Alkynes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
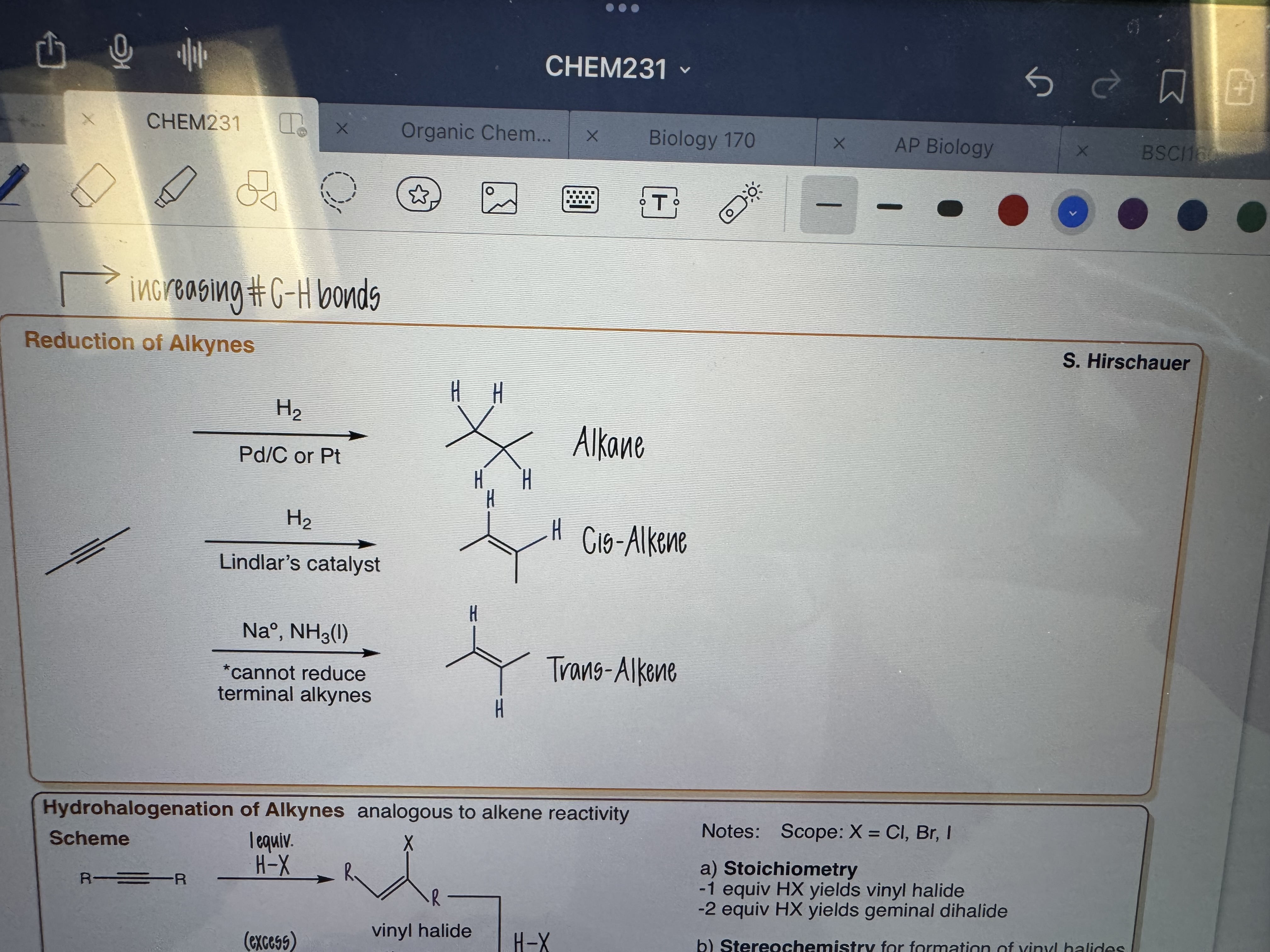
Reductions of Alkynes
a) 1) H2 2) Pd/Pt
b) 1) H2 2) Lindlars Catalyst
c) 1) Na^• , NH3(I)
- Cannot reduce terminal alkynes
Can be cis or trans
