A levels chemistry: arenes
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
what are arenes
hydrocarbons based on the benzene ring
what are aromatic compounds
compounds possessing benzene ring or other molecular structures that resemble benzene in structure and chemical behaviour
when does the nomenclature not end with benzene
benzaldehyde (-CHO)
phenol (-OH)
phenylamine (-NH2)
benzoic acid (-COOH)
benzenesulfonic acid (-SO3H)
when is the benzene ring treated as a substituent
when
the substituent on the benzene ring has more than 6 carbon atoms
the highest priority functional group is not a substituent on the benzene ring
eg -OH is not on the benzene ring, then it is not a phenol but a phenyl alcohol
phenyl and substituted phenyl groups are called aryl groups
physical properties of benzene
colourless liquid
characteristic odour
non-polar, insoluble in water, less dense than water
pre dominant IMF: id-id forces
soluble in all organic solvents and is a good solvent for organic compounds
burns with a smoky and luminous flame
due to its relatively high carbon content (C:H = 1:1)
undergoes incomplete combustion
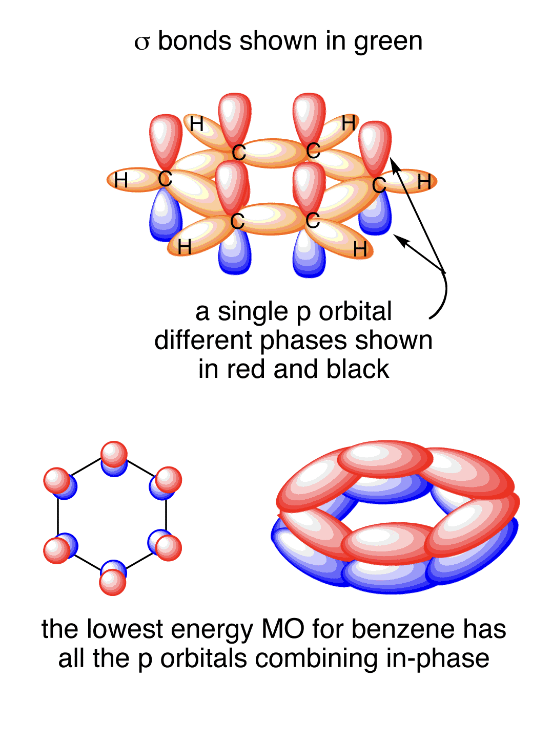
explain the resonance structure of benzene
to minimise electronic repulsion, the 3 regions of electron density about each C atom in a benzene molecule adopt a trigonal planar geometry
Hence, all bond angles in the benzene ring are 120 degrees
it is a planar molecule
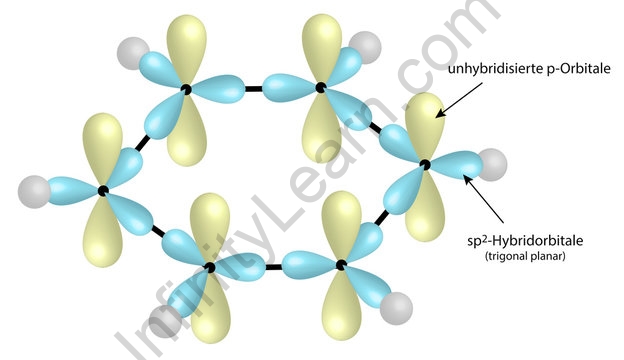
each of the 6 carbon atoms in benzene is sp2 hybridised, comprising 3 sp2 hybrid orbitals and 1 unhybridised p orbital
2 sp2 hybrid orbitals are used to overlap head-on with the sp2 hybrid orbitals of 2 adjacent C atoms to form 2 C-C sigma bonds
1 sp2 hybrid orbital is used to overlap head-on with the 1s orbital of the H atom to form the C-H sigma bond
each singly filled p orbital overlaps side-on with the adjacent p orbital on either side
