Buda: Academic Chemistry CH 5.2- Electron Configuration
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|

17 Terms
How many energy levels are there?
seven
atomic orbitals
the regions around the nucleus within which the electrons have the highest probability of being found
Energy level location determines ______________.
reactivity
What is another name for the first sublevel?
"s" sublevel
What is the shape of the "s" sublevel orbital?
spherical
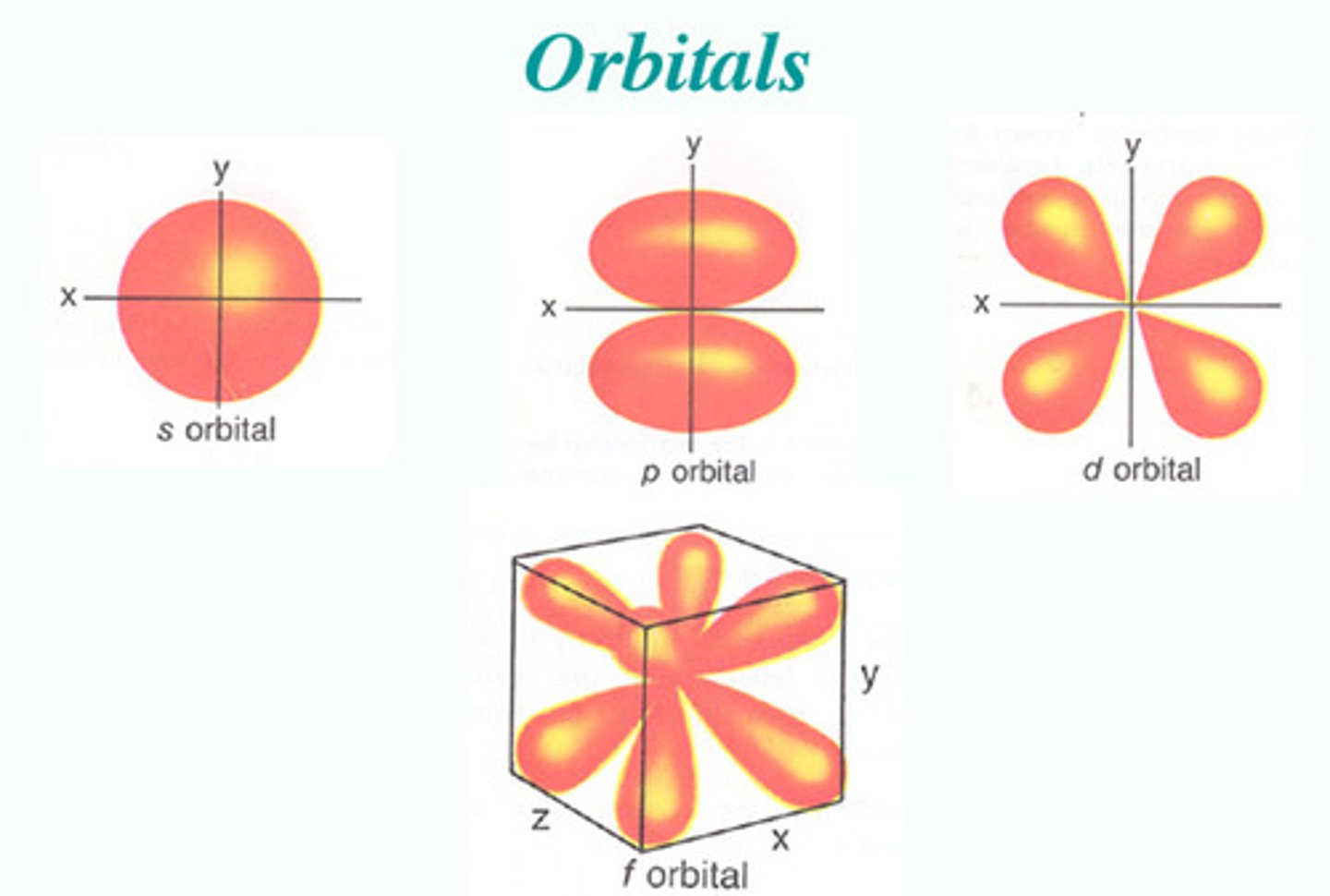
How many orbitals are in the "s" sublevel?
one
What is another name for the second sublevel?
"p" sublevel
How many orbitals are in the "p" sublevel?
three
Aufbau Principle
- each electron occupies the lowest level available.