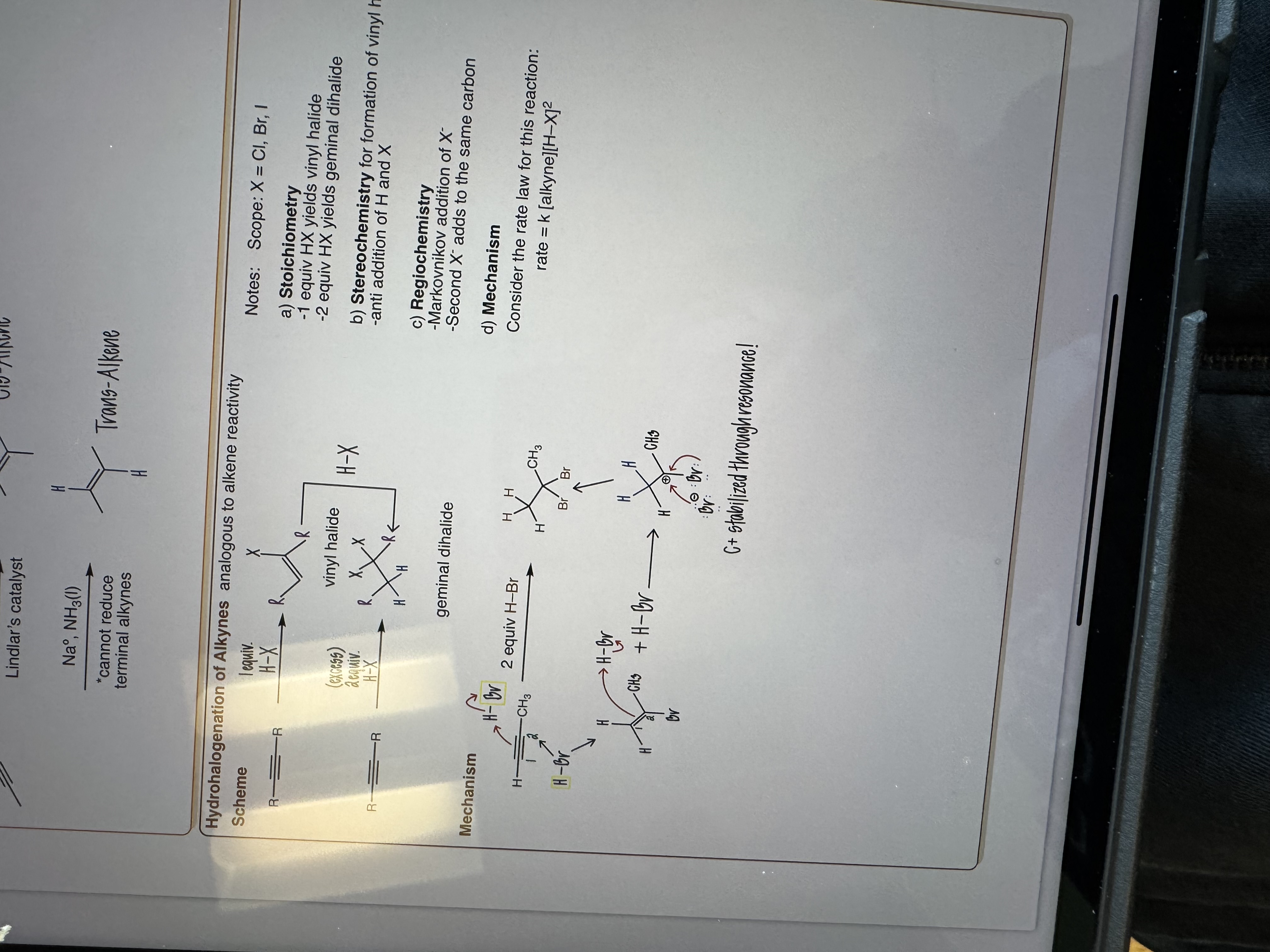
Hydrohalogen of Alkynes
H-X
Excess is 2 equiv
Scope: X = Cl, Br, I
Stoichiometry: 1 eq hx yields vinyl halide (1 bonded to C) , 2 equiv HX yields germinal halide (2 bonded to same C)
Stereochemistry for Formation of vinyl halides: Anti addition of H and X