this continuous side-on overlap of the p-orbitals results in a cloud of delocalised pi electrons above and below the plane of the ring
ie resonance is present
evidence for resonance in benzene
all the carbon-carbon bonds in benzene are identical and equal in length. This agrees with the description of benzene as a resonance hybrid of the two Kekulé structures
the measured carbon-carbon bond length in benzene is intermediate between the length of a C-C bond and that of a C=C bond
this indicates that the carbon-carbon bonds have partial double bond character
pi electron density is evenly distributed
delocalisation of pi electrons (ie pi bond is averaged out across the 6 C atoms)
Why does benzene undergo substitution rather than addition reactions
If benzene undergoes addition reactions, the overall aromatic character is destroyed. The extra stability associated with the delocalisation of the 6 pi electrons is lost
Hence, the majority of reactions that benzene undergoes involve the substitution in the ring. The delocalisation of the six pi electrons in the continuously overlapping p-orbitals, and hence its aromatic character, is retained
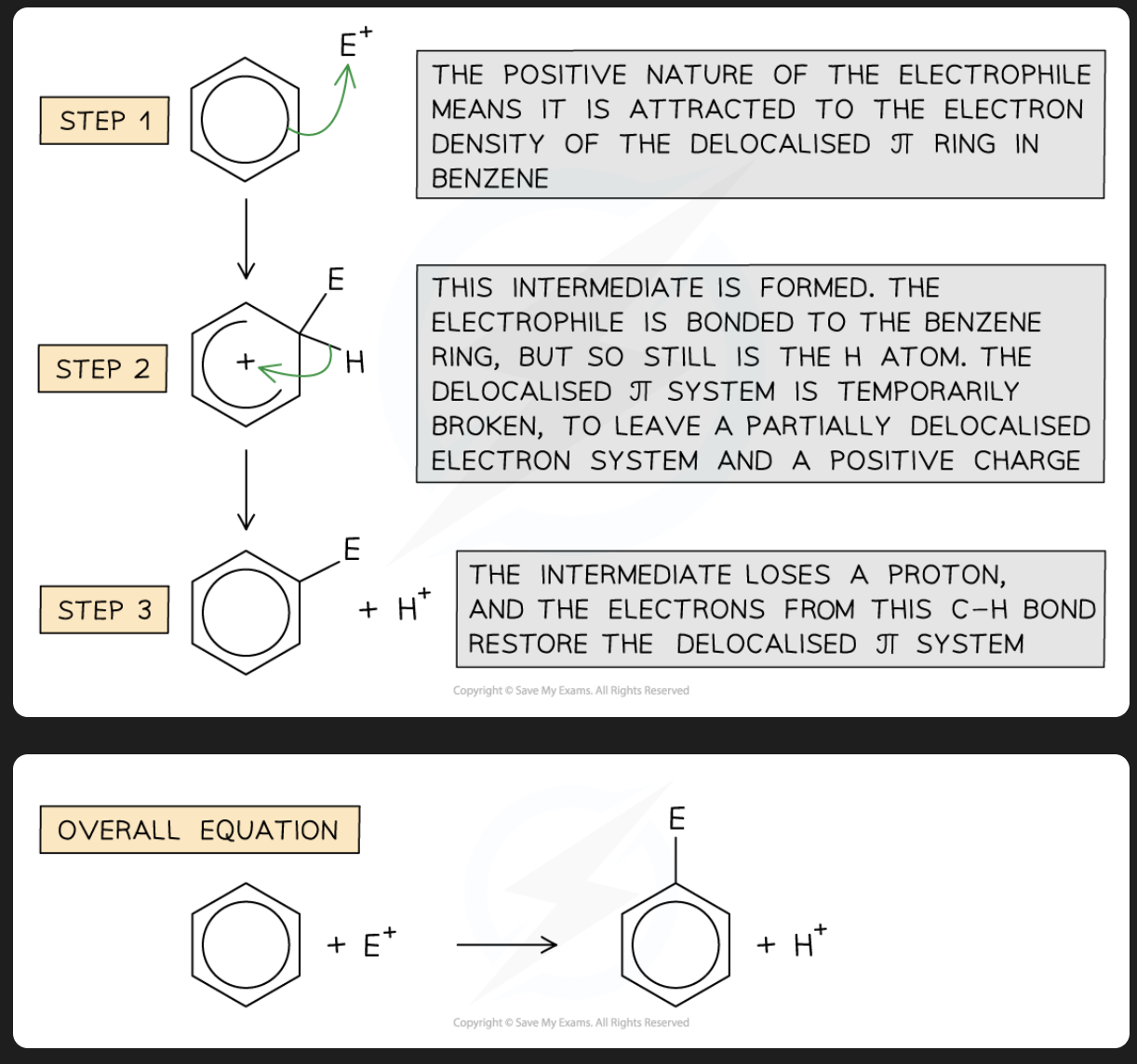
explain the electrophilic substitution mechanism
benzene ring is electron rich
due to the availability of 6 pi electrons
benzene is a nucleophile, attracts electrophiles
Generation of electrophile, E+
the electrophile E+ is first generated
Mechanism: electrophilic addition
Step 1
the electrophile attacks the electron-rich benzene ring
this involves the movement of 2 pi electrons from the benzene ring to the electrophile, forming a sigma bond to one carbon atom of the benzene ring
this carbon atom is sp3 hybridized, the rest remain sp2 hybridized
a carbocation is formed.
the carbocation is resonance-stabilised but not aromatic
this step is the slow step
ie the rate-determining step
because it involves the destruction of the aromatic character of the benzene ring.
the extra stability associated with the delocalisation of 6 pi electrons is lost
Step 2
the carbocation intermediate loses a proton from the carbon atom that bears the electrophile
the two electrons that bonded this proton to carbon become part of the delocalised pi-electron system
the aromatic character of the benzene ring is restored
the substituted product is formed
this step is a fast step
reagents, conditions, product and mechanism of reaction of benzene with concentrated nitric acid
reagent: concentrated HNO3, concentrated H2SO4
role of H2SO4: Bronsted-Lowry acid catalyst
Condition: 55-60 degrees celsius (or heat, depending on the substituents)
Product: nitrobenzene (a yellow oil)
mechanism: electrophilic substitution
what is a bronsted-lowry acid
a proton donor
what is a bronsted-lowry base
a proton acceptor
how is the electrophile generated during the nitration of benzene
2 H2SO4 + HNO3 ⇌ NO2+ + 2 H2SO4- + H3O+
in the nitrating mixture, HNO3 acts as a Bronstead-Lowry base and accepts a proton from the Bronsted-Lowry acid H2SO4
H2SO4 + HNO3 ⇌ H2NO3+ + HSO4-
the reaction leads eventually to the formation of the nitronium ion, NO2+
H2NO3+ ⇌ NO2+ + H2O
H2SO4 + H2O —> HSO4- + H3O+
adding up all 3 equations gives:
2H2SO4 + HNO3 ⇌ NO2+ + 2HSO4- + H3O+
explain the electrophilic substitution mechanism for the nitration of benzene
step 1: (electrophilic attack by NO2+) (rate-determining step)
the NO2+ ion acts as an electrophile and attacks the electron-rich benzene ring.
2 of the 6 pi electrons in the benzene ring are used to form the C-N bond.
Hence the aromatic character of the ring is destroyed.
this is the slow step/rate-determining step of the mechanism
a carbocation is formed.
the C atom bearing the -NO2 group is sp3 hybridized
shape wrt to this atom is tetrahedral
the rest of the C atoms remain sp2 hybridized
Step 2: (loss of proton from carbonation) (fast step)
the unstable carbocation loses a proton to the HSO4- ion to form nitrobenzene, thus regaining the aromatic character.
H2SO4 is regenerated
H2SO4 acts as a Bronsted-Lowry acid catalyst
reagents, conditions, product and mechanism of reaction of benzene with concentrated nitric acid to form 1,3-dinitrobenzene
reagent: concentrated HNO3, concentrated H2SO4
Condition: heat
Product: 1,3-dinitrobenzene (major product)
minor products: 1,2-dinitrobenzene, 1,4-dinitrobenzene
mechanism: electrophilic substitution
reagents, conditions, product and mechanism of reaction of benzene with concentrated nitric acid to form 1,3,5-trinitrobenzene
reagent: concentrated HNO3, fuming H2SO4
Condition: very high temperatures for several days
Product: 1,3,5-trinitrobenzene (major product)
mechanism: electrophilic substitution
* yield of the reaction is only about 40%
what is a lewis acid
electron pair acceptor
what is a lewis base
electron pair donor
how is electrophile generated in the halogenation of benzene
AlCl3/FeCl3/AlBr3/FeBr3 accepts a lone pair of electrons from the halogen molecule, generating the Cl+/Br+ electrophile
AlCl3/FeCl3/AlBr3/FeBr3 functions as a lewis acid
FeCl3 can be generated in situ from Fe and Cl2 and FeBr3 from Fe and Br2
2Fe + 3Cl2 —> 2FeCl3
2Fe + 3Br2 —> 2FeBr3
Note
AlCl3 reacts readily with water
AlCl3 (s) + 6H2O(l) —>[Al(H2O)6]3+ (aq) + 3Cl- (aq)
[Al(H2O)6]3+ (aq) + H2O(l) ⇌ [Al(H2O)5(OH)]2+ (aq) + H3O+ (aq)
hence the reaction with AlCL3 can only proceed under anhydrous condition
I2 is too unreactive and F2 reacts too violently
![<p>AlCl3/FeCl3/AlBr3/FeBr3 accepts a lone pair of electrons from the halogen molecule, generating the Cl+/Br+ electrophile</p><ul><li><p>AlCl3/FeCl3/AlBr3/FeBr3 functions as a lewis acid</p></li></ul><p>FeCl3 can be generated in situ from Fe and Cl2 and FeBr3 from Fe and Br2 </p><ul><li><p>2Fe + 3Cl2 —> 2FeCl3</p></li><li><p>2Fe + 3Br2 —> 2FeBr3</p></li></ul><p>Note</p><ul><li><p>AlCl3 reacts readily with water</p><ul><li><p>AlCl3 (s) + 6H2O(l) —>[Al(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup> (aq) + 3Cl- (aq)</p></li><li><p>[Al(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup> (aq) + H2O(l) ⇌ [Al(H<sub>2</sub>O)<sub>5</sub>(OH)]<sup>2+</sup> (aq) + H3O+ (aq)</p></li></ul></li><li><p>hence the reaction with AlCL3 can only proceed under anhydrous condition </p></li><li><p>I2 is too unreactive and F2 reacts too violently </p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/29514597-6432-490d-9cb2-817d527b1359.jpeg)
reagents, conditions, product and mechanism of reaction of benzene with halogen (Br2 or Cl2) to form chloro/bromobenzene
reagents: FeCl3 or AlCl3 (anhydrous) or FeBr3 or AlBr3 or Fe
role of AlCl3/FeCl3/AlBr3/FeBr3: lewis acid catalyst
condition: room temperature, anhydrous condition if using AlCl3
product: chlorobenzene or bromobenzene (and HCl/HBr)
mechanism: electrophilic substitution
In the presence of a suitable lewis acid catalyst such as AlCl3 or FeCl3, benzene undergoes electrophilic substitution reaction with chlorine at room temperature
Similarly, benzene reacts with bromine to form bromobenzene and hydrogen bromide in the presence of AlBr3 or FeBr3
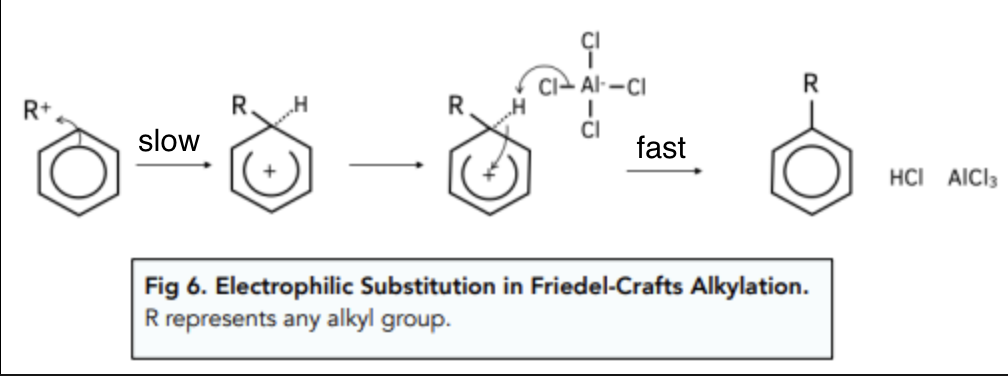
what is friedel-crafts alkylation
alkylation with a halogenoalkane (RX) and a trace amount of anhydrous AlCl3 as catalyst
role of AlCl3: lewis acid catalyst
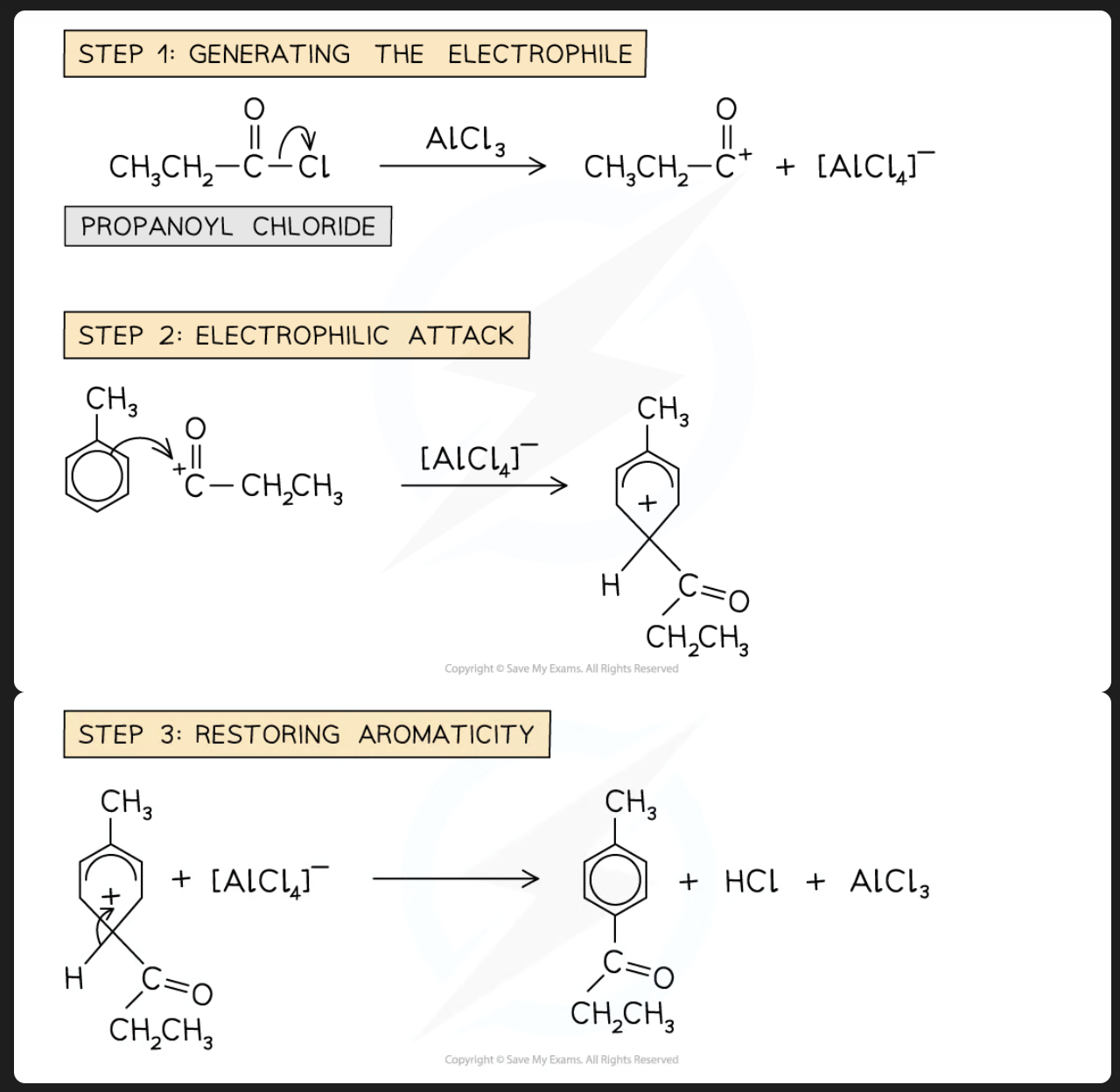
what is friedel-crafts acylation
acylation is similar to that of alkylation except that an acid halid (RCOX or ArCOX) instead of a halogenoalkane (RX) is used. The acid halide provides the acyl group (-COR) needed for the reaction
role of AlCl3: lewis acid catalyst
reagents and conditions for nitration of substituted benzene rings
compound | reagents | conditions |
|---|---|---|
nitrobenzene (least reactive; -NO2 group is deactivating) | conc HNO3 | conc H2SO4 catalyst, heat (»55°C) |
chlorobenzene (-Cl group is weakly deactivating) | conc HNO3 | conc H2SO4 catalyst, heat (> 55°C) |
benzene ring | conc HNO3 | conc H2SO4 catalyst, 55-60°C |
methylbenzene (-CH3 group is weakly activating) | conc HNO3 | conc H2SO4 catalyst, 30°C |
phenol (most reactive; -OH group is activating) | DILUTE HNO3 | room temperature |
explain what an activating group is
electron-donating
makes the benzene ring more reactive towards electrophilic substitution reactions