Pauli Exclusion Principle
- each atomic orbital can hold a maximum of two electrons
- two electrons in the same orbital must have opposite spin
Hund's Rule
- electrons must be distributed in equal-energy orbitals before a 2nd electron can be added to the same orbital
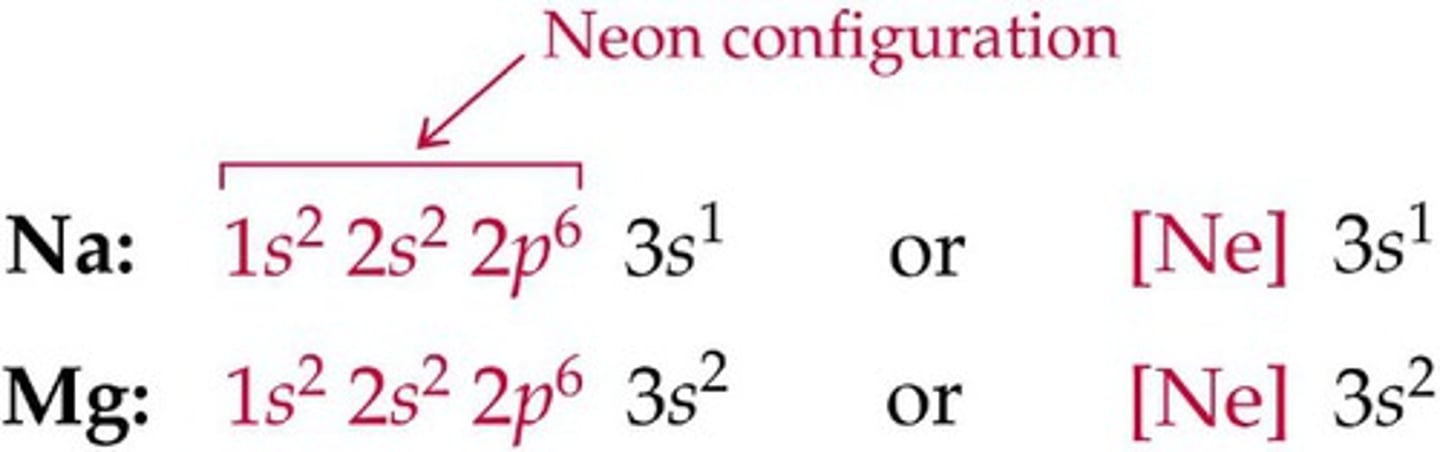
Short Hand Configuration
using the symbol of the Noble gas that comes before the element
valence electrons
electrons on the outermost energy level of an atom
What is the maximum amount of valence electrons can a configuration contain?
8
What orbitals are valence electrons located in?
S & P orbitals
Lewis Dot Structure
diagram of a molecule using dots to represent valence electrons
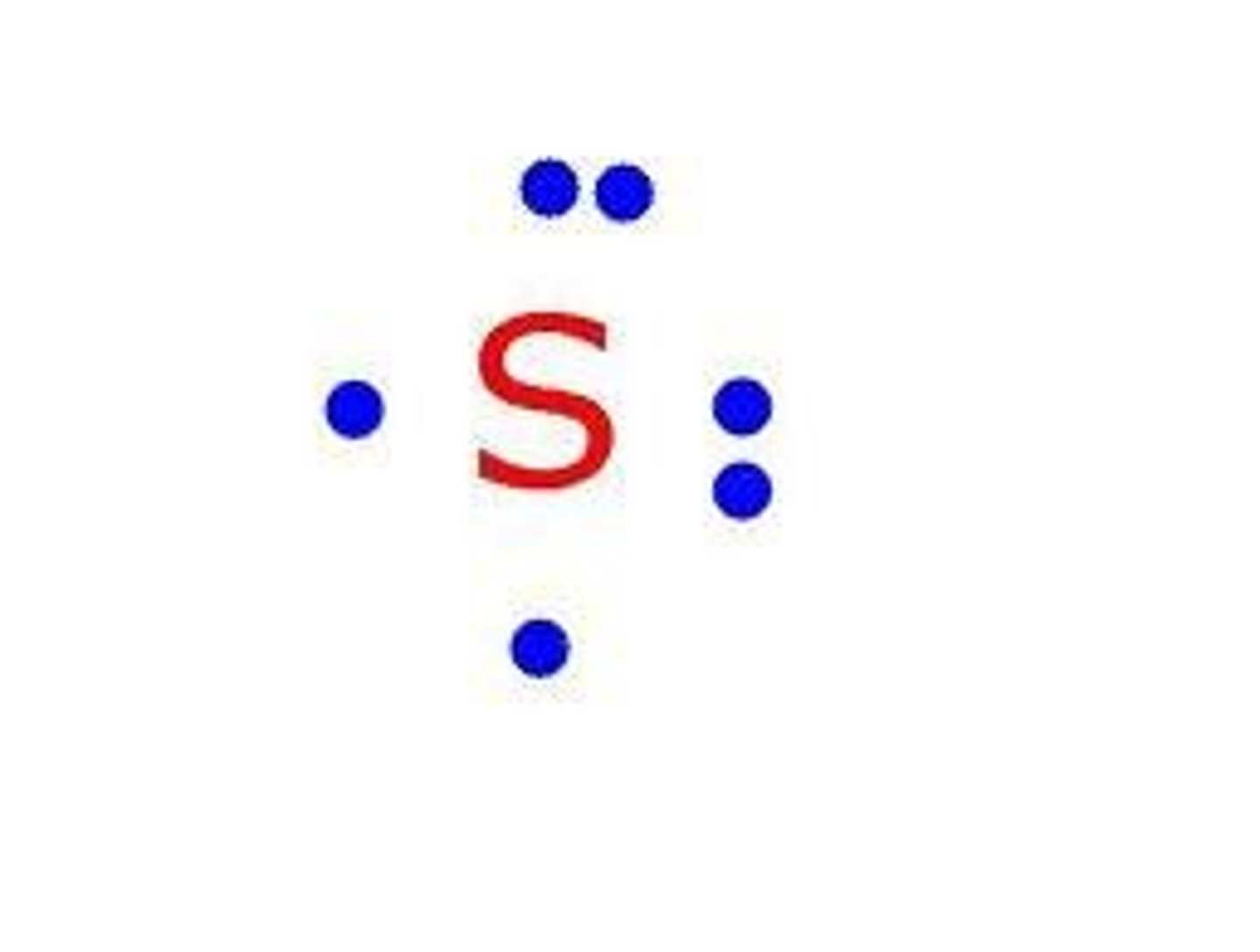
How do you distribute valence electrons around the element symbol?
first valence electron: goes above it
second valence electron: goes to the right of it
third valence electron: goes below it
fourth valence electron: goes to the left of it
fifth valence electron: (repeats) goes above it