Regiochemistry: Markovnikov addition of 1st X, 2nd X adds to same carbon
Mechanism: rate = k(alkyne)(H-X)²
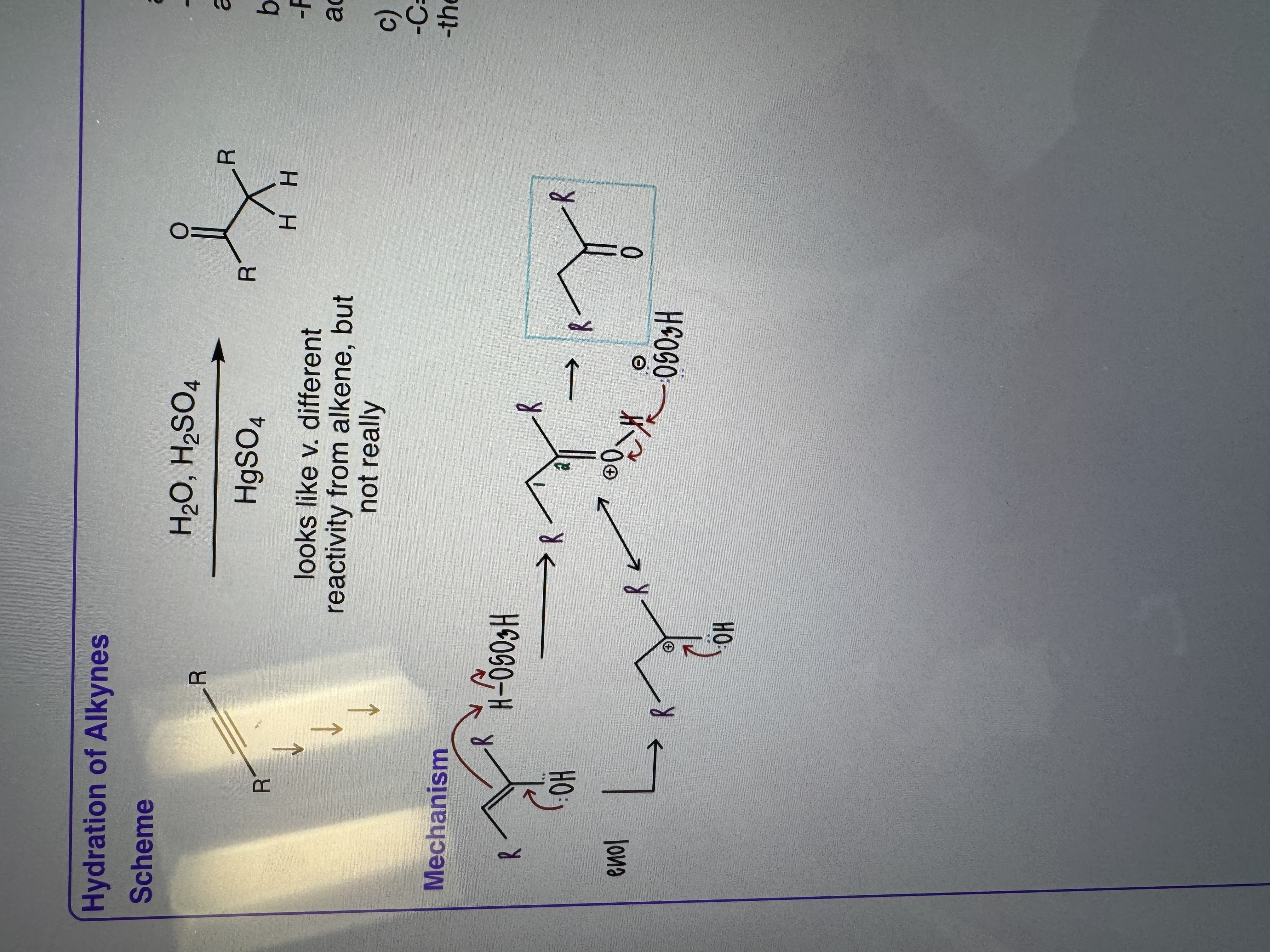
Hydration of Alkynes
a) Reagents
Step 1) H2O, HgSO4 catalyst
Step 2) H2SO4 (or H3O+) for alkynes
b) Regioselectivity
For terminal alkynes, Markovnikov
addition of water occurs
c) keto/enol tautomerization
-C=C DB less stable than C=O DB therefore the enol form is less stable than the keto form
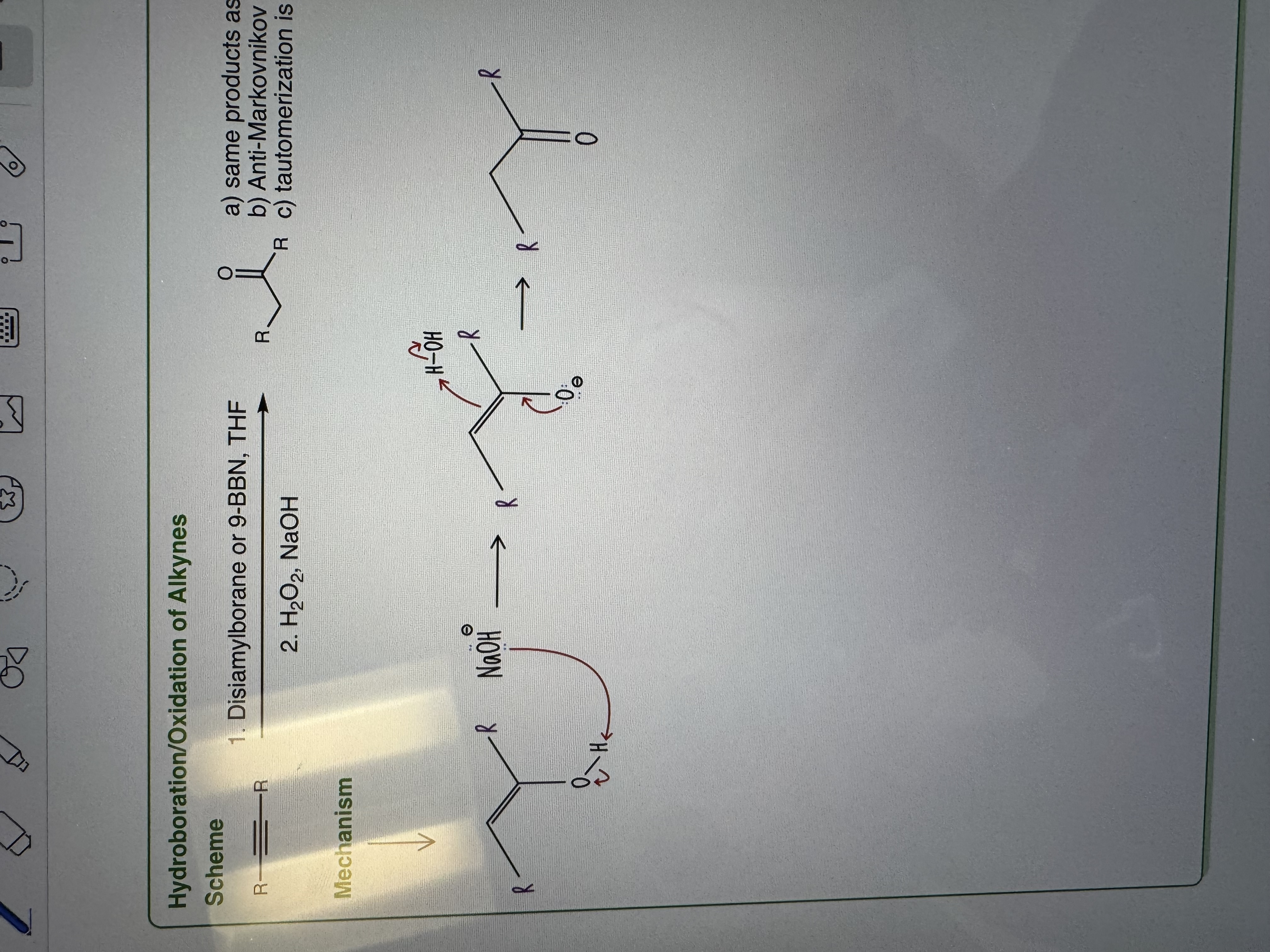
