esters
1/12
Earn XP
Description and Tags
don't know if you need to know the diff ways of making esters
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
13 Terms
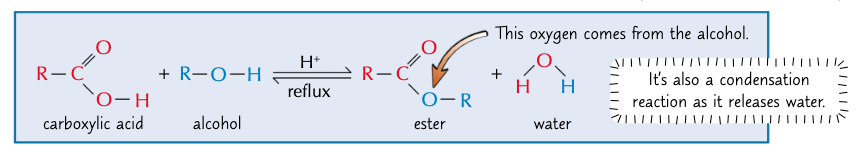
give the general eqn for the formation of esters and state the conditions - what is this process called?
conc H2SO4 catalyst
esterification (condensation reaction)
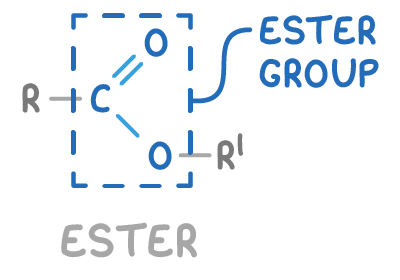
what is the ester functional group?
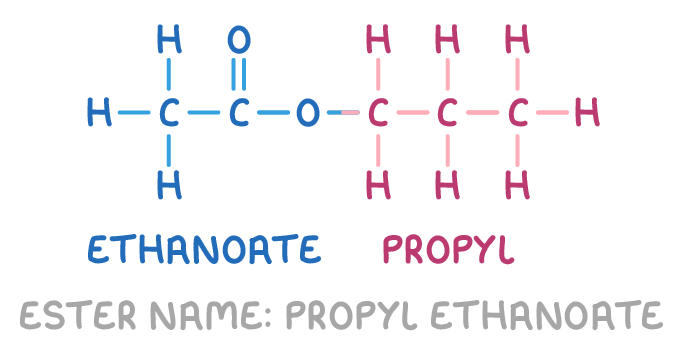
how do we name esters?
first part - from alcohol used (on RHS)
second part - from carboxylic acid used (on LHS)
give 4 uses of esters:
solvents
plasticisers
perfumes
food flavourings
give the general eqn for the acid hydrolysis of an ester - state the conditions and products:
dilute acid (typically H2SO4) catalyst
heated under reflux
products = carboxylic acid and alcohol
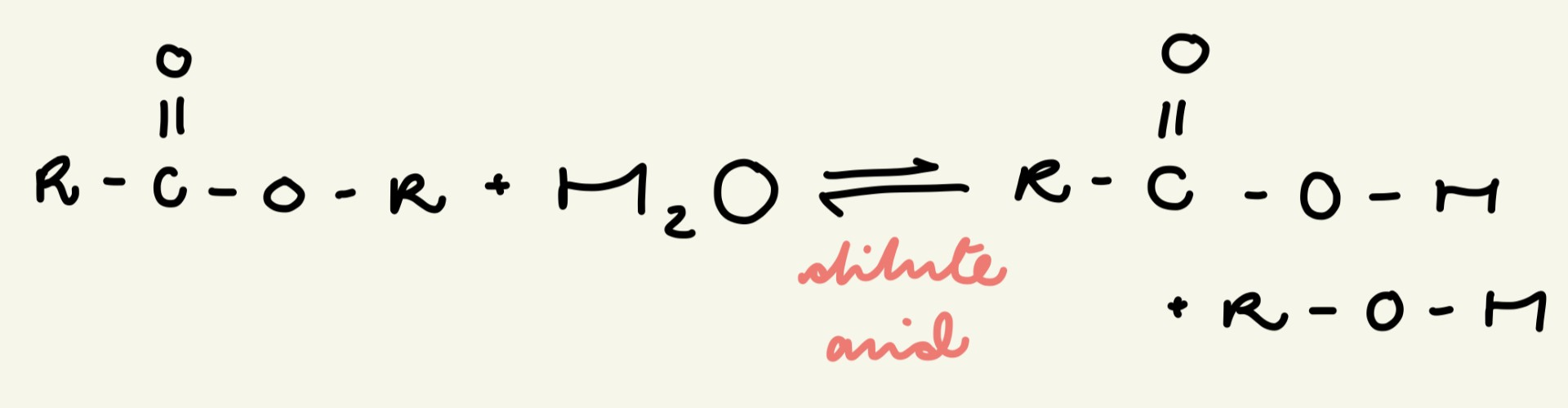
give the general eqn for the base hydrolysis (saponification) of an ester - state the conditions and products:
heated under reflux
products = salt (of the carboxylic acid) and alcohol

give and justify 4 safety measures necessary when heating under reflux in the formation of esters:
use a fume cupboard - to avoid breathing in harmful/toxic/corrosive compounds
add anti-bumping granules - to ensure smooth boiling/reduce size of bubbles
use an electric heater/water bath - as compounds are flammable
wear gloves - compounds may be corrosive
why might we shake the reaction mixture with aqueous sodium carbonate after making esters? what precaution should be taken when doing this and why?
to neutralise/react with XS acid
you should remove the stopper to prevent a buildup of pressure
which alcohol are animal fats and vegetable oils made from in esterification?
propane-1,2,3-triol (glycerol)
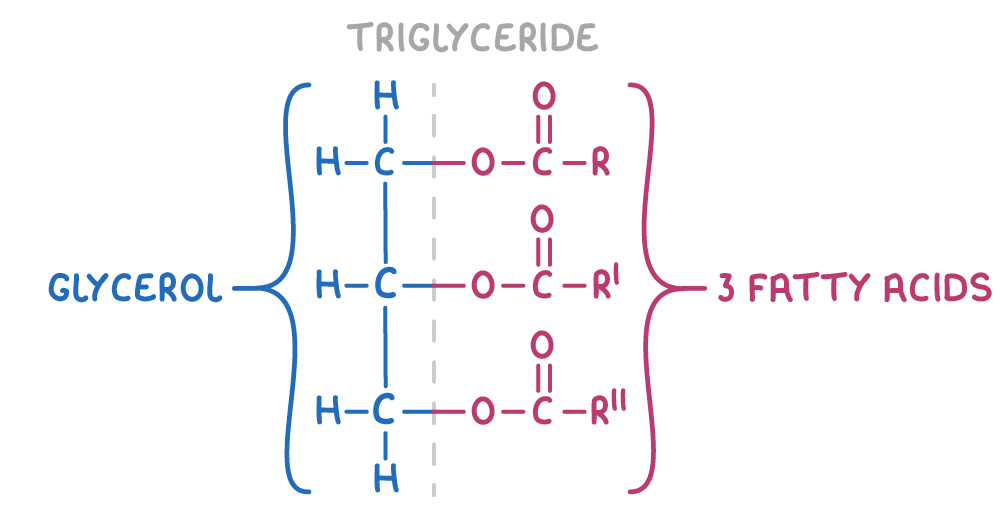
give the general structure of a triglyceride (lipid):
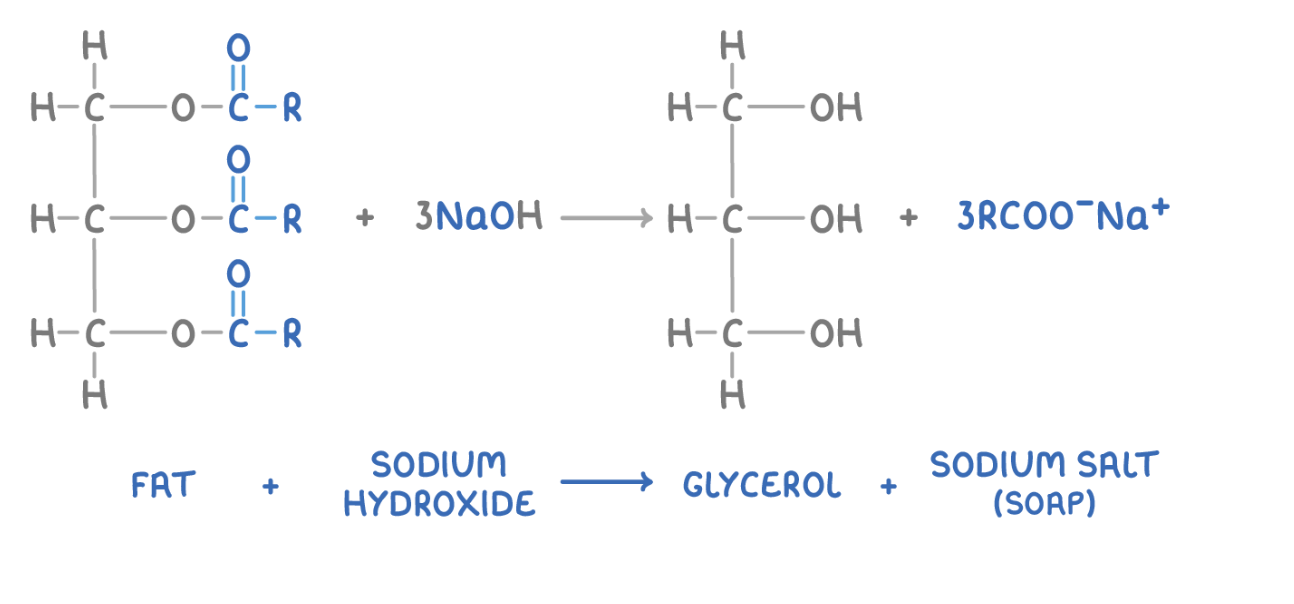
give and explain one example where base hydrolysis may be used and give the word and symbol eqn:
