rp2 + calorimetry
1/13
Earn XP
Description and Tags
plastic cup - conversion of anhydrous copper (II) sulfate into hydrated copper (II) sulfate
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
how could we reduce the % uncertainty in rp2 w/o changing the apparatus?
increase mass of substance/increase conc of solutions
so ΔT greater
why may the experimental value for the enthalpy of combustion be lower than it should have been in the calorimetry practical?
incomplete combustion
thermal energy/heat loss
evaporation of alcohol
what is the formula for uncertainty?
± (no. of readings taken x smallest increment/2)
what is the formula for % uncertainty?
(uncertainty / volume or mass measured) x 100
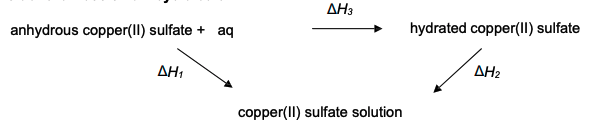
provide the Hess cycle for the conversion of anhydrous copper (II) sulfate and water to hydrated copper (II) sulfate:
summarise the method for the measurement of the enthalpy change for the conversion of anhydrous copper (II) sulfate and water to hydrated copper (II) sulfate:
weigh out approx 4g - record precise mass - of anhydrous copper(II) sulfate in a dry stoppered weighing bottle
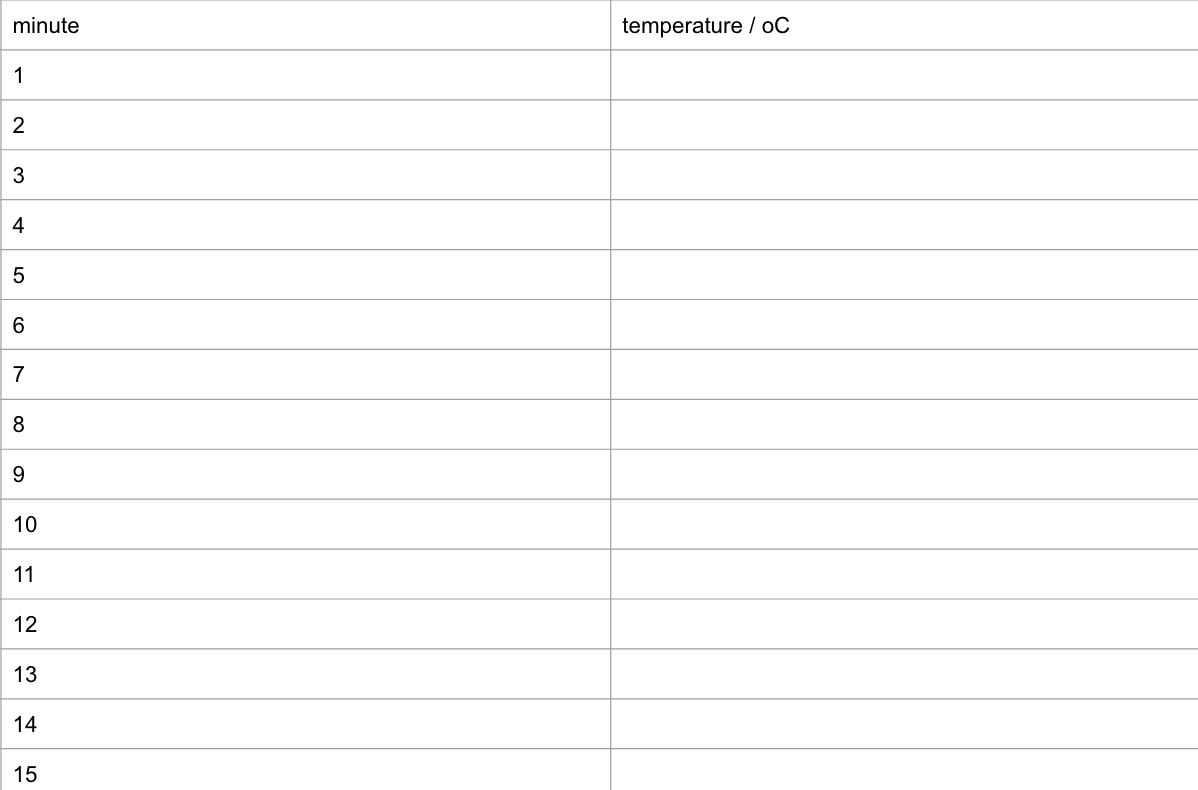
construct a suitable table of results to allow you to record temperatures at minute intervals up to 15 minutes
using a measuring cylinder, pour 25 cm³ of deionised water into an insulating cup and record its temperature at the beginning ( t = 0), start the timer and then record the temperature again every minute, stirring the liquid continuously.
at the fourth minute, add the powdered anhydrous copper(II) sulfate rapidly to the water in the insulating cup and continue to stir, but do not record the temperature
at the fifth minute and for every minute up to 15 minutes, stir and record the temperature of the solution in the insulating cup
plot a graph of temperature against time and draw two separate best fit lines; one, which joins the points before the addition, and one, which joins the points after the addition, extrapolating both lines to the fourth minute.
use your graph to determine the temperature change at the fourth minute, which theoretically should have occurred immediately on addition of the solid
repeat for hydrated copper sulfate - instead use approx 6.25 g
what sources of error could there be in rp2? how could these errors be minimised?
heat loss to surroundings (ignored in this experiment) - insulate cup further by adding a lid/wrapping cup w/ foil
incomplete/slow reaction means exact temperature rise can be difficult to find as cooling occurs as reactions progresses - use electronic temperature sensor and data logging software to plot graph accurately
what should the graph for the endothermic reaction look like? which reaction is this?
water + hydrated copper (II) sulfate
what should the graph for the exothermic reaction look like?
water + anhydrous copper (II) sulfate
why can the enthalpy change of the conversion of anhydrous copper (II) sulfate to hydrated copper (II) sulfate not be measured directly?
it is impossible to add the precise amount of water required for the reaction to occur
it is difficult to measure the temp change of a solid
why do we take temperature readings at regular intervals instead of just measuring the start and end temperature?
temperature changes continuously as heat is always being lost to the surroundings
why do we extrapolate back to 4 mins instead of just taking the initial and final temperature?
more heat energy is lost at the point of reaction, especially when the reaction is highly exothermic
the enthalpy change of neutralisation for a reaction between a strong acid and base should be identical regardless of the strong acid and base used - suggest why:
all strong acids and bases are completely ionised in dilute solution
construct a suitable results table for rp2: