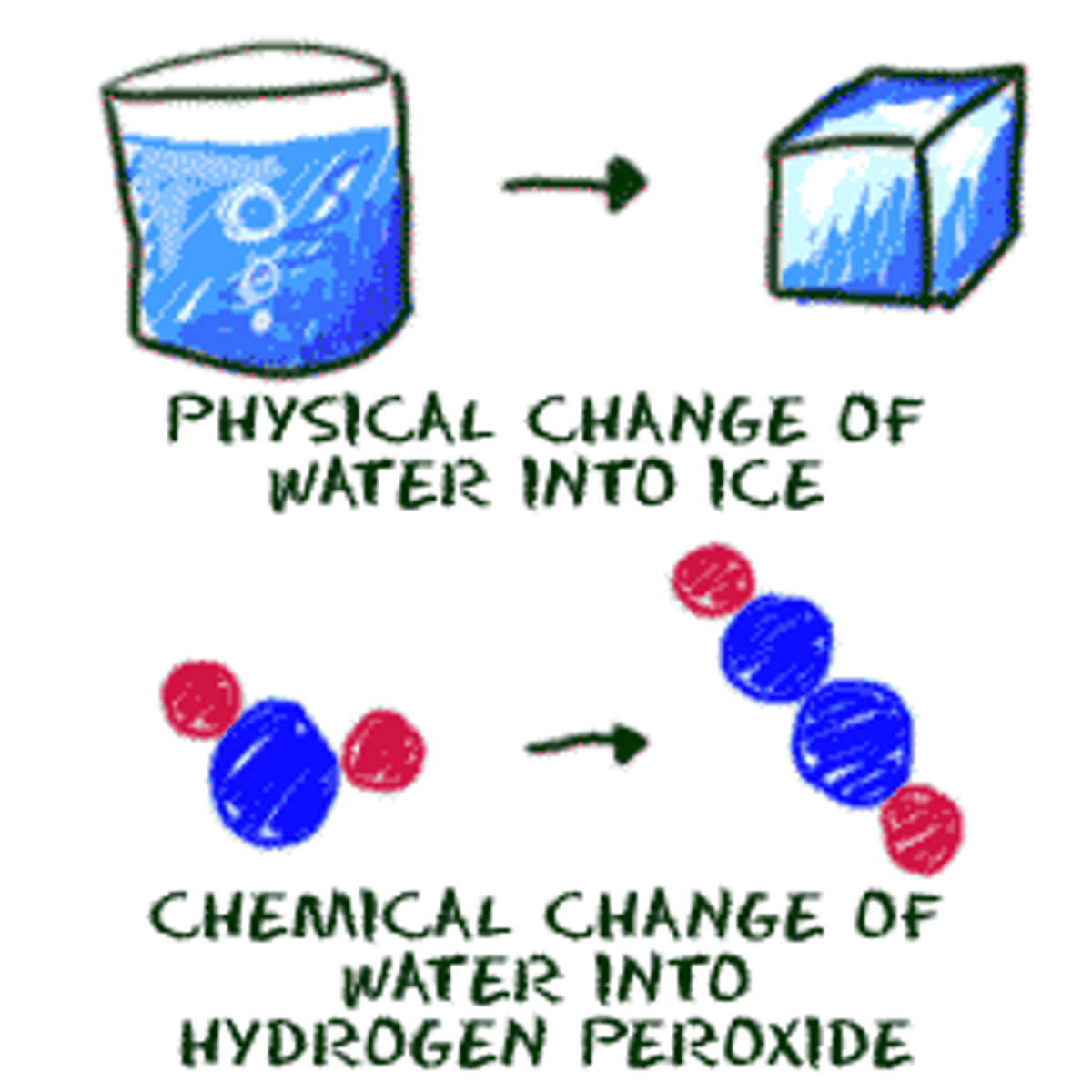
Physical change
When a substance recovers its original properties if the change is reversed
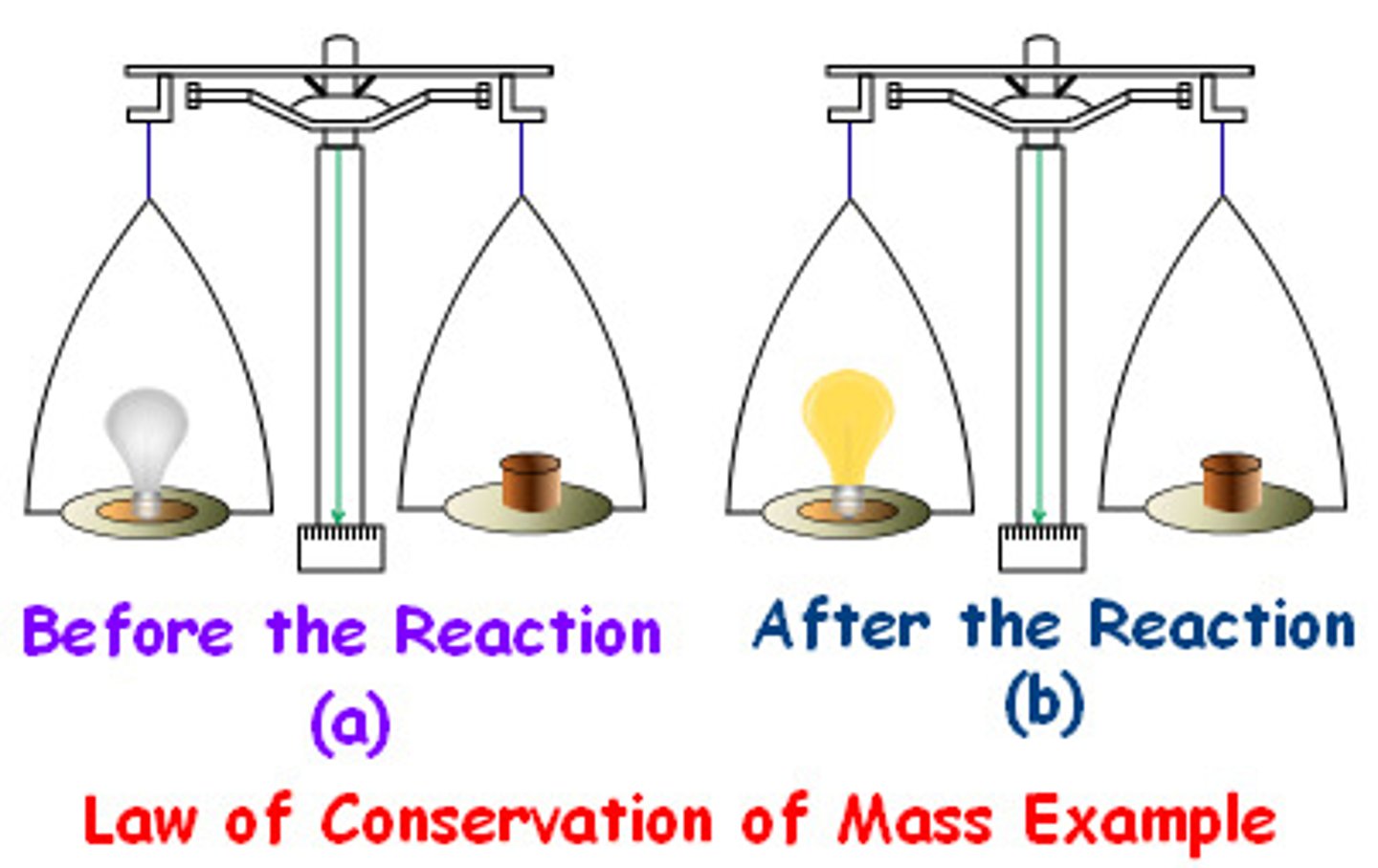
Conservation of mass
Mass cannot be created or destroyed during a physical or chemical change
Internal energy
The total kinetic energy and potential energy of all the particles (atoms and molecules) that make up a system
The energy change when a substance is heated or cooled
The internal energy of the particles increases or decreases
The energy change when a substance's temperature changes
The kinetic energy of the the particles increases or decreases
The energy change when a substance changes state
The particles are gain or lose potential energy because bonds between them break or form