making soap (saponification) - reacting a lipid w/ a strong base forms salts of the fatty acids
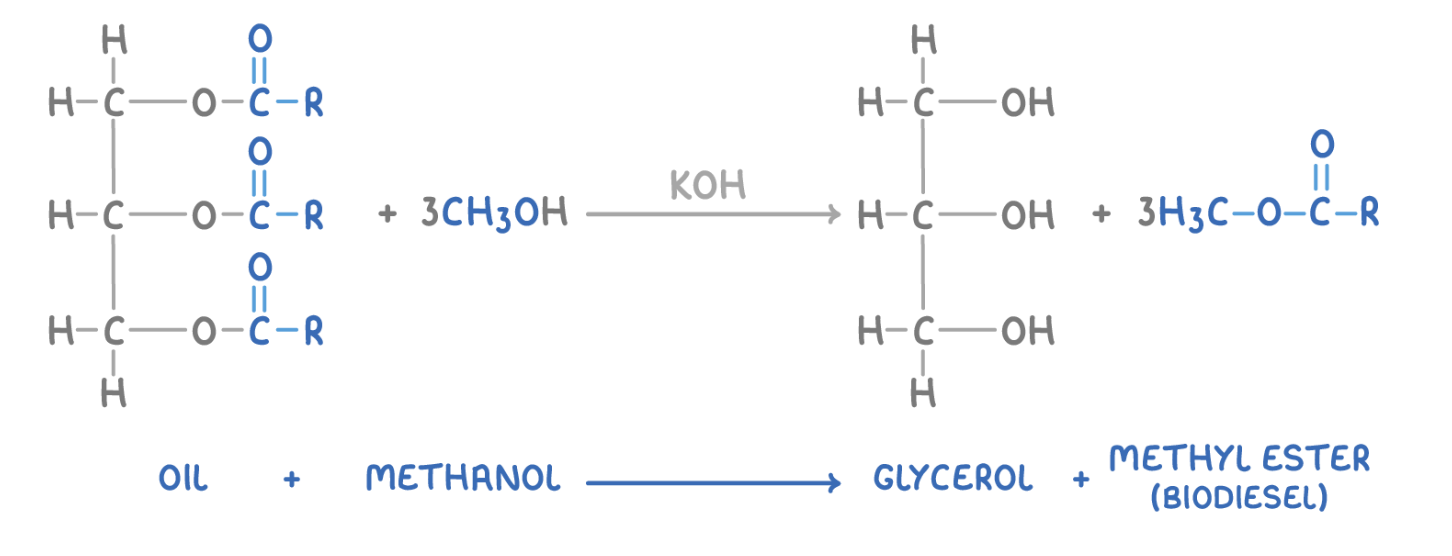
what are biodiesels? how are they made?
biodiesel = mixture of methyl esters of long-chain carboxylic acids
produced by reacting vegetable oils w/ methanol in the presence of a catalyst
give the word and symbol eqn for making biodiesels: