chem exam 2
0.0(0)
0.0(0)
Card Sorting
1/102
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
103 Terms
1
New cards
The mother of a very fussy and colicky one-year-old notices that her child’s black T-shirt is crusted over with dried salt after being outside on an extremely warm day. What (inborn) disorder could this be a sign of? What labs to order?
Cystic fibrosis
Sweat Chloride testing – Cystic fibrosis mutations
Sweat Chloride testing – Cystic fibrosis mutations
2
New cards
A 27 year old trainer in a local gym experiences acute and worsening chest pain. His labs come back with a total creatine kinase (CK) activity that is four-fold elevated as compared with the normal upper limit of the reference range. What additional labs would be informative in this setting?
- CK-MM (muscles)
- CK-MB (heart)
- CK-BB (brain)
- Caused by strenuous exercises – CK testing of the blood
- CK-MB (heart)
- CK-BB (brain)
- Caused by strenuous exercises – CK testing of the blood
3
New cards
Given pCO2 and HCO3, calculate and interpret pH levels using the Henderson-Hasselbalch buffer equation
- PH calculation given pCO2 and HCO3
- PH = 6.1 + log (HCO3)/(H2CO3)
- PH = 6.1 + log (HCO3)/ (0.03 x pCO2)
- H2CO3 = 0.03 x pCO2
- PH = 6.1 + log (HCO3)/(H2CO3)
- PH = 6.1 + log (HCO3)/ (0.03 x pCO2)
- H2CO3 = 0.03 x pCO2
4
New cards
expected ABG results (pCO2, pO2, HCO3-, pH) and potassium values for the following: Metabolic Acidosis
Metabolic acidosis: pH
5
New cards
expected ABG results (pCO2, pO2, HCO3-, pH) and potassium values for the following: Metabolic Alkalosis
Metabolic alkalosis: pH >7.45, high PCO2 (>44), high HCO3 (>27), K level low
6
New cards
For the following acid-base imbalances, list expected ABG results (pCO2, pO2, HCO3-, pH) and potassium values for the following: Respiratory Acidosis
Respiratory acidosis: pH < 7.35, high PCO2 (>44), high HCO3 (>27)
7
New cards
For the following acid-base imbalances, list expected ABG results (pCO2, pO2, HCO3-, pH) and potassium values for the following: Respiratory Alkalosis
Respiratory alkalosis: pH > 7.45, low PCO2 (
8
New cards
anion gap equation
Na – CL + HCO3
9
New cards
high anion gap?
metabolic acidosis
10
New cards
Explain what compensatory mechanisms arise in the context of Metabolic Acidosis
Metabolic acidosis: respiratory alkalosis increased respiratory rate to decrease PCO2; Kussmaul breathing) – associated with hyperkalemia
11
New cards
Explain what compensatory mechanisms arise in the context of Metabolic Alkalosis
Metabolic alkalosis: respiratory acidosis (slow breathing) (renal and respiratory) – associated with hypokalemia
12
New cards
Explain what compensatory mechanisms arise in the context of: Respiratory Acidosis
Respiratory acidosis: metabolic alkalosis (renal – slow bicarbonate reclamation) – COPD, overdose
13
New cards
Explain what compensatory mechanisms arise in the context of: Respiratory Alkalosis
