PHYS 1.19- antigen presentation
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
what is the specific way in which T cells recognize antigens?
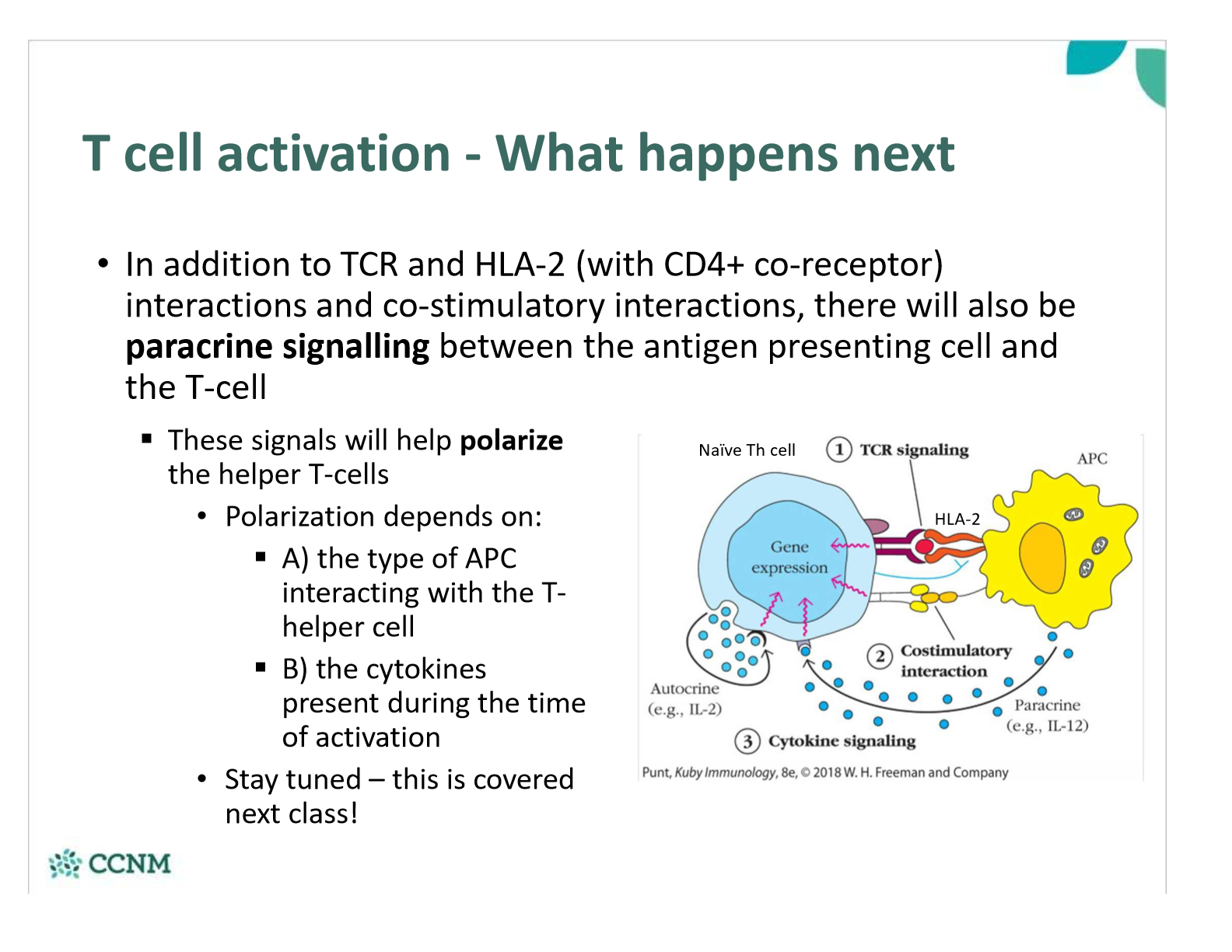
•T-cells recognize antigens in a specific fashion:
§Antigens must be presented to a T-cell in order for them to recognize the antigen
§Antigens are presented by being bound to particular proteins found on other cells
•In mice and rats, these are called MHC (major histocompatibility) proteins
•In humans they’re called the HLA (human leukocyte antigen) proteins
•A wide variety of cells can present antigens using HLA proteins
how do HLA proteins help helper T cells?
§The HLA protein can help a T-cell distinguish between foreign antigen and self antigen
•And thus, should know to only mount a response against foreign antigens
how do HLA proteins differ from lymphocyte receptors?
•Although HLA proteins are bound to antigens, they are not genetically “shuffled” like lymphocyte receptors
§Meaning that the antigen is not bound particularly tightly to these proteins
§This also implies that these proteins can present a wide variety of antigens to a wide variety of lymphocytes
§There are also a wide variety of genes/proteins called “MHC-like”
•They have a wide of range of functions beyond simply presenting antigens.
compare the different roles of the 2 HLA protein classes
•There are two types of HLA proteins:
§HLA class I
§HLA class II
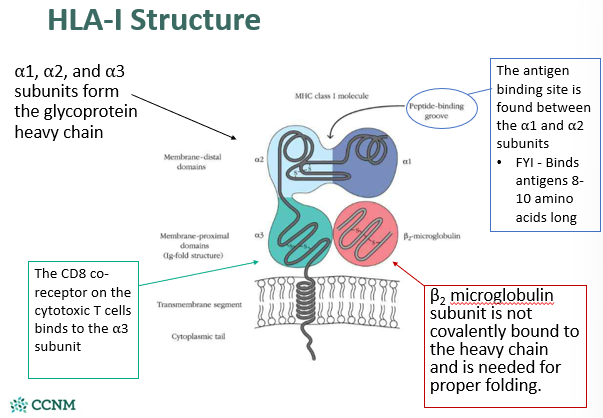
•HLA class I proteins interact with cytotoxic T-cells and binds intracellular antigens
§Interact with a CD8 co-receptor on the cytotoxic T-cell
•HLA class II proteins interact with T-helper cells and binds extracellular antigens
§Interacts with CD4 co-receptor on the T-helper cell
describe the structure of HLA-1
describe the structure of HLA-2

explain how HLA molecules are polymorphic
•Each individual expresses about 6 different class I molecules and 12 different class II molecules
§HLA type I molecule subtypes are all indicated by the designation A, B, or C
§HLA type II molecule subtypes are all indicated by the designation “D”
what is the clinical application of HLA molecules polymorphism?
§There are hundreds and hundreds of different allelic variants of class I and class II molecules in the human population
•Differing allelic variants of class I and II HLA proteins is the main reason why we usually reject organs from randomly-matched organ donors
§The presence of particular allelic variants have been associated with increased risk of some autoimmune diseases
•Eg. HLA-B27 predisposes a person to ankylosing spondylitis
•Eg. HLA-DQ8 predisposes a person to celiac disease
on what cells are HLA-1 molecules expressed on?
