Shapes of Molecules and VSEPR Theory
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Electron Pair repulsion theory
the shape of a molecule or ion is governed by the arrangement of the electron pairs around the central atom
VSEPR Theory
groups of electrons around the atom will repel each other and therefor take up positions in space as far apart as possible
group of electrons are;
shared pair forming a bond
4 electrons forming a double bond
6 electrons forming a triple bond
unshared pair of electrons - lone pair
order of repulsion
lone pair lone pair > bonding pair - lone pair > bonding pair - bonding pair
lone pairs decrease the b.p - b.p angle by
2-2.5 degrees
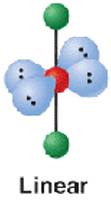
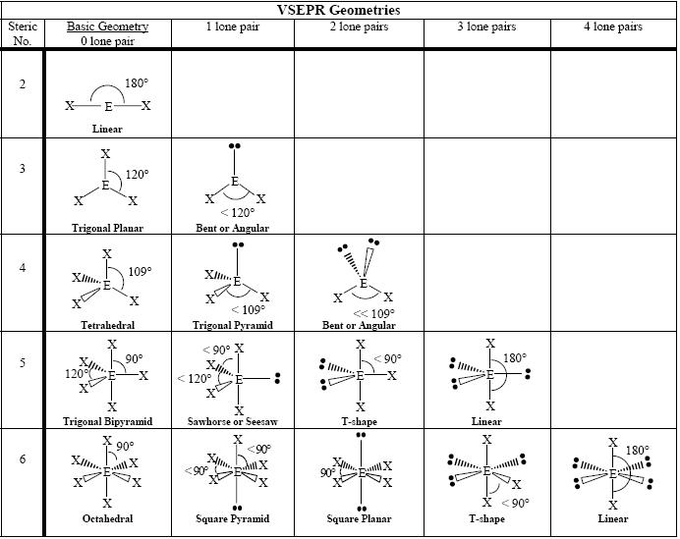
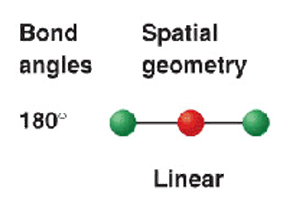
2 electron groups (2.bp)
shape name, bond angle, examples
linear
180*
BeCl2
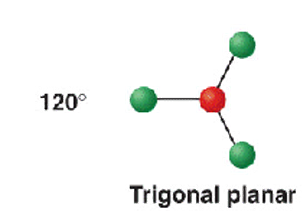
3 electron groups - 3 b.p
shape name, bond angle, examples
trigonal planar
120
BF3 BCl3
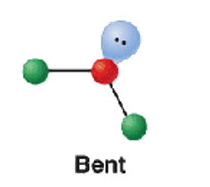
3 electron groups - 2 b.p + 1 l.p
shape name, bond angle, examples
bent/ v shape
120
SO2
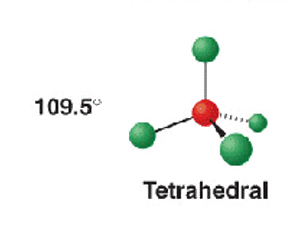
4 electron groups - 4 b.p
shape name, bond angle, examples
tetrahedral
CH4
109.5
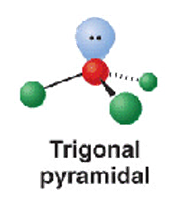
4 electron groups - 3 b.p + 1 l.p
shape name, bond angle, examples
trigonal pyramidal
107
NH3 and H3O+
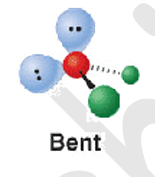
4 electron groups - 2.bp -2.bp
shape name, bond angle, examples
bent
105
h20
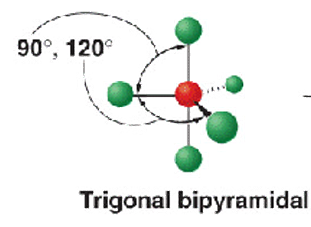
5 electron groups - 5 b.p
shape name, bond angle, examples
trigonal bipyramidal
90 and 120
PCl5
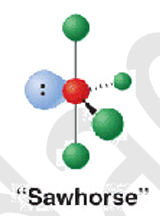
5 electron groups - 4 b.p - 1.l.p
shape name, bond angle, examples
seesaw or sawhorse
90 120
SF4 and XeO2F2
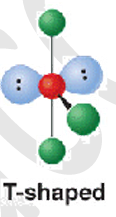
5 electron groups - 3 b.p + 2 l.p
shape name, bond angle, examples
t- shaped
90
ClF3
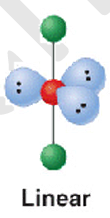
5 electron pairs; 2 b.p + 3 l.p
shape name, bond angle, examples
linear
180
I3-
6 electron pairs - 6 b.p
shape name, bond angle, examples
octahedral
90
SF6
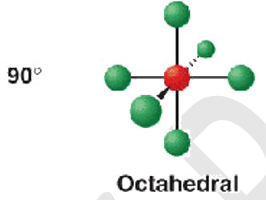
6 electron pairs - 5 b.p + 1 l.p
shape name, bond angle, examples
square pyramidal
90
XeOF4
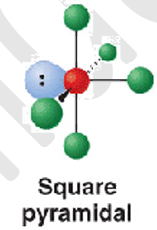
6 electron pairs - 4 b.p + 2 l.p
shape name, bond angle, examples
square planar
90
ClF4- and XeF4
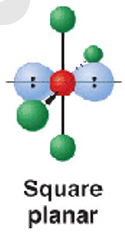
6 electron pairs- 3.bp + 3 l.p
shape name, bond angle, examples
T- shaped
90
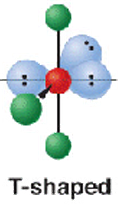
6 electron groups 2 B.P + 4l.p
shape name, bond angle, examples
linear
180