topic 1 - states of matter and mixtures
0.0(0)
0.0(0)
Card Sorting
1/18
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
1
New cards
atom
the smallest neutral part of an element that can take part in chemical reactions
2
New cards
attractive forces
the weak forces of attraction between molecules
3
New cards
boiling point
the temperature at which an object boils
4
New cards
chemical properties
how a substance reacts with other substances
5
New cards
melting point
the temperature at which an object melts
6
New cards
molecule
particle consisting of two or more atoms joined by covalent bonding
7
New cards
particle
a tiny piece of matter that everything is made out of
8
New cards
particle model
a theory to explain the different properties and observations of solids, liquids and gases
9
New cards
physical change
a change in which no new substances are formed - like changes of state
10
New cards
states of matter
solid, liquid, gas
11
New cards
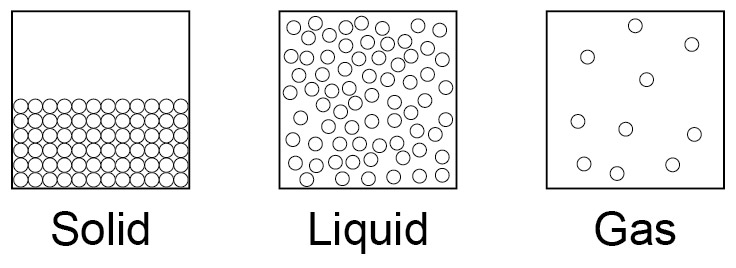
structure of a solid
* particles are closely packed (touching)
* particles are in a regular pattern
* keep their shape
* particles are in a regular pattern
* keep their shape
12
New cards
movement of a solid
* particles are vibrating on the spot
* particles do not move past each other
* particles do not move past each other
13
New cards
energy of a solid
low energy
14
New cards
structure of a liquid
* particles touch
* random/no pattern
* takes the shape of the container
* random/no pattern
* takes the shape of the container
15
New cards
movement of a liquid
* particles touch
* some gaps
* move freely
* some gaps
* move freely
16
New cards
energy of a liquid
medium energy
17
New cards
structure of a gas
* no pattern
* fill the container
* fill the container
18
New cards
movement of a gas
* move freely
* rapid and random
* rapid and random
19
New cards
energy of a gas
high energy