•HLA-1 types are expressed on almost all nucleated cells
§Level of expression differs – highest expression level found on lymphocytes
•50,000 HLA-1 proteins on lymphocyte cell membranes
•Much, much less on fibroblasts, muscle cells, hepatocytes, and most neurons
•Other cells have intermediate levels of expression
§Cells without nuclei do not express HLA-1
what is the function of HLA-1 molecules?

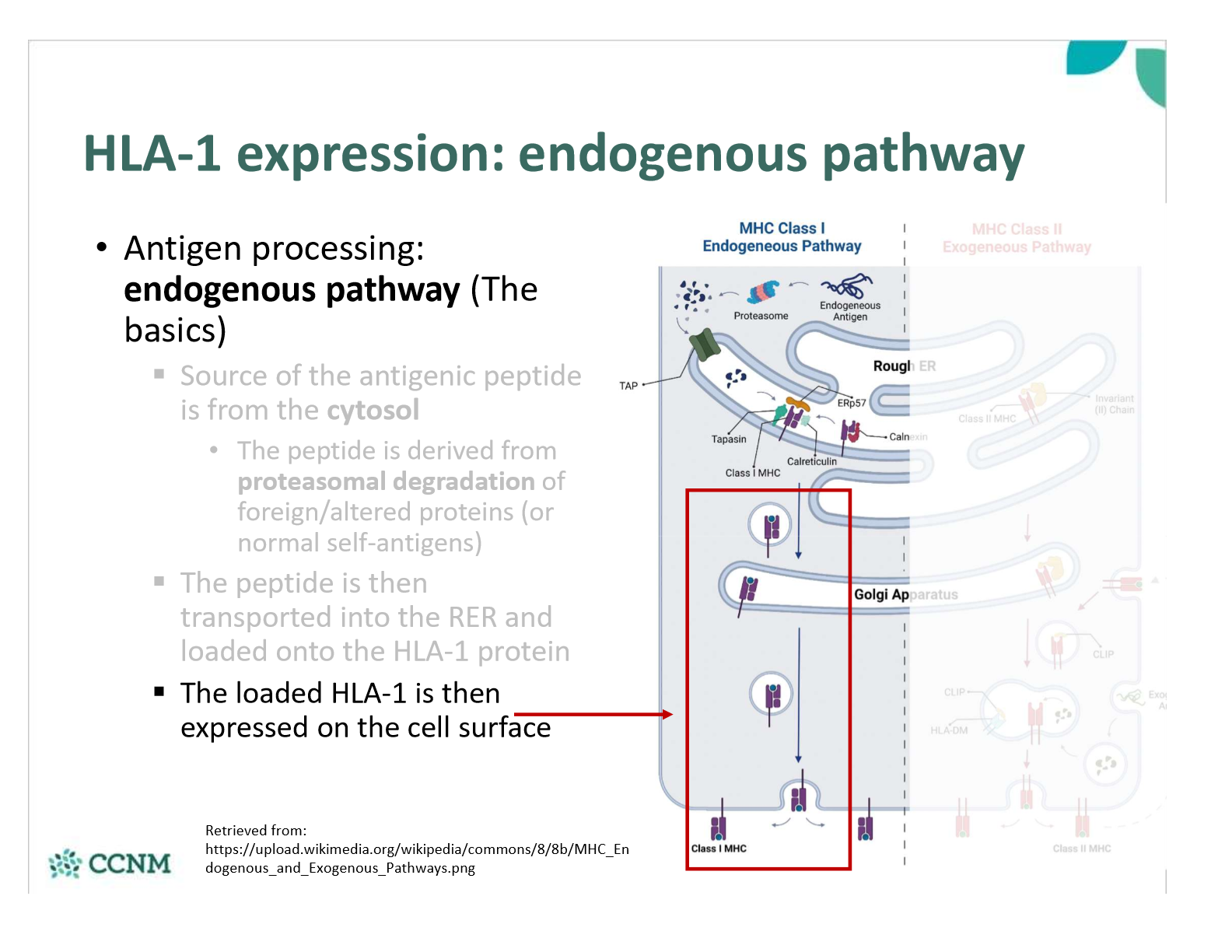
explain step 1 in the endogenous pathway?
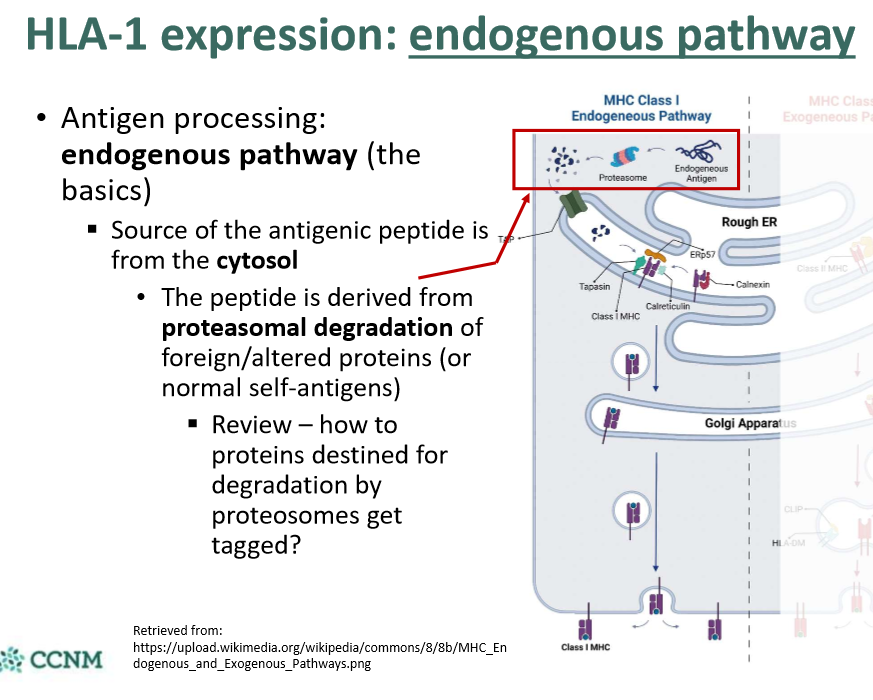
explain step 2 in the endogenous pathway?