Hydroboration/Oxidation
1) Disiamylborane or 9-BBN, THF
2) H2O2, NaOH
-same products as hydration if internal alkyne
b) Anti-Markovnikov addition of OH if terminal alkyne
c) tautomerization is base-catalyzed
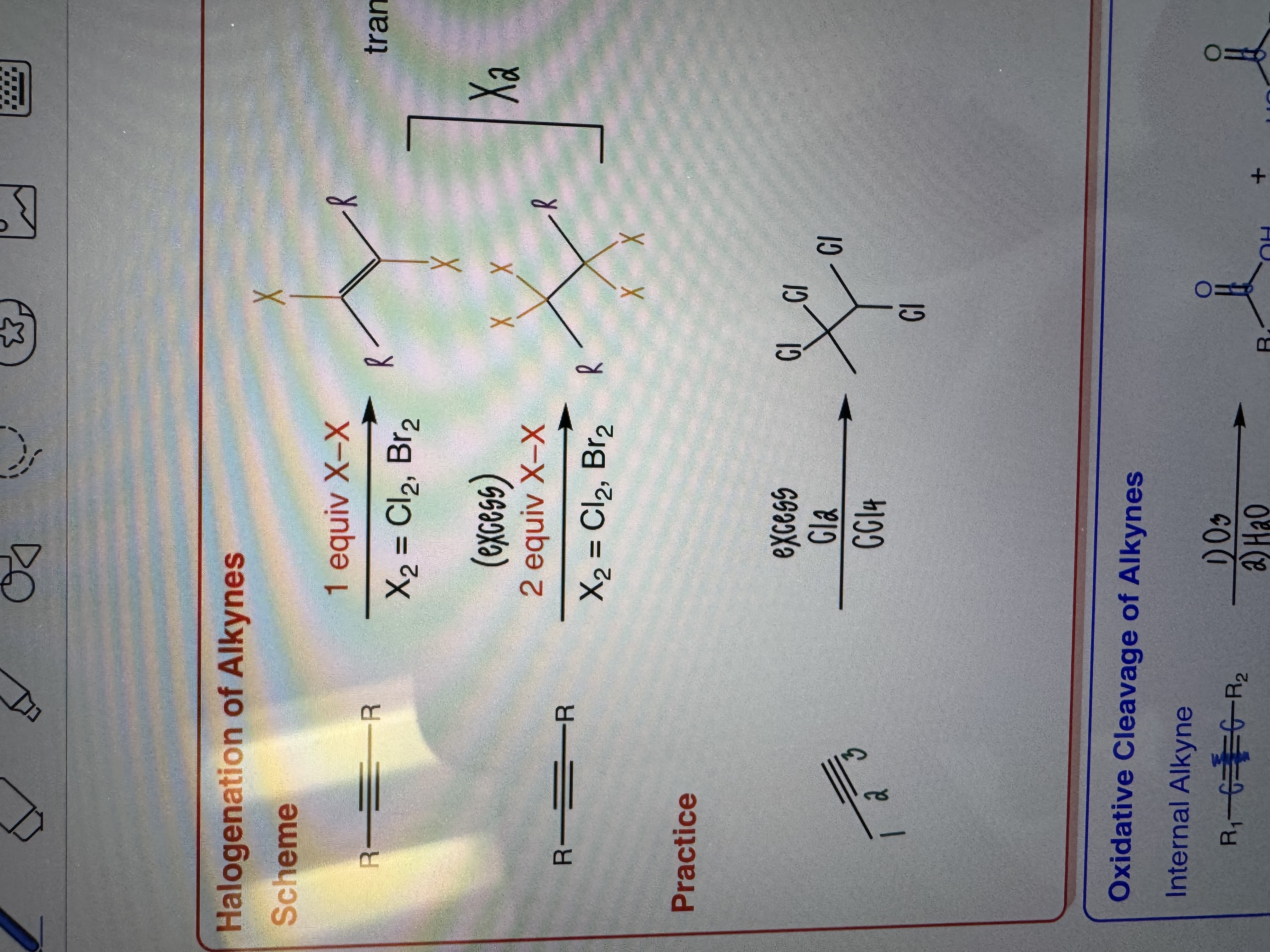