Respiratory alkalosis: metabolic acidosis (dumping of bicarb) – caused by hyperventilation
14
New cards
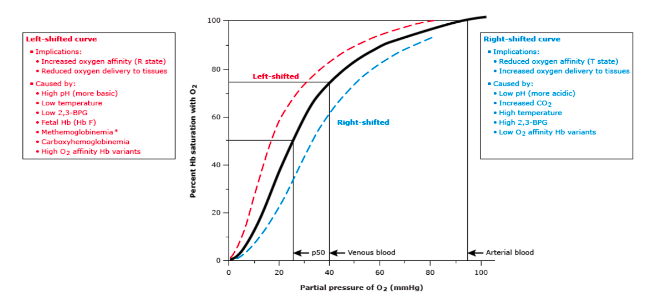
List factors that shift the oxyhemoglobin dissociation curve to the right and the consequences in regard to oxygen delivery to the periphery
Low pH, decreased O2 affinity (T state), increased oxygen delivery (low pH, increased CO2, high temp, high 2,3-BPG, low O2 affinity hemoglobin variants)
15
New cards
List factors that shift the oxyhemoglobin dissociation curve to the left and the consequences in regard to oxygen delivery to the periphery
High PH, increased O2 affinity (R state), decreased oxygen delivery (high pH, decreased CO2, low temp, low 2,3-BPG, high O2 affinity Hemoglobin variant, fetal hemoglobin (HB F))
16
New cards
Identify the p50 in mmHg from inspection of an oxyhemoglobin dissociation curve
- P50 is the partial pressure of oxygen that causes hemoglobin to be 50% saturated
- 50% of Hemoglobin saturation determines the P50 partial pressure (mmHg)
- 50% of Hemoglobin saturation determines the P50 partial pressure (mmHg)
17
New cards
Explain how HCO3 works as a volatile buffer
HCO3 takes up protons from HB and becomes carbonic acid
In the lungs carbonic acid (H2CO3) is converted to CO2 and water by carbonic anhydrase
In the lungs carbonic acid (H2CO3) is converted to CO2 and water by carbonic anhydrase
18
New cards
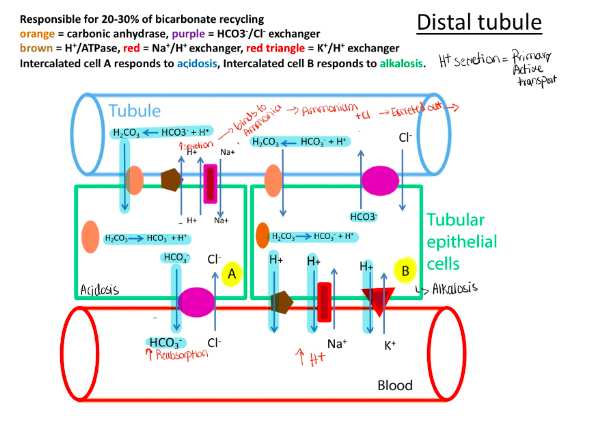
Describe how the kidney handles acid or base excess using ammonia, carbonic anhydrase, the anion exchanger (HCO3/Cl), the H+ ATPase and the Na+/H+ and K/H+ exchanger
Proximal tubule: 70-80% of HCO3 recycling – HCO3 reabsorption – secondary active transport
Distal tubule: 20-30% of HCO3 recycling – H+ secretion
Respiratory alkalosis– low PCO2 causes high pH which causes low HCO3 reabsorption and low H + secretion – causing compensation
- Respiratory acidosis – high PCO2 causes low PH and compensate by high HCO3 reabsorbing and high H+ secretion
- Metabolic acidosis – if H+ high and HCO3 low causes kidney to compensate by increasing H+ secretion and increasing HCO3 absorption
- Metabolic alkalosis – if H+ low and HCO3 high causes kidney to compensate by decreasing H+ secretion and decreasing HCO3 reabsorption
Distal tubule: 20-30% of HCO3 recycling – H+ secretion
Respiratory alkalosis– low PCO2 causes high pH which causes low HCO3 reabsorption and low H + secretion – causing compensation
- Respiratory acidosis – high PCO2 causes low PH and compensate by high HCO3 reabsorbing and high H+ secretion
- Metabolic acidosis – if H+ high and HCO3 low causes kidney to compensate by increasing H+ secretion and increasing HCO3 absorption
- Metabolic alkalosis – if H+ low and HCO3 high causes kidney to compensate by decreasing H+ secretion and decreasing HCO3 reabsorption
19
New cards
How does an oximeter work and what does it measure?
Uses absorbance Maxima of Hb derivatives and differential equations to quantify (%): Oxyhemoglobin, Deoxyhemoglobin, carboxyhemoglobin (CO-Hb), Methemoglobin (Fe-Hb)
- spectrophotometry (Hb variants change color of blood)
- spectrophotometry (Hb variants change color of blood)
20
New cards
Explain why lactate is the final end product of anaerobic glycolysis
So by converting pyruvate to lactate, that is a reduction that requires NADH, cytosolic NADH, and therefore liberates NAD to really participate in the glyceraldehyde-3 phosphates step. So it's a way of keeping anaerobic glycolysis going in the cytoplasm of the muscle and rbc.
21
New cards
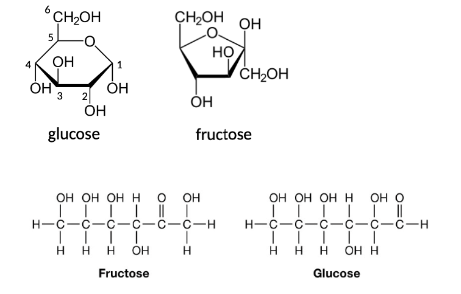
monosaccharides
pentose (5 carbons – ribose), hexoses (6 carbon – glucose, fructose, galactose)
22
New cards
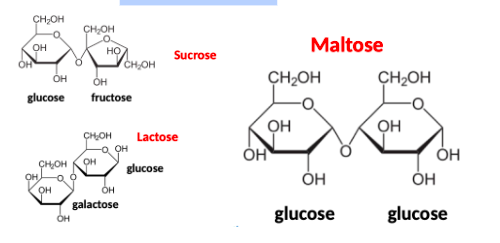
disaccharides
sucrose, lactose, maltose
23
New cards
glycogen
one form in which body fuel is stored; stored primarily in the liver and broken down into glucose when needed by the body
24
New cards
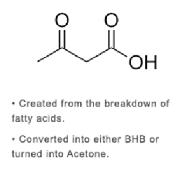
acetoacetate
25
New cards
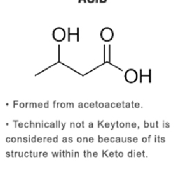
B-hydroxybutyrate
26
New cards
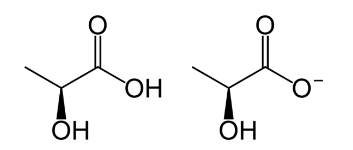
lactate
27
New cards
Identify biological activities of insulin
-secreted by pancreatic beta cells
-Inhibits glucagon
-Induces glucokinase
-Increases transport of glucose into liver, skeletal muscle, and adipose tissue
-Inhibits glucagon
-Induces glucokinase
-Increases transport of glucose into liver, skeletal muscle, and adipose tissue
28
New cards
Identify biological activities of Glucagon
-secreted by pancreatic alpha cells in islet (a response to decreasing plasma glucose)
-quickly raises blood sugar and inhibits insulin secretion. -signals carbohydrate starvation causes the liver to export glucose
-Inhibits glycolysis and uptake of glucose by liver
-Stimulates gluconeogenesis, glycogenolysis and mobilization of fatty acids from adipose cells
Medication for people with hypoglycemia
-quickly raises blood sugar and inhibits insulin secretion. -signals carbohydrate starvation causes the liver to export glucose
-Inhibits glycolysis and uptake of glucose by liver
-Stimulates gluconeogenesis, glycogenolysis and mobilization of fatty acids from adipose cells
Medication for people with hypoglycemia
29
New cards
Identify biological activities of cortisol
adrenal steroid hormone – high levels promote gluconeogenesis – starvation state hormone – favored by untreated diabetes mellitus.
30
New cards
Identify biological activities of GLP-1 (Glucagon-like peptide-1
inhibits glucagon release from alpha cells in pancreatic islets. GLP-1 analogs used for treatment in type 2 diabetes
31
New cards
Identify biological activities of GIP (Gastric inhibitory peptide)
glucose-dependent insulinotropic polypeptide. GIP analogs used for type 2 diabetes
32
New cards
Identify biological activities of DPP-4 (dipeptidyl peptidase-4):
rapidly degrades GLP-1 and GIP. DPP-4 inhibitors use for treatment of type 2 diabetes.