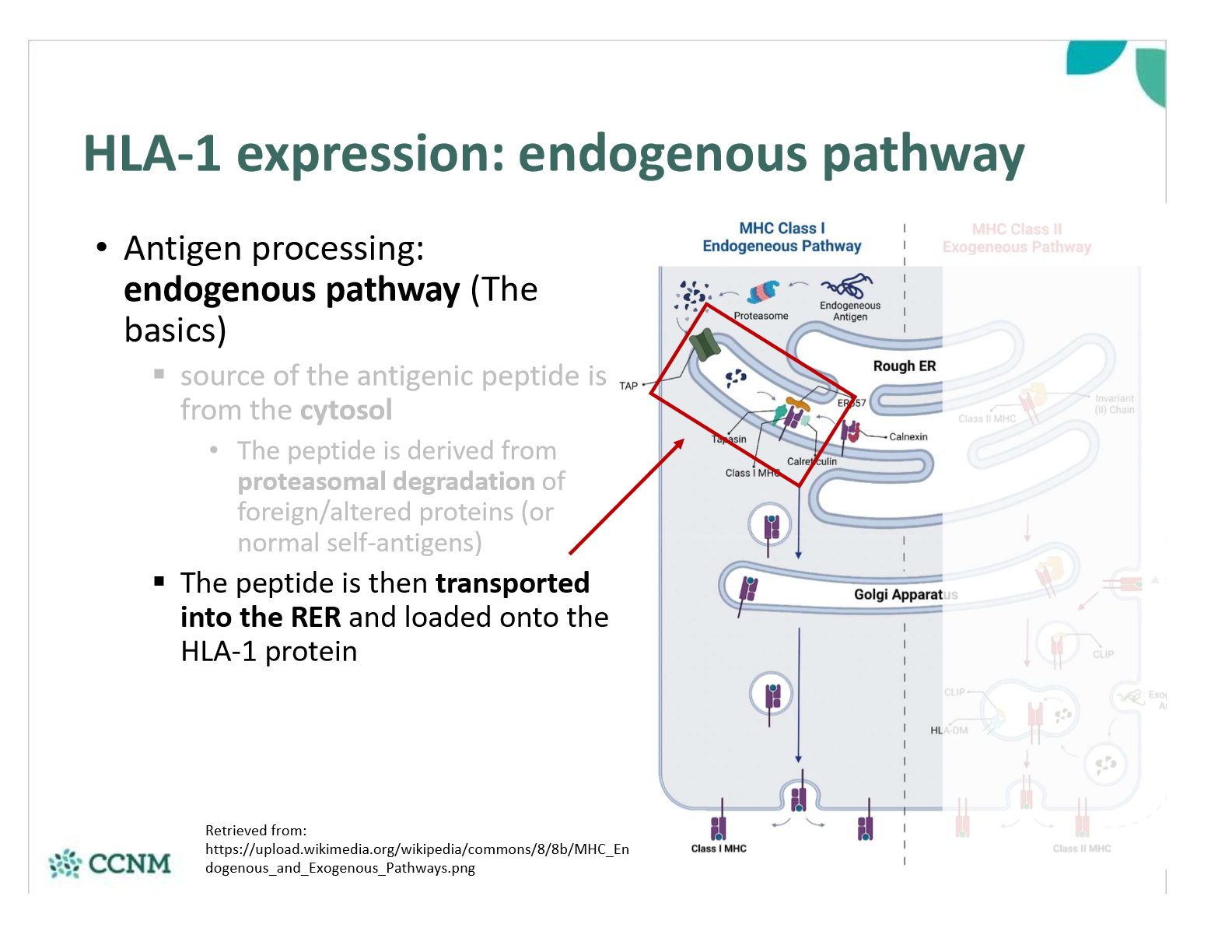
explain step 3 in the endogenous pathway?
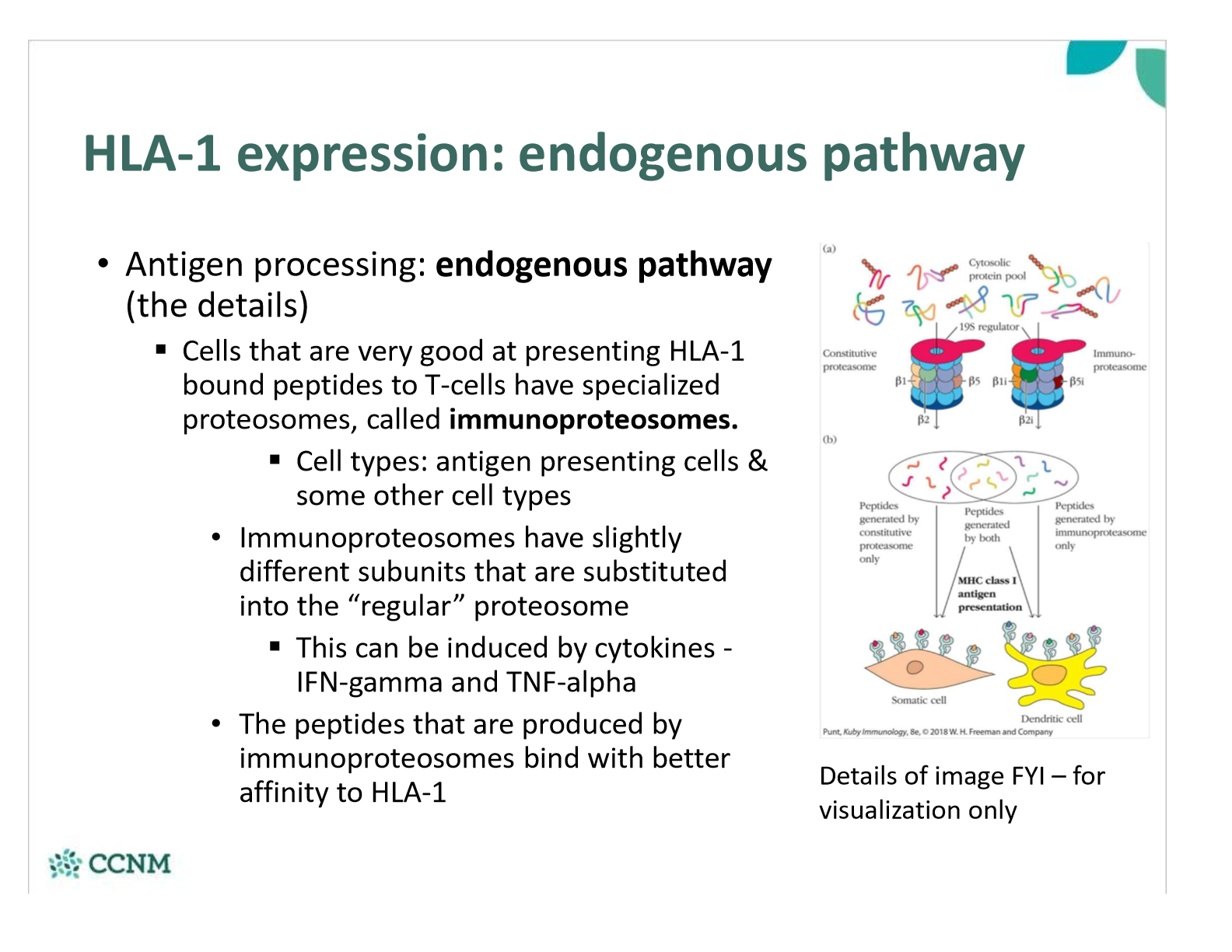
what are immunoproteosomes? how are they involved in the endogenous pathway?