Halogenation of Alkynes
1 or 2 equiv X-X
X2 = Cl2, Br2
-One equiv yield trans vinyl dihalide
-Two equiv yields tetrahalide
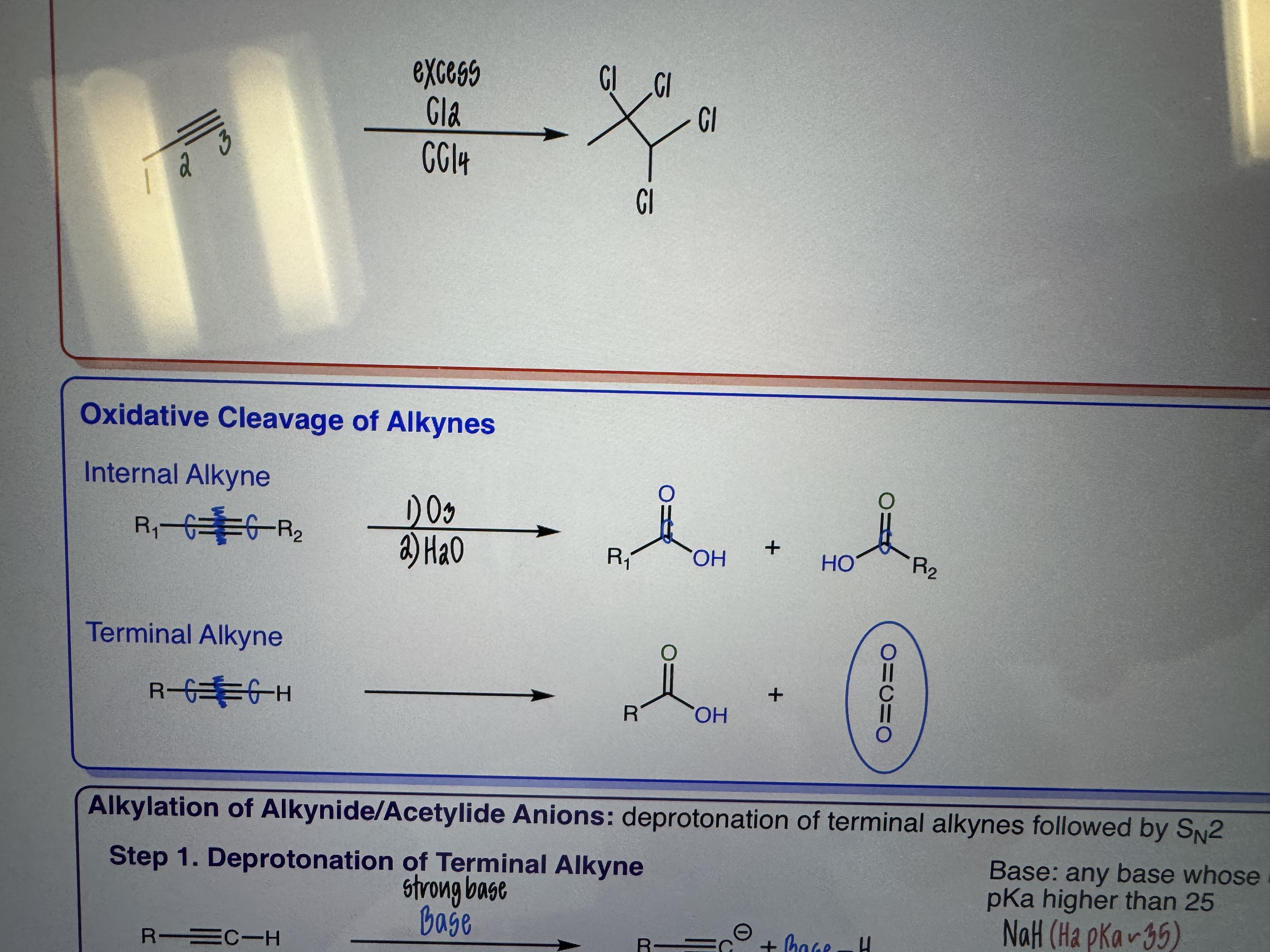
Oxidative Cleavage of Alkynes
1) O3
2) H2O
Internal and Terminal