- Boost insulin secretion in individuals with type 2 diabetes
- Boost insulin secretion in individuals with type 2 diabetes
33
New cards
Type 1 DM
immune mediated diabetes. Pancreatic beta cell destruction (immune destruction of pancreas) (B and T cell mediated). Autoantibodies against stuff in islet cells. Get insulin replacement.
34
New cards
Type 2 DM
insulin resistance and impairment of insulin secretion
35
New cards
Gestational DM
transient effect of placental hormones
36
New cards
MODY
rare autosomal dominant mutations in hepatic nuclear factors (HNF14, HNF4A) and glucokinase
37
New cards
Identify risk factors for type 2 DM and long term complications
-geographical, ethnicity, body mass index, inactivity, obesity. Nicotine (smoking) is a direct vascular toxin for coronary vascular disease which is a component of diabetes
-Long-term complications: retinopathy, nephropathy, and coronary vascular disease
-Long-term complications: retinopathy, nephropathy, and coronary vascular disease
38
New cards
long term complications of type 1
etinopathy, nephropathy, and coronary vascular disease
39
New cards
Hemoglobin A1c screening is performed to screen for type 2 diabetes but not for type 1. Why?
A1C reflects average blood glucose value in the last 2-3 months. Useful in diagnosing diabetes and monitoring long-term efficacy of glucose control.
- There is low sensitivity for type 1 diabetes.
- There is low sensitivity for type 1 diabetes.
40
New cards
Interpret HA1c % in the context of non-diabetic individuals, and those with pre-diabetes and diabetes mellitus
- Non-diabetic individuals:
41
New cards
Contrast HA1c and fructosamine in regard to the time period of glucose control they reflect
-HA1C: A1C reflects average blood glucose value in the last 2-3 months
-Fructose amino only over the last few weeks because of the circulating half-life of red cells and albumin
-Fructose amino only over the last few weeks because of the circulating half-life of red cells and albumin
42
New cards
Identify HA1c measurement techniques and common interferences
- Measurement techniques: column chromatography method, electrophoresis, high pressure liquid chromatography (HPLC), immunoassay and more.
- Common interferences: hemoglobin variants like Hemoglobin S and hemoglobin C. Hemolytic anemia (short half-life or life span of RBCs – sickle cells – 14 days in HBS).
- Common interferences: hemoglobin variants like Hemoglobin S and hemoglobin C. Hemolytic anemia (short half-life or life span of RBCs – sickle cells – 14 days in HBS).
43
New cards
common autoantibodies included in working up individuals with type 1 diabetes
islet cell antibodies, glutamic acid decarboxylase (GAD-65), insulin autoantibodies (IAA), and IA-2A, and protein tyrosine phosphate
44
New cards
List ADA guidelines for the diagnosis of type1
Fasting Plasma glucose >126mg/dL (7.0mmol/L), 2-H Plasma Glucose >200 mg/dL, Plasma blood glucose recommended for acute onset of type 1 in individuals with symptoms of hyperglycemia (random plasma glucose >200 mg/dL (11.1 mmol/L)).
45
New cards
List ADA guidelines for the diagnosis of type 2
FPG >126 mg/dL. 2-H PG >200mg/dL, A1C >6.5%, patients with hyperglycemia – random plasma glucose >200 mg/dL
46
New cards
List ADA guidelines for the diagnosis of pre-diabetes
BMI, overweight – A1C >5.7 %, FPG >126 mg/dL. 2-H PG >200mg/dL
47
New cards
List ADA guidelines for the diagnosis of gestational DM
when oral glucose tolerance testing with plasma glucose measurement fasting (92 mg/dl), 1 hr into fasting (180 mg/dl), 2 hr into (153 mg/dL).
- If plasma glucose level 1 hr after non-fasting >130 mg/dL and more.
- If plasma glucose level 1 hr after non-fasting >130 mg/dL and more.
48
New cards
What are the expected values for glucose, pH, bicarbonate, sodium, potassium and pCO2 in the setting of diabetic ketoacidosis?
Glucose: hyperglycemia – high glucose
- PH: low
- Bicarbonate: low
- PCO2: low (compensation)
- Sodium: low (hyponatremia)
- Potassium: high (hyperkalemia) – cells take H+ and exchange for potassium
- PH: low
- Bicarbonate: low
- PCO2: low (compensation)
- Sodium: low (hyponatremia)
- Potassium: high (hyperkalemia) – cells take H+ and exchange for potassium
49
New cards
Define reducing sugar and the test utilized to detect them in urine
- Reducing sugar – can open glucose ring to expose free aldehyde (which carries reduction of copper) (lactose and maltose)
– non-reducing sugar is sucrose.
- Benedict’s test
– non-reducing sugar is sucrose.
- Benedict’s test
50
New cards
Recognize immediate action values for glucose on the high and low ends
high end: >300 mg/dL
low end:
low end:
51
New cards
List common methodologies utilized to measure glucose in serum and urine
-Glucose is a reductant (reducing substance) – copper reduction – oldest
-Glucose oxidase technique: utilized by glucometers and urine dipstick patches
-Hexokinase technique – more specific than glucose oxidase
-Glucose oxidase technique: utilized by glucometers and urine dipstick patches
-Hexokinase technique – more specific than glucose oxidase
52
New cards
exothermic (exergonic) reaction
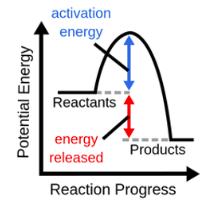
53
New cards
endothermic (endergonic) reaction
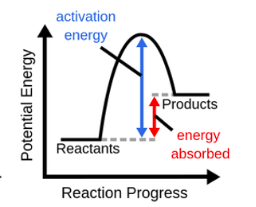
54
New cards
Identify conditions or parameters that satisfy the requirements for using Michaelis-Menten kinetics to analyze enzyme activity
- Enzymes with no extensive allosteric regulator – good candidate
- Has one substrate and one product – classical enzyme – good candidate
- Restricts kinetics to less complex allosteric enzymes.
- Has one substrate and one product – classical enzyme – good candidate
- Restricts kinetics to less complex allosteric enzymes.
55
New cards
Define the following: Km
measures substrate binding affinity for enzyme
low km (mmol) = high substrate binding, high Km = low substrate binding
low km (mmol) = high substrate binding, high Km = low substrate binding
56
New cards
Define the following: Vmax
- Substrate concentration at which ½ Vmax is achieved
-enzyme saturated with substrate and substrate concentration higher than km. Maximal velocity
-enzyme saturated with substrate and substrate concentration higher than km. Maximal velocity
57
New cards
Contrast competitive and non-competitive enzyme inhibition in regard to how they alter Km or Vmax.
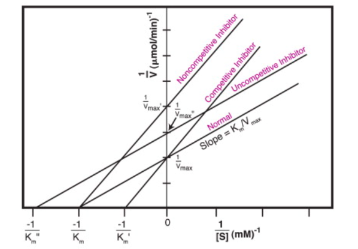
58
New cards
Interpret Lineweaver-Burke plots to determine whether an enzyme inhibitor is active in a competitive or non-competitive fashion
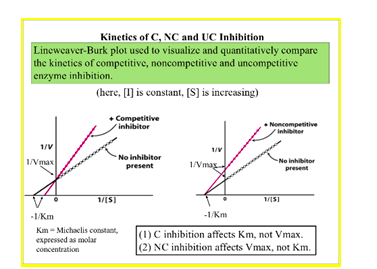
59
New cards
1st order kinetics
substrate concentration much less than km
not used for clinical labs bc substrate has to be higher (it's too low)
not used for clinical labs bc substrate has to be higher (it's too low)
60
New cards
2nd order kinetics
-used in clinical labs
-rate is only dependent on enzyme concentration and independent of substrate concentration
-rate is only dependent on enzyme concentration and independent of substrate concentration
61
New cards
electron carrier
accept and donate electrons from and to various enzymes
62
New cards
NAD+/NADH
reduction of NAD+ and oxidation of NADH
63
New cards
NDP+/NADPH
-reduction of NADP+ and oxidation of NADPH
-absorbs light at 350 nm
-absorbs light at 350 nm
64
New cards
decreasing absorbance
NAD to NADH, NADP to NADPH
65
New cards
increasing absorbance
NADH to NAD, NADPH to NADP
66
New cards
Draw a graph showing the following changes in enzyme activities (AST, LDH, CK, CKMB) and other markers of cardiomyocyte injury (Troponin T and Troponin I) over a four-week time course after symptoms of MI (ACS) occur.
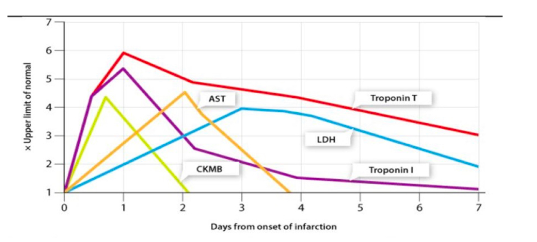
67
New cards
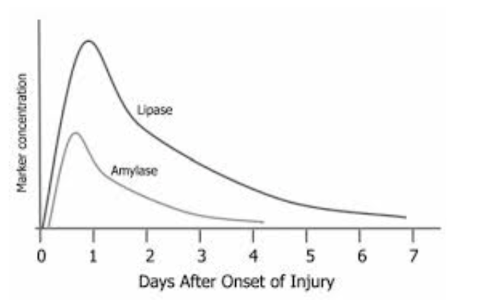
Draw a graph showing the following changes in enzyme activities (Lipase, Amylase) over a 3-week period after symptoms of acute pancreatitis occur.
Lipase and amylase – markers of acute pancreatitis
68
New cards
Biliary tree disease markers and Hepatobiliary enzyme
Alkaline phosphatase (ALP), y-Glutamyltranspeptide (yGT)
69
New cards
ALP/y-GT
induction of enzymes due to stasis or obstruction of the biliary tract
70
New cards
ALP
cleaves phosphate group from substrates, mild increase in parenchymal liver disease, high in children and during pregnancy, a marker of bone turnover and bone disease. Increases due to increased expression of enzymes
71
New cards
Y-GT
plasma form derived from liver
Has highest sensitivity as a marker of biliary obstruction. Is more liver selective.
Induced by salicylates and alcohol. Used to monitor drinking.
Increased in acute pancreatitis, MI, diabetes
Has highest sensitivity as a marker of biliary obstruction. Is more liver selective.
Induced by salicylates and alcohol. Used to monitor drinking.
Increased in acute pancreatitis, MI, diabetes
72
New cards
Hepatocellular injury
Alanine aminotransferase (ALT),
Aspartate aminotransferase (AST)
Aspartate aminotransferase (AST)
73
New cards
ALT
longer half-life than AST, liver disease elevates more ALT
74
New cards
AST/ALT
viral hepatitis causes an increase in AST and ALT (very high)
liver cell integrity and liver cell death
liver cell integrity and liver cell death
75
New cards
List LDH isoforms and where they are expressed
LDH 1 (heart muscle, RBC)
LDH 2 (WBC)
LDH 3(lung)
LDH 4 (pancreas, kidney, placenta)
LDH 5 (liver, skeletal muscle)
LDH 2 (WBC)
LDH 3(lung)
LDH 4 (pancreas, kidney, placenta)
LDH 5 (liver, skeletal muscle)
76
New cards
CK isoforms and where they are expressed
CKMM (skeletal muscle),
CKMB (heart),
CKBB (brain)
CKMB (heart),
CKBB (brain)
77
New cards
What is the most specific marker for cardiomyocyte injury?
