Biochem Exam 1 Quesitons
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Pyruvic acid is a monoprotic acid with a pKa value of 2.6. What are the concentrations of the protonated (HA) and deprotonated (A-) forms of pyruvic acid in a 50 mM aqueous solution of pyruvic acid when the pH = 3.3?
a. [HA] = 42mM, [A-] = 8mM
b. [HA] = 40mM, [A-] = 10mM
c. [HA] = 25mM, [A-] = 25mM
d. [HA] = 8mM, [A-] = 42mM
e. [HA] = 5mM, [A-] = 45mM
d. [HA] = 8mM, [A-] = 42mM
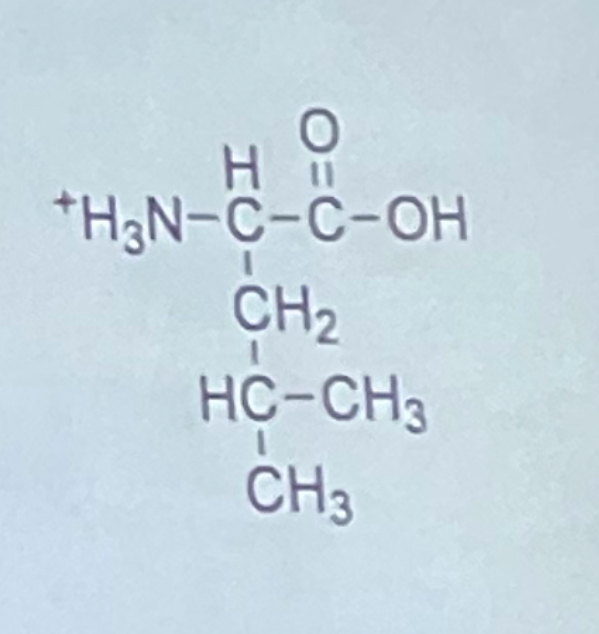
What is the one letter code for the amino acid shown at the right?
a. I
b. L
c. M
d. Q
e. V
b. L
Which of the following is a correct description of oleic acid?
a. it is a C18 saturated fatty acid
b. it is a C18 omega-6 fatty acid
c. it is a C16 omega-3 fatty acid
d. it is a C18 omega-9 fatty acid
e. it is a C16 omega-6 fatty acid
d. it is a C18 omega-9 fatty acid
What is the pH of an aqueous solution of 5.0 × 10^-7 M HCl?
a. 5.10
b. 6.22
c. 6.30
d. 7.00
e. 6.90
b. 6.22
Which of the following IS NOT contained within the amide plans of the peptide backbone?
a. Cα carbon
b. amide nitrogen
c. side chain carbons
d. carbonyl carbon
e. none, all are included
c. side chain carbons
The symbol Ψ in a Ramachandran plot provides what information about a polypeptide?
a. the amino acid residues that form α-helices
b. the amino acid residues that form β-sheets
c. the rotational angle between the amide nitrogen & the carbonyl
d. the rotational angles between the carbonyl & the Cα
e. the rotational angles between the side chains & the Cα
d. the rotational angles between the carbonyl & the Cα

Shown below are two disaccharides. Which of the following descriptions of the linkage found in these two disaccharides is correct?
a. A is 1,2 and B is 3,2
b. A is 1,3 and B is 3,3
c. A is 1,3 and B is 4,3
d. A is 1,2 and B is 4,2
e. A is 5,3 and B is 4,4
b. A is 1,3 and B is 3,3
This questions deals with the hydrophobic effect. Choose the correct letter that best fills in the blanks and completes the sentence.
The association is driven because the water is more ________ when the non-polar molecules are associated with each other, and lowering the temperature would _________ the strength of the hydrophobic effect.
a. ordered; increase
b. ordered; decrease
c. disordered; increase
d. disordered; decrease
e. hydrophilic; not impact
d. disordered; decrease
If the following section of a polypeptide is folded into an α-helix, to which amino acid is the carbonyl group of valine hydrogen bonded?
V-S-A-D-E-G-L
a. Leu
b. Ser
c. Val
d. Glu
e. Asp
d. Glu
Lipid anchored proteins containing farnesyl and geranyl groups are attached to proteins via which of the following linkages?
a. amide bond
b. ether bond
c. ester bond
d. thioether bond
e. thioester bond
d. thioether bond
Shown below are the first twenty amino acids of liver alcohol dehydrogenase:
M-S-T-Y-G-Q-V-I-R-C-K-A-A-I-V-W-K-P-G-A
Which of the following statements is NOT correct?
a. cyanogen bromide treatment produces free methionine
b. chymotrypsin treatment yields a tripeptide
c. chymotrypsin treatment yields a tetrapeptide
d. trypsin treatment yields tripeptide
e. trypsin treatment yields a dipeptide
b. chymotrypsin treatment yields a tripeptide
To which amino acid are carbohydrates chemically linked to form glycoproteins?
a. Q
b. C
c. H
d. N
e. Y
d. N
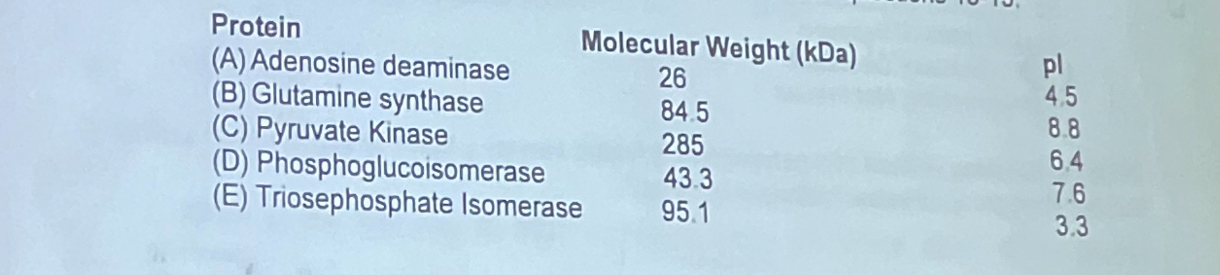
Shown below are the physical and chemical properties of five different proteins. Use this information to pick the protein that fits the descriptions listed in questions 13-15.
Which protein migrates furthest upon gel electrophoresis in SDS?
a. adenosine deaminase
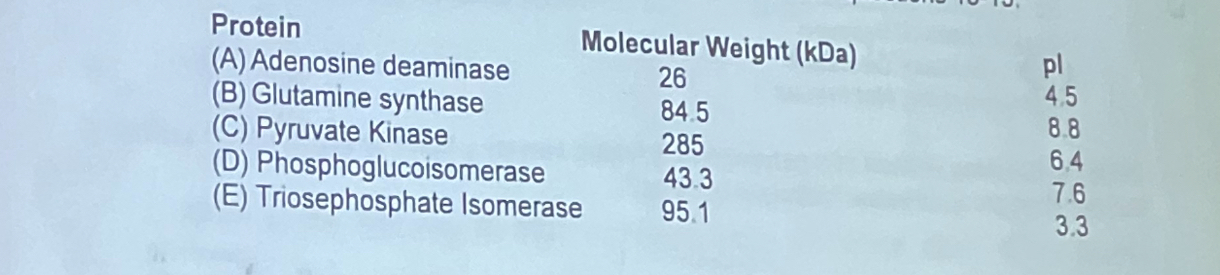
Shown below are the physical and chemical properties of five different proteins. Use this information to pick the protein that fits the descriptions listed in questions 13-15.
Which protein elutes second from a cation exchange column?
a. adenosine deaminase
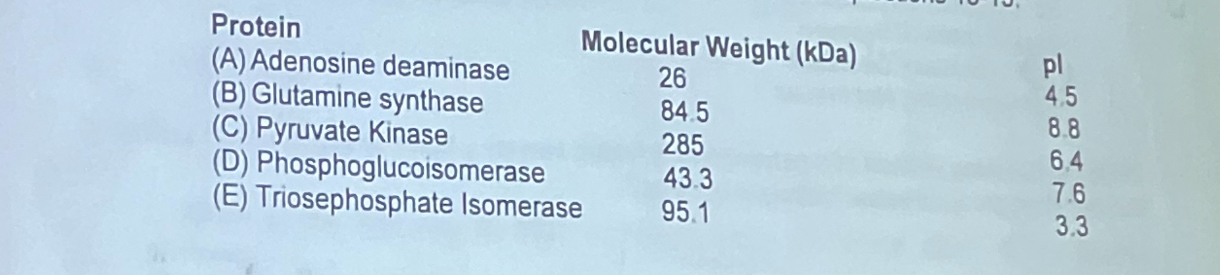
Shown below are the physical and chemical properties of five different proteins. Use this information to pick the protein that fits the descriptions listed in questions 13-15.
Which protein elutes first from a size exclusion column?
c. pyruvate kinase
Shown below is the primary sequence of a peptide. Use this information to answer questions 16-18
W-H-I-S-P-E-R
What are the pKa values of the ionizable groups found in this peptide?
a. 3, 3.9, 6, 8, 10.1, 13
b. 2, 3.9, 6, 8, 12.5, 13
c. 3, 4.3, 6, 8, 9.5, 13
d. 2, 4.3, 6, 9.5, 12.5, 13
e. 3, 4.3, 6, 8, 12.5, 13
e. 3, 4.3, 6, 8, 12.5, 13
Shown below is the primary sequence of a peptide. Use this information to answer questions 16-18
W-H-I-S-P-E-R
What is the estimated isoelectric point for this peptide?
a. 3.58
b. 5.15
c. 7
d. 10.25
e. 12.75
c. 7
Shown below is the primary sequence of a peptide. Use this information to answer questions 16-18
W-H-I-S-P-E-R
What is the estimated net change at pH 6.0 and pH = 10.1, respectively?
a. +0.5 and -1
b. +0.5 and -1.5
c. +1 and -1
d. +1 and -0.5
e. 0 and -1
a. +0.5 and -1
Shown below are four peptides, labeled A-E. Use these peptides to answer 19-20
a. Q-F-Y-A
b. I-K-M-D
c. A-R-G-L
d. E-P-C-N
e. V-I-T-S
Which of the peptides would be positively charged at pH 7.0?
c. A-R-G-L
Shown below are four peptides, labeled A-E. Use these peptides to answer 19-20
a. Q-F-Y-A
b. I-K-M-D
c. A-R-G-L
d. E-P-C-N
e. V-I-T-S
Which of the peptides can form disulfide bonds?
d. E-P-C-N