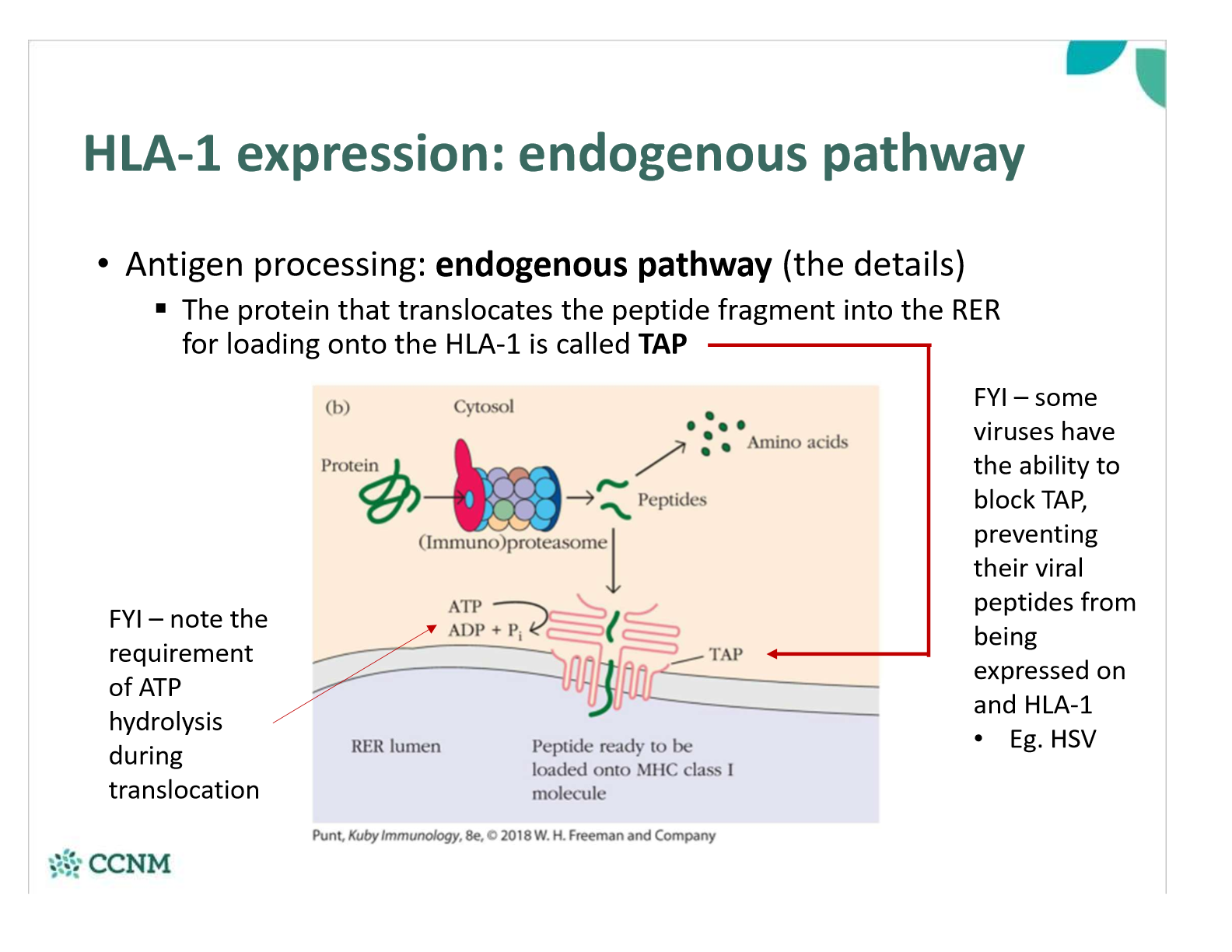
what is the role of TAP protein
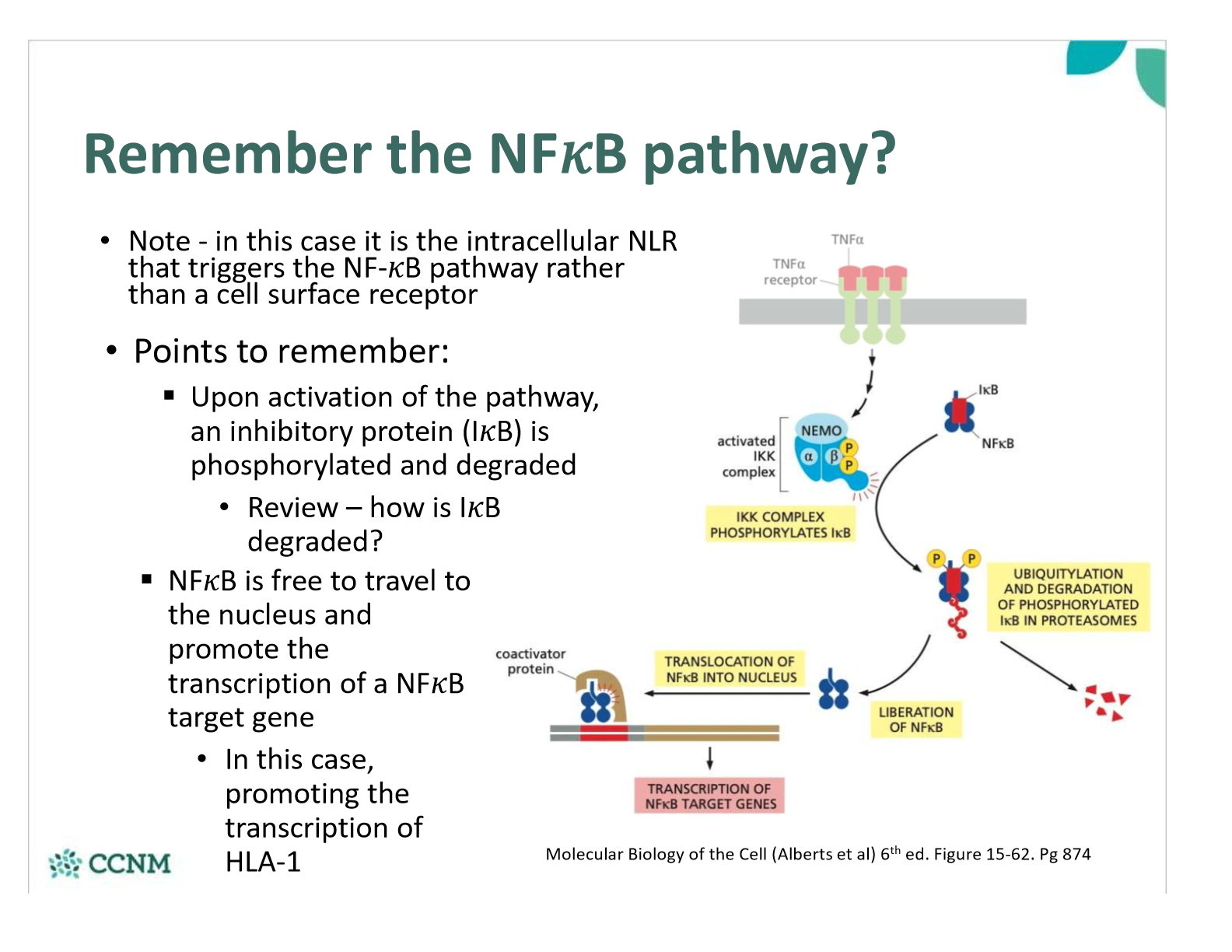
how do intracellular invaders effect transcription of HLA-1?

How is the NFkB pathway involved?
how do cytokines effect expression of HLA-1 molecules?
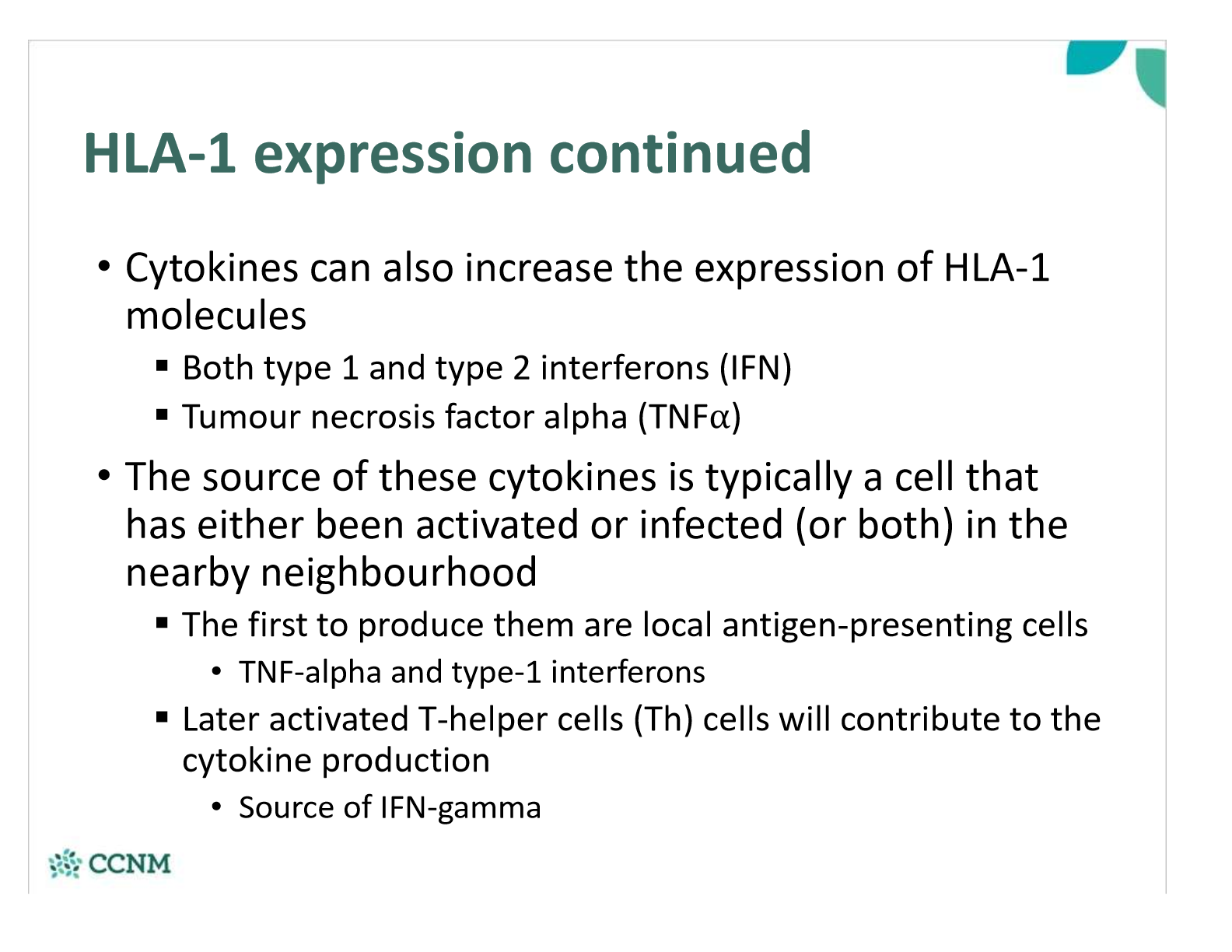
what happens once a peptide-bound HLA-1 is expressed on the cell surface?
what types of cells express HLA-2?
compare HLA-2 expression in dendritic cells, macrophages, B-cells and non-professional APCs

by which pathway do HLA-2 proteins bind extracellular antigens?
exogenous pathway
which cytokines up-regulate HLA-2 expression

what is the first step of the exogenous pathway?
phagocytosis
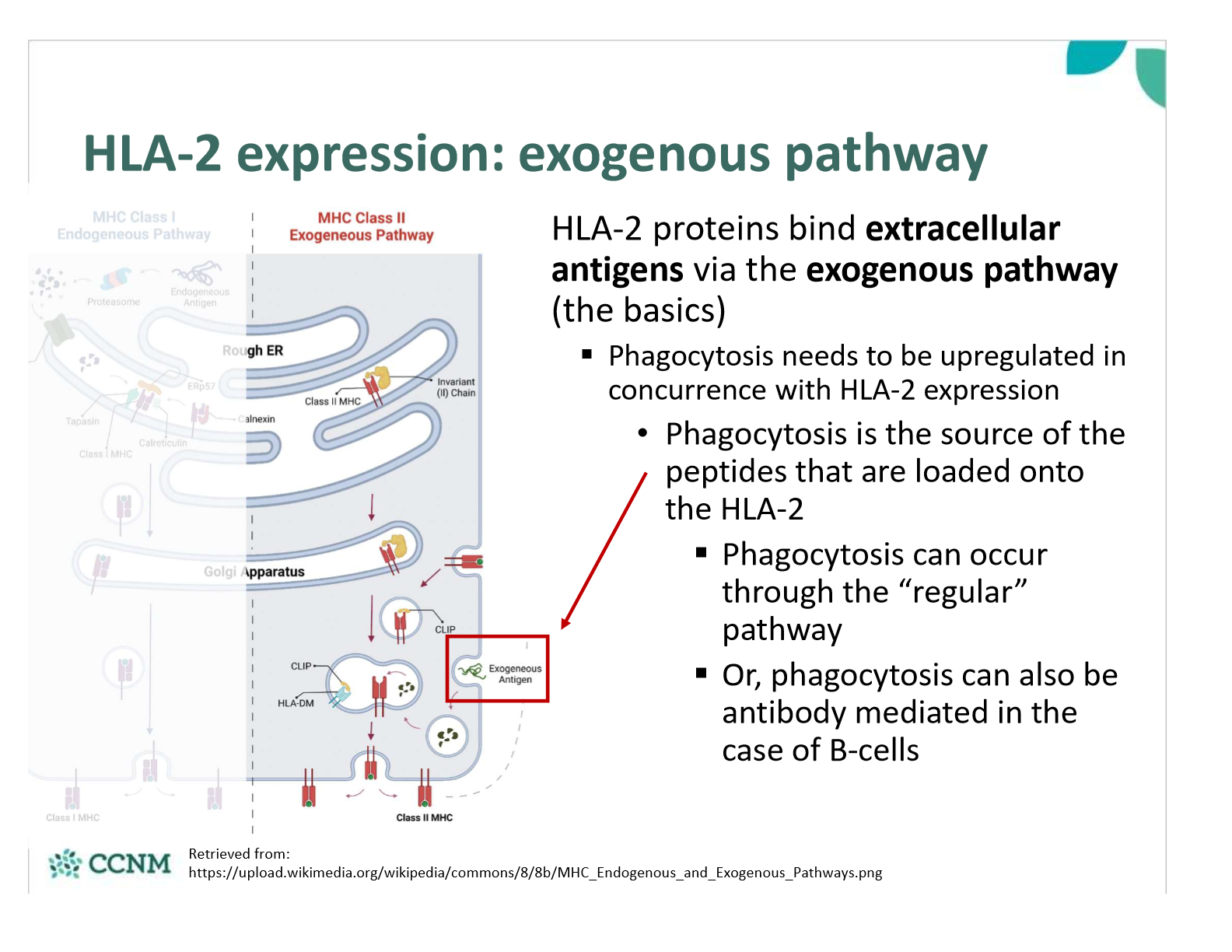
what is step 2 of the exogenous pathway?
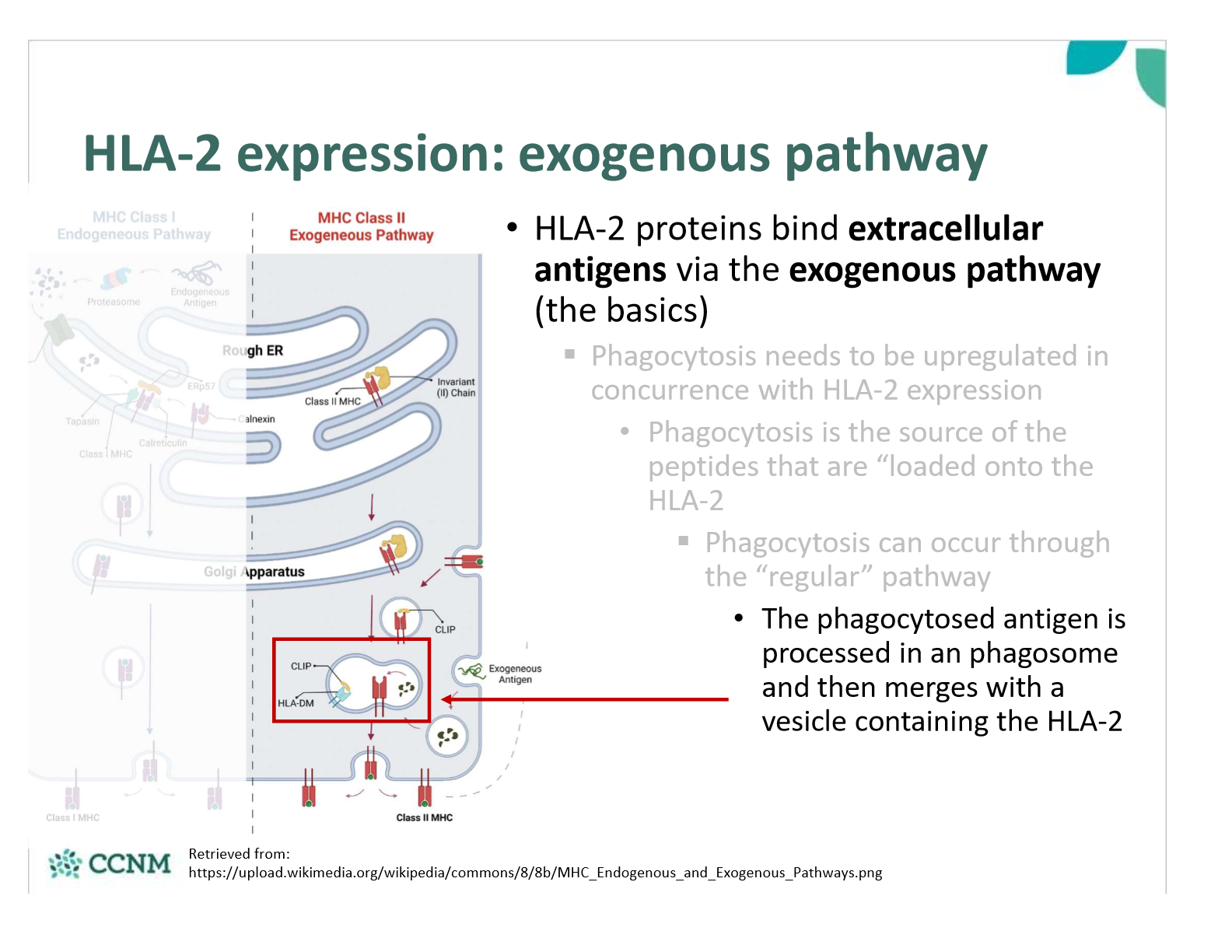
what is step 3 of the exogenous pathway

explain how antibodies are used in the exogenous pathway in B cells
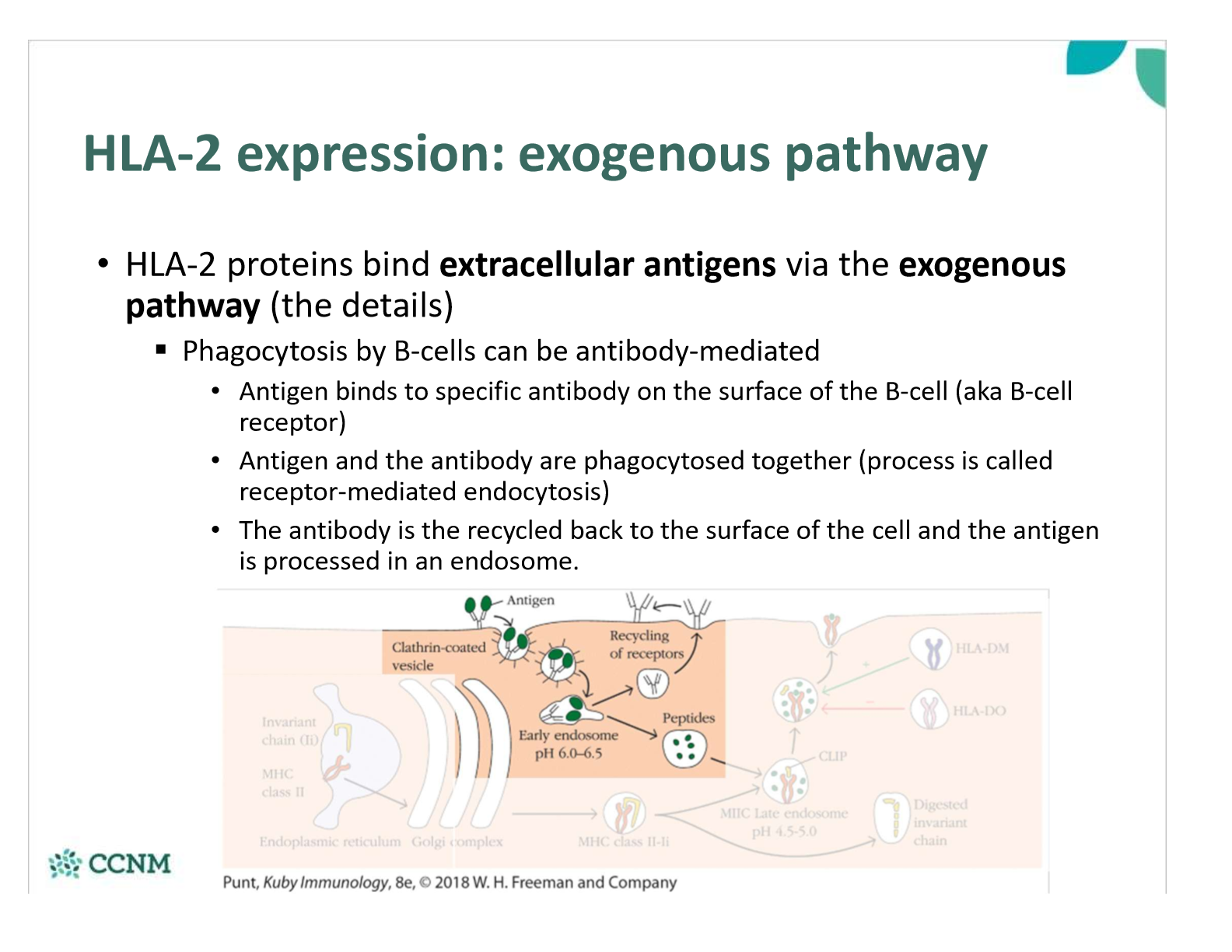
how do we ensure a cystolic antigen is not loaded onto HLA-2 (rather than HLA-1) in the RER?
In the RER, the HLA-2 protein associates with the invariant chain (FYI - aka CD74)
This prevents a cytosolic antigen from being loaded onto the HLA-2 while in the RER
As the HLA-2 containing vesicle merges with the phagosome/ endosome containing the antigen, the invariant chain is chopped up
The “chopped version” is called CLIP, and it will remain bound to the HLA-2 peptide binding region until displaced
When a peptide binds with sufficient affinity to HLA-2, CLIP is displaced
HLA-2 with bound extracellular antigen is then expressed on the surface of the cell
what determines whether antigenic peptides associate with HLA-1 or HLA-2? are there every exceptions?
§The mode of antigen entry into cells and the site of antigen processing determine whether antigenic peptides associate with:
•HLA class I molecules in the rough endoplasmic reticulum
•HLA class II molecules in endocytic compartments
•Under certain circumstances however, exogenous antigens may be assembled with MHC class I molecules via cross-presentation and vice versa.
in what circumstances are there exceptions?
Exogenous antigens can be presented by HLA-1, and endogenous antigens can be presented by HLA-2 in some circumstances:
1. An infected cell dies and is phagocytosed
•Viral particles in the cytosol of the infected cell will be presented on HLA-2 of the phagocyte (eg. Macrophage)
§Thus an “intracellular” antigens becomes extracellular antigens
•Autophagy can also results in peptides from the cytosol being presented on HLA-2
2. Cross-presentation – not fully understood
•Likely a blend of the exogenous and endogenous pathways
§Dendritic cells are best at cross presentation, but all antigen presenting cells can do it.
§Antigens can leak out of the endosome after phagocytosis into the cytosol, then the antigen can be presented to HLA-1
•Allows antigen presenting cells to either sequentially or simultaneously activate both T-helper and cytotoxic T cells
Once a peptide-bound HLA-2 is expressed on the surface of a cell, what happens next?
It can bind a CD4+ T-cells, ie. a helper T-cell, and activate it
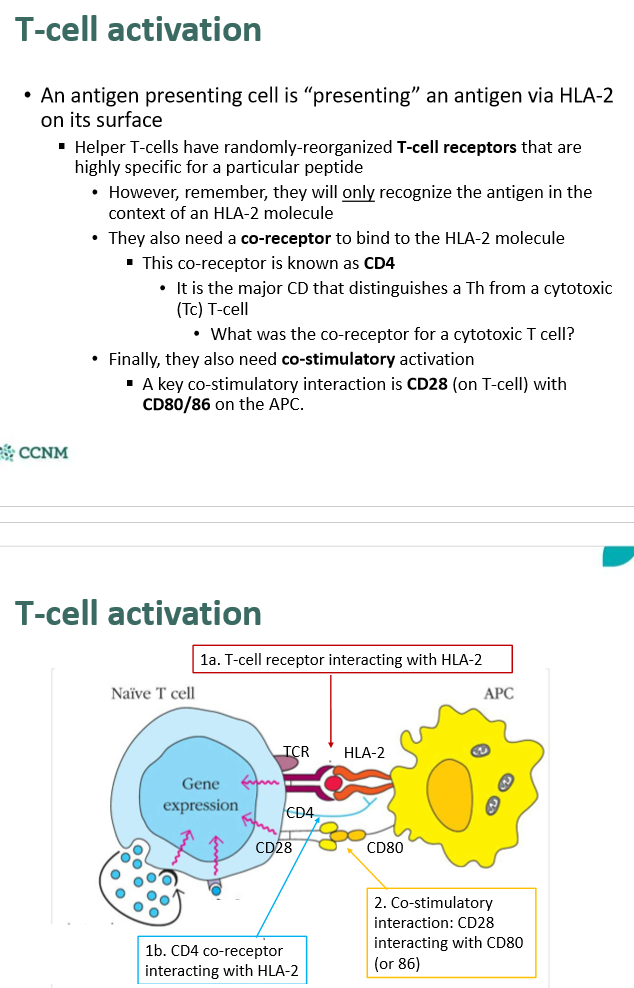
explain how T-cells get activated
which cells express CD80/86?
How does CD28 signaling add to the effects of TCR signaling described previously?