increases the electron density in the benzene ring and makes the ring more susceptible to electrophilic attack
helps to disperse the positive charge on the intermediate carbocation and stabilise the carbocation
Ea for step 1 is smaller —> faster rate of reaction for slow step —> faster rate of reaction
explain what a deactivating group is
electron-withdrawing
makes the ring less reactive than benzene towards electrophilic substitution reactions
decreases the electron density in the ring and makes the ring less susceptible to electrophilic attack
intensifies the positive charge on the intermediate carbocation and destabilises the carbocation
Ea for step 1 is larger —> slower rate of reaction for slow step —> slower rate of reaction
explain why methylbenzene requires a lower temperature to carry out nitration
Methylbenzene is more reactive than benzene towards electrophilic substitution as the -CH3 group is an electron-donating group and hence an activating group. It increases the electron density in the benzene ring and makes it more susceptible to electrophilic attack
what is inductive effect
donation or withdrawal of electrons through sigma bonds due to the EN difference between atoms
more EN atom will attract the bonding electrons closer to itself —> shift in electron density towards the more EN atom
explain electron-donating via inductive effect
eg - CH3
alkyl groups have a sp3 carbon
compared to the sp2 carbon in benzene ring, sp3 orbitals have less s character and its electrons are less tightly held and more easily donated to the benzene ring
higher s character of sp2 C atoms: stronger attraction for bonding e-
the substituent is said to be electron donating by inductive effect
explain electron-withdrawing via inductive effect
eg -Cl
atoms such as O, N and Cl are more EN than C atom (on benzene ring)
they pull electron density away from C though the sigma bond
the substituent is said to be electron-withdrawing by inductive effect
explain what resonance effect is
a resonance effect is the donation or withdrawal of electrons through pi bonds due to the continuous side-on p-orbital overlap of the substituent and the benzene ring.
This results in the delocalisation of electrons, either towards or away from the benzene ring
explain electron-donating via resonance effect
eg -OH
if a substituent has a lone pair of electrons on the atom that is directly attached to the benzene ring
the lone pair is usually in a p-orbital and
can be delocalised into the ring
net shift of electrons into the benzene ring since the substituent p orbital has >1 pi electron for that atom attached to the benzene ring
the substituent is said to be electron-donating by resonance effect
explain electron-withdrawing via resonance effect
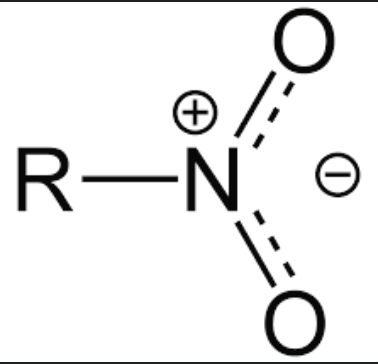
eg -C=O, -NO2
if a substituent is directly attached to the benzene ring by an atom that is doubly or triply bonded to a more EN atom, the pi electrons of the benzene ring can be delocalised onto the substituent
in -C=O, the pi electrons in the delocalised pi electron cloud shift towards O (EN of O > EN of C)
in -NO2, the pi electrons in the delocalised pi electron cloud shift to O (EN of O > EN of N)
Each substituent is unsaturated and does not have an atom with a lone pair bonded directly to the benzene ring
C in -C=O only has one electron in its unhybridised p orbital, so do its O atoms
N in -NO2 only has one electron in its unhybridised p orbital, so do its O atoms
the substituent is said to be electron-withdrawing by resonance effect
In chlorobenzene, is the electron-withdrawing, inductive effect of Cl greater or the electron-donating, resonance effect of Cl stronger
inductive effect of Cl is stronger
Cl is in Period 3 and its p-orbitals are more diffuse, leading to a less effective side-on overlap with the p-orbitals of the benzene ring.
Hence, the overall effect of the -Cl group in chlorobenzene is electron-withdrawing (by inductive effect)
The -Cl group decreases the electron density in the benzene ring and makes the ring less susceptible to electrophilic attack
it is said to be a deactivating group
In phenol, is the electron-withdrawing, inductive effect of Cl greater or the electron-donating, resonance effect of Cl stronger
The resonance effect is stronger than the inductive effect
the ability to donate e- back through the pi bond > the e- withdrawing effect through sigma bond
hence, the overall effect of the -OH group in phenol is electron-donating
the -OH group increases the electron density in the benzene ring and makes the ring more susceptible to electrophilic attack
it is said to be a strongly activating group
combustion reaction
benzene ring + 15/2 O2(g) —> 6 CO2(g) + 3 H2O(l)
reagents, conditions and product of reduction of benzene ring
Reagents: H2(g)
conditions: Ni catalyst, high temperature, high pressure
product: cycloalkane
reactions that benzene does not undergo
oxidation
bromination
hydrogenation at room temperature
what reactions can methylbenzene undergo
electrophilic substituition reactions
FRS
oxidation
reagents, conditions, product and observations for oxidation of alkyl side chain of alkylbenzene
Acidic conditions
reagent: KMnO4 (aq), H2SO4 (aq)
condition: heat/heat under reflux
product: -CH3 change to -COOH
-CH2Ch3 change to -COOH + CO2
observations: purple KMnO4 decolourises (if it is LR), formation of white ppt of benzoic acid upon cooling
Alkaline conditions
reagent: KMnO4 (aq), NaOH (aq)
condition: heat/heat under reflux
product: -CH3 change to -COO-Na+
-CH2Ch3 change to -COO-Na+ + CO2
observations: purple KMnO4 decolourises (if it is LR), formation of brown-black ppt of MnO2, formation of white ppt of benzoic acid upon cooling
critieria needed for oxidation of alkyl side chain on benzene
the benzylic carbon atom must be directly bonded to H or O atom
ie the C atom directly bonded to the benzene ring must be directly bonded to a H or O atom as well