troponins – selective cardiac markers – not selective for heart attack
78
New cards
What is the most specific marker for Acute pancreatitis
lipase (more pancreatic specific) and amylase
79
New cards
What is the most specific marker for Liver injury
ALT (more specific) and AST
80
New cards
What is the most specific marker for Hepatobiliary disease
y-GT (more specific) and ALP
81
New cards
What enzyme isoform is useful as a marker of osteoblast activity?
bone isoform – bALP (bone specific alkaline phosphatase activity)
82
New cards
How does PTH stimulate calcium mobilization in bone
PTH receptor 1 (PTHR1) in osteoblasts.
Increases RANKL in OB which binds to RANK on osteoclast (OC) membrane (reduces bone turnover). Increases bone resorption.
High extracellular calcium in skeleton + proteins regulated by vitamin D promote bone mineralization
Increases RANKL in OB which binds to RANK on osteoclast (OC) membrane (reduces bone turnover). Increases bone resorption.
High extracellular calcium in skeleton + proteins regulated by vitamin D promote bone mineralization
83
New cards
How does PTH stimulate calcium mobilization in kidney tubules
increases Ca reabsorption and phosphorus excretion by decreasing insertion of Type II Na+/P cotransporters (NPT2a, NPT2c) in apical membrane of proximal tubule.
84
New cards
How does PTH stimulate calcium mobilization in the intestine
secondary fashion –
ca between cells in intestinal
PTH sense calcium by calcium sensing receptor (CaR)
ca between cells in intestinal
PTH sense calcium by calcium sensing receptor (CaR)
85
New cards
How does PTH affect renal handling of phosphate
increases phosphorus excretion –
blocks renal phosphate reabsorption
blocks renal phosphate reabsorption
86
New cards
How does the parathyroid sense calcium?
activating mutations of CaR will lower set point and Inactivity mutations will cause a high set point due to particular mutation inherited
87
New cards
Describe bound and free forms of calcium in plasma
Free: measured by electrodes – 48% free (biologically active in feedback loops) and parathyroid
Bound: 46% bound to albumin. Doesn’t affect the feedback loop.
- 6 % bound to small anions (citrate, phosphate, lactate)
Bound: 46% bound to albumin. Doesn’t affect the feedback loop.
- 6 % bound to small anions (citrate, phosphate, lactate)
88
New cards
Adjust a total calcium for a low albumin
For every 1 g/dL decrease in albumin (4.0g/dL) – total calcium is adjusted upwards by 0.8 mg/dL
Example: total calcium is 7.5 mg/dL. If an albumin concentration of 2.5 g/dL is measured, the adjusted total calcium is 8.7 mg / dL
Example: total calcium is 7.5 mg/dL. If an albumin concentration of 2.5 g/dL is measured, the adjusted total calcium is 8.7 mg / dL
89
New cards
How does FGF work to lower phosphate levels
-FGF-23 (Fibroblast Growth Factor-23) produced by osteocytes and bone lining cells.
Synthesis regulated by plasma, PTH, and calcitriol – secreted in response to hyperphosphatemia
-Has phosphate dumping effect – blocks renal phosphate reabsorption
Synthesis regulated by plasma, PTH, and calcitriol – secreted in response to hyperphosphatemia
-Has phosphate dumping effect – blocks renal phosphate reabsorption
90
New cards
How does FGF work to affect vitamin D activation
-Inhibits active calcitriol production by inhibiting vitamin D 1-hydroxylase and stimulating vitamin 24-hydroxylase
-Favors destruction of 125 vitamin D dihydroxy and vitamin D 25 hydroxy
-Favors destruction of 125 vitamin D dihydroxy and vitamin D 25 hydroxy
91
New cards
Describe diseases associated with increased FGF-23 and altered set points of the CaSR
-ADHR = Autosomal dominant hypophosphatemic rickets due to resistance proteolysis of FGF-23
-TIO = tumor induced osteomalacia – due to paraneoplastic syndrome (tumor ectopically producing FGF-23) in FGF-23
- Autosomal dominant Hypocalcemia – CasR – PTH deficiency, Ca/vitamin D deficiency, vitamin D resistant, mg depletion, pseudohypoparathyroidism, high calcium set point
- Familial hypocalciuric Hypercalcemia – CasR – primary hyperparathyroidism, increased calcitriol, increased bone reabsorption (Paget’s disease), vitamin D 24 hydroxylase deficiency
-TIO = tumor induced osteomalacia – due to paraneoplastic syndrome (tumor ectopically producing FGF-23) in FGF-23
- Autosomal dominant Hypocalcemia – CasR – PTH deficiency, Ca/vitamin D deficiency, vitamin D resistant, mg depletion, pseudohypoparathyroidism, high calcium set point
- Familial hypocalciuric Hypercalcemia – CasR – primary hyperparathyroidism, increased calcitriol, increased bone reabsorption (Paget’s disease), vitamin D 24 hydroxylase deficiency
92
New cards
Identify recommended ranges for 25-OH vitamin D (calcidiol)
Storage form - Around 30 at least (below 20 is a vitamin D deficiency)
93
New cards
Use that chart from the Ca/Pi PowerPoint presentation to identify individuals with: primary hyperparathyroidism, primary hypoparathyroidism and the hypercalcemia of malignancy.
High calcium, normal PTH == something wrong with the feedback loop (included in hyperparathyroidism because non-intact feedback loop is affecting parathyroid gland)
Hypoparathyroidism == lost parathyroid tissue from surgeries
Red = intact feedback loop!!!!!
Blue hollow = no feedback loop (no parathyroid)
Green = intact feedback loop
Blue solid = non-intact feedback loop
Hypoparathyroidism == lost parathyroid tissue from surgeries
Red = intact feedback loop!!!!!
Blue hollow = no feedback loop (no parathyroid)
Green = intact feedback loop
Blue solid = non-intact feedback loop

94
New cards
Identify enzymes that activate and degrade calcitriol
Activate = Vit D 1 hydroxylase (activated by PTH)
Degrade = Vit D 24 dehydroxylase (activated by FGF-23)
Degrade = Vit D 24 dehydroxylase (activated by FGF-23)
95
New cards
Rhabdo (compartment syndrome)
Releases myoglobin (toxic, has free iron)
Releases potassium
CKMM
Creatinine (check renal function)
Releases potassium
CKMM
Creatinine (check renal function)
96
New cards
C-peptide
Secreted with secretion of insulin (cleaved from insulin on secretion)
Longer half-life than insulin
Marker of quality of insulin secretion
Differentiate from CRP (inflammation marker)
Longer half-life than insulin
Marker of quality of insulin secretion
Differentiate from CRP (inflammation marker)
97
New cards
What is Cinacalcet? How does it work?
Mimics activity of calcium when bound to CasR. Given to chronic kidney disease patients – acts like calcium and has inhibitory effect on secretion of PTH.
Drug to prevent tertiary hyperparathyroidism in chronic kidney disease
Drug to prevent tertiary hyperparathyroidism in chronic kidney disease
98
New cards
Primary hyperparathyroidism
High PTH, High Calcium and low phosphorus
caused by adenoma in parathyroid gland (benign tumor – not malignant)
caused by adenoma in parathyroid gland (benign tumor – not malignant)
99
New cards
Secondary hyperparathyroidism
chronic kidney disease
High potassium and high phosphorus
High phosphorus, low Calcium and high PTH
High potassium and high phosphorus
High phosphorus, low Calcium and high PTH
100
New cards
Tertiary hyperparathyroidism
High calcium and high PTH
parathyroid glands become autonomous (big and overactive)- consistently secreting PTH
Osteoporosis
parathyroid glands become autonomous (big and overactive)- consistently secreting PTH
Osteoporosis