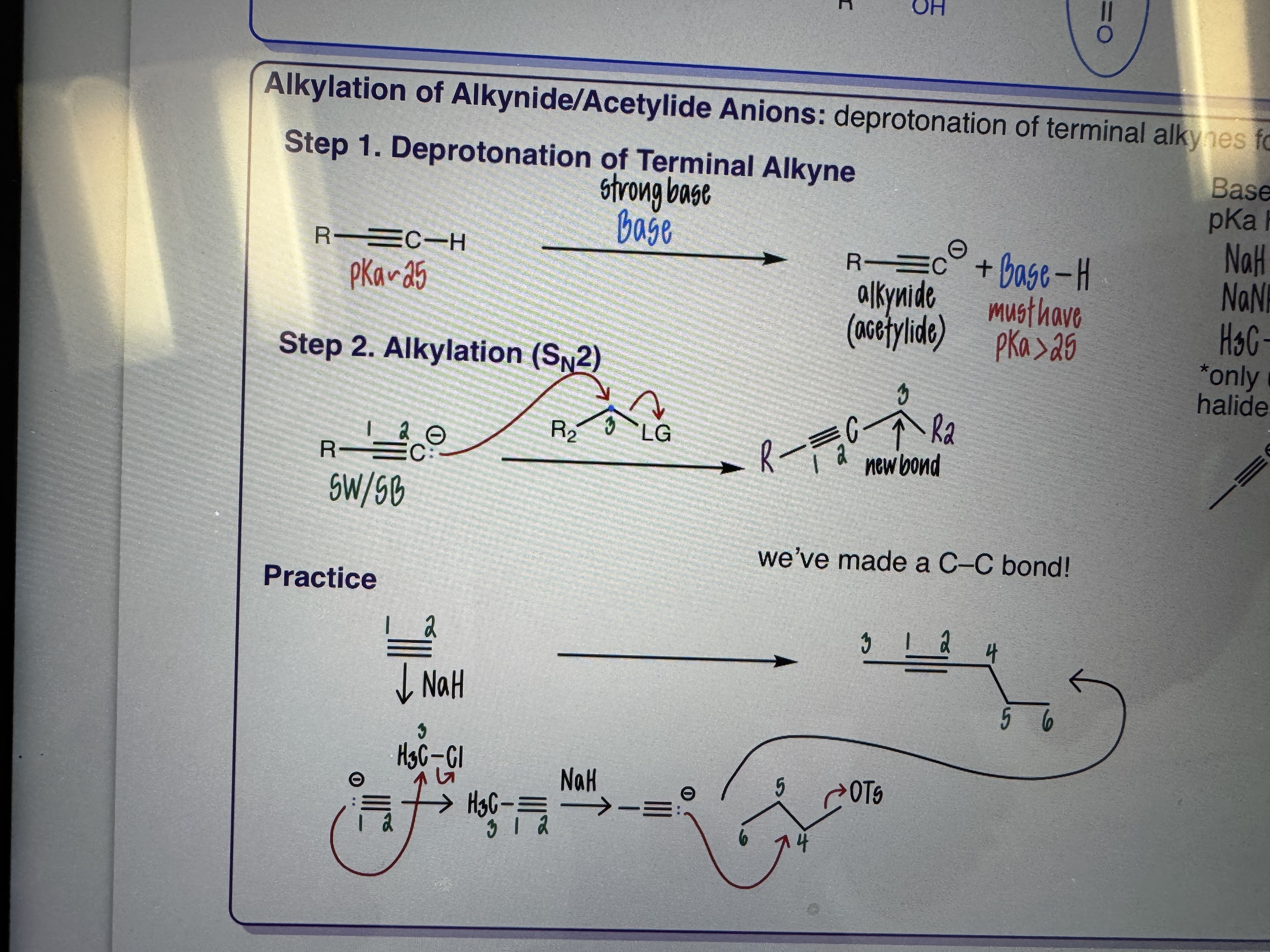
Alkylation of Alkynide/Acetylide Anions
Deprotonation of terminal alkynes followed by SN2
Base: any base who’s conjugate acid has a pKa higher than 25
NaH
NaH2
H3C-Li
Only useful with methyl or primary alkyl halides (otherwise E2 major)