1. Enhances the strength of signal between the T-cell and the Antigen presenting cell
§Initiates a cell-signaling cascade to promote T cell survival and proliferation
•CD28 will recruit PI3 Kinase
compare cSMAC and pSMAC interactions between the T-call and the APC
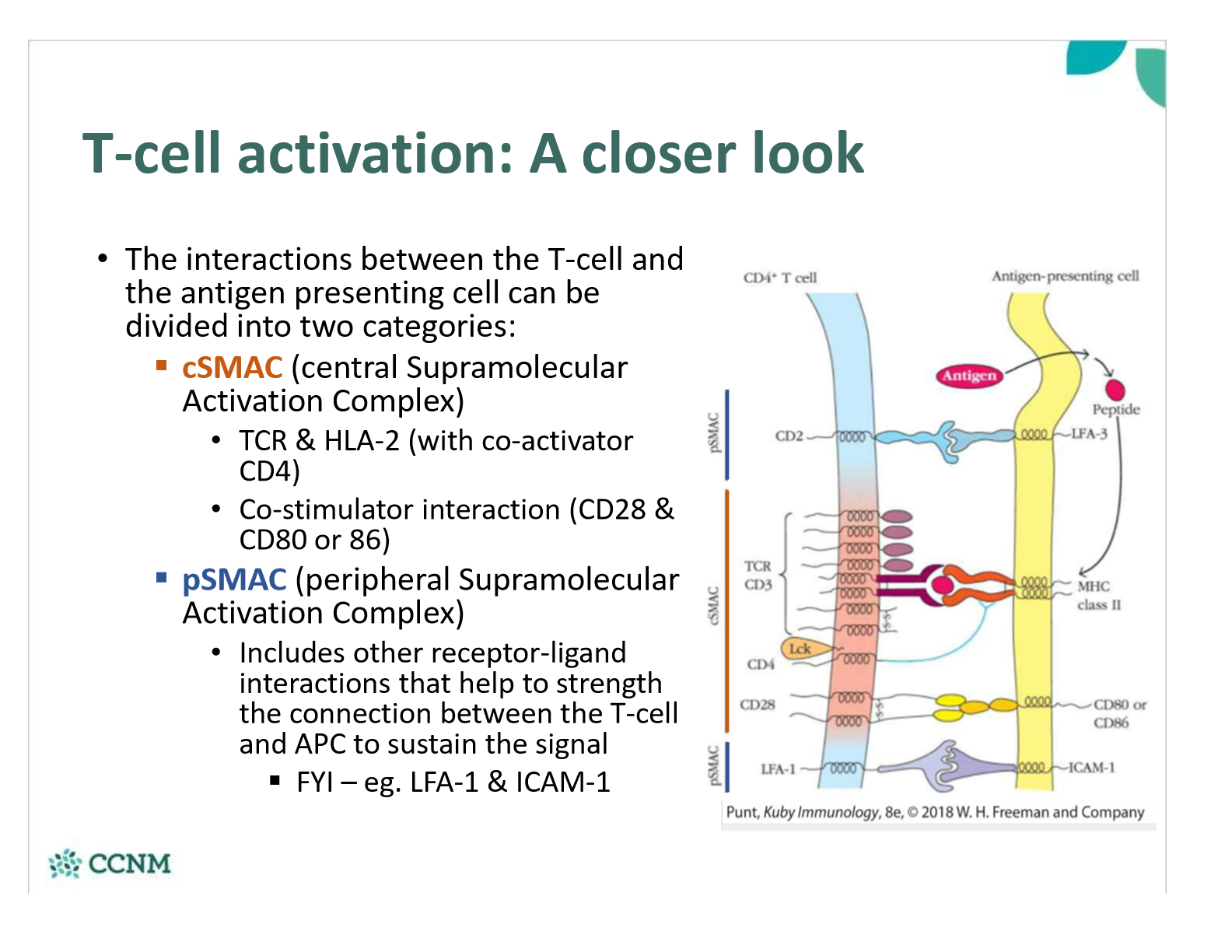
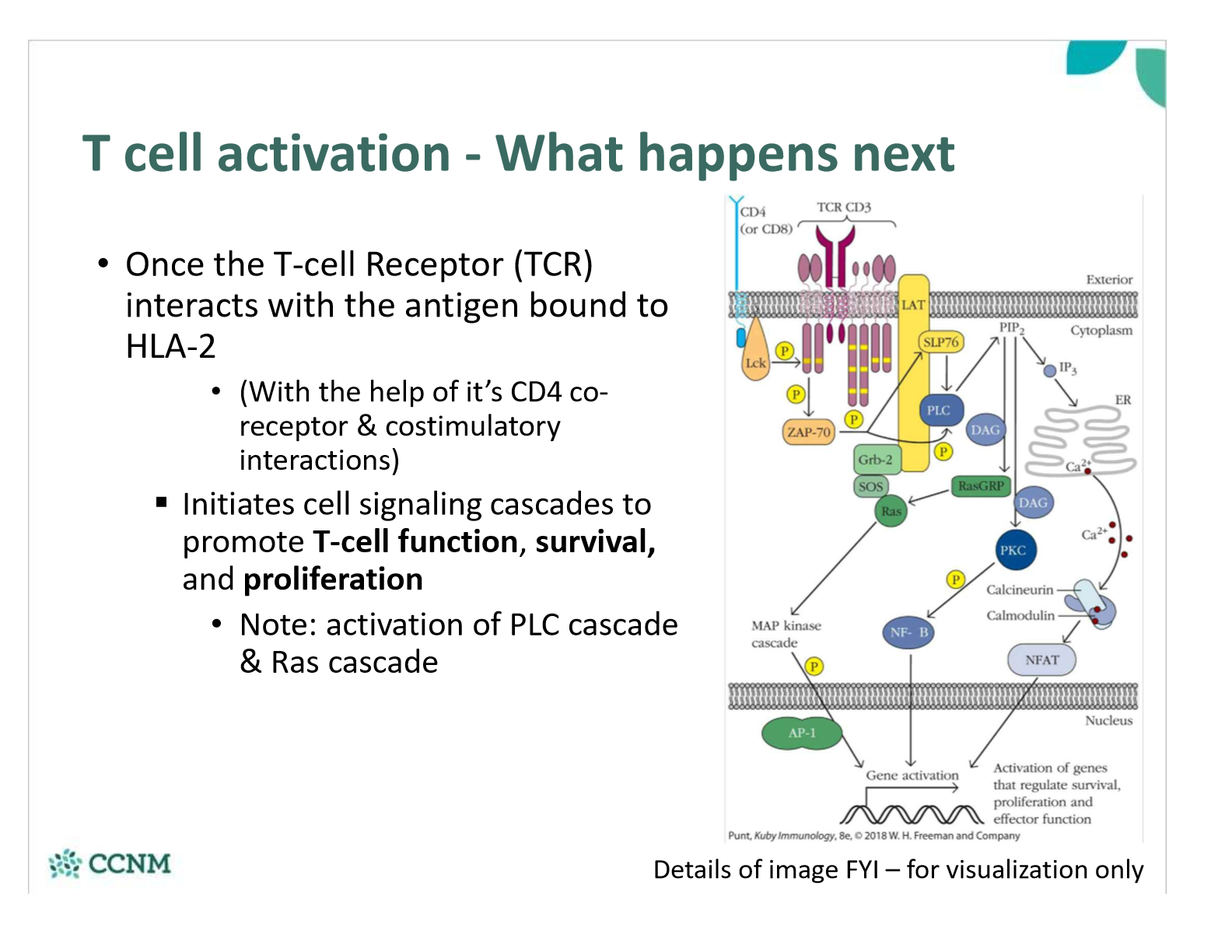
What happens once the T-cell Receptor (TCR) interacts with the antigen bound to HLA-2
explain the effect of paracrine signaling that occurs between APC and T-cell