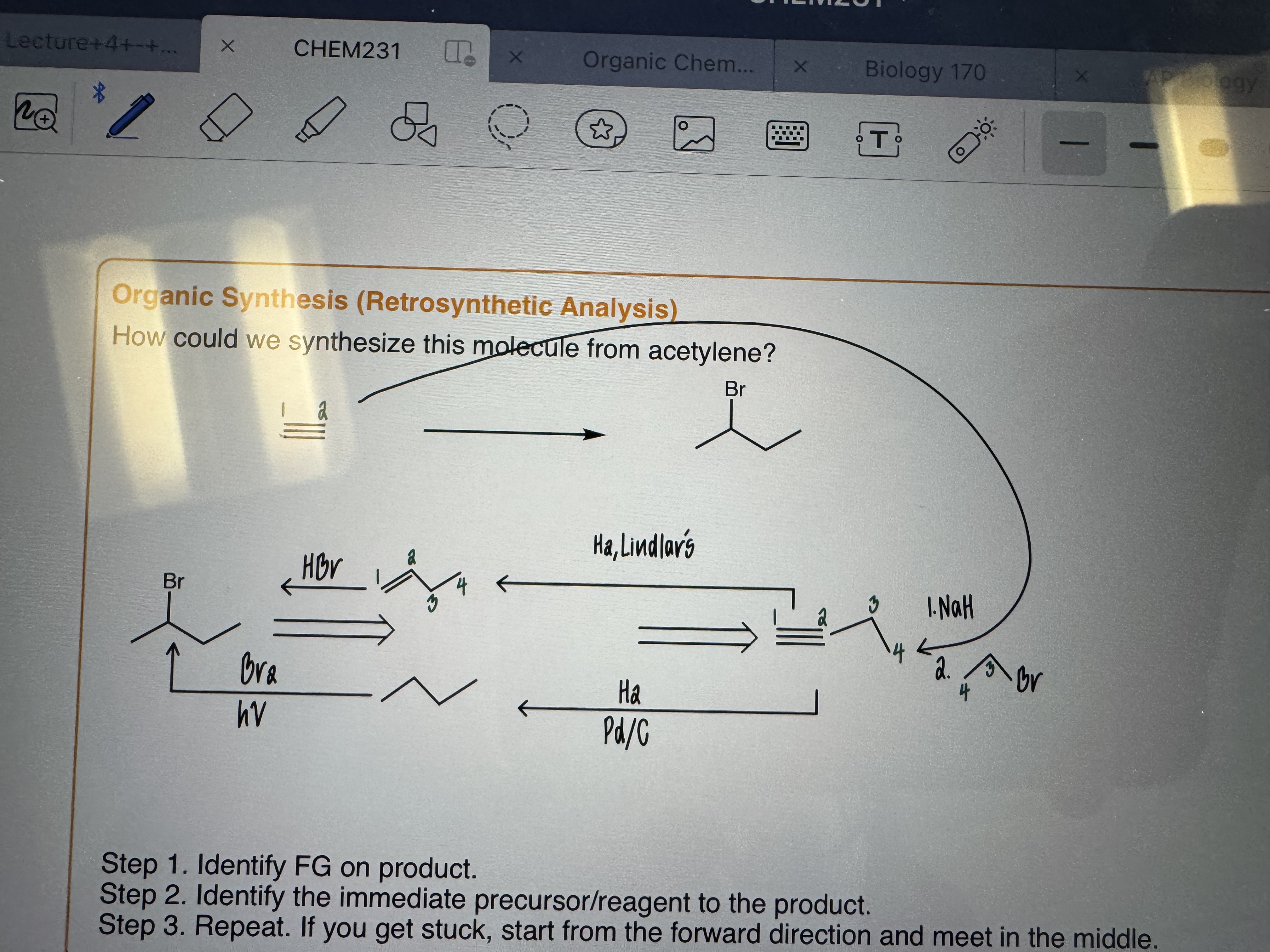
Organic Synthesis (Retrosynthetic Analysis)
Identity FG in product
Identify the immediate precursor/reagent to the product.
Repeat. If you get stuck, start from the forward direction and meet in the middle.