Energetics (exo and endo reactions)
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
What happens during any reaction?
Bonds are being broken and made
Breaking bonds requires the input of/releases energy
Making bonds requires the input of/releases energy
Breaking bonds requires the input of energy
Making bonds releases energy
Define what is meant by the term ‘exothermic reaction’
A reaction that releases thermal energy (heat) to the surroundings
Define what is meant by the term ‘endothermic reaction’
A reaction that absorbs thermal energy (heat) from the surroundings and so cause a decrease in the temp of the surroundings
Explain how the energy of the products compare to the energy in the reactants in an exothermic reaction? What happens to the temp of the surroundings AND the reaction mixture?
What about an endothermic reaction?
Exothermic
The products of the reaction have less (chemical) energy than the reactants
In the reaction, chemical energy (stored in the bonds of chemicals) is released and converted to heat energy
Temp increases bc chemical energy has been converted into thermal energy
Endothermic
The products of the reaction have more (chemical) energy than the reactants
In the reaction, heat energy of the surroundings is absorbed and converted into chemical energy (energy stored in the bonds of the chemicals)
Temp decreases heat energy has been converted into chemical energy
Exothermic or endothermic?
Combustion
Photosynthesis
Thermal decomposition
Respiration
Some types of electrolysis
Neutralisation of acids with alkalis
Reactions of metals with acids
The thermit process (a type of displacement between a metal and a metal oxide)
Combustion — exo
Displacement — exo
Photosynthesis — endo
Thermal decomposition — endo
Respiration — exo
Some types of electrolysis — endo
Neutralisation of acids with alkalis — exo
Reactions of metals with acids — exo
The thermit process (a type of displacement reaction between a metal and a metal oxide) — exo
Define the term enthalpy change (ΔH)
What is the unit?
The amount of heat energy taken in or given out in a chemical reaction (difference between the energy of the products and the energy of the reactants)
kJ/mol or kJ/mol-1
ΔH is given as a minus or plus sign to show whether heat is being given out or absorbed by the reaction.
The plus sign represents an ____ reaction
The minus sign represents an ____ reaction
What does this tell us?
Mg(s) + H2SO4 (aq) → MgSO4(aq) + H2(g) ΔH = -466.9 kJ/mol
The plus sign represents an endothermic reaction
The minus sign represents an exothermic reaction
You always look at it from the point of view of the reactants
466.9kJ of heat is given out when one mole of magnesium reacts this way
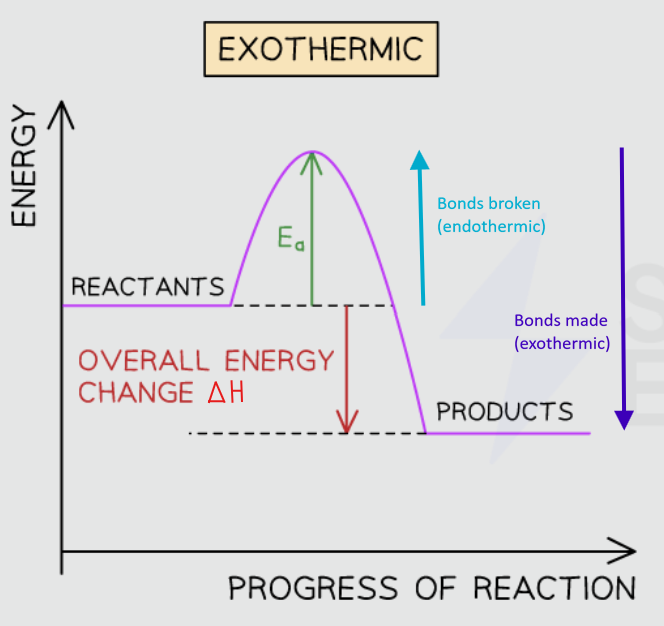
TRIPLE: Draw the energy profile diagram for an exothermic reaction
Describe the trends
Products have less energy than the reactants (products are more stable than the reactant, so the change in energy is negative. This is represented with a downwards arrow
Overall energy is released in this reaction
Activation energy is needed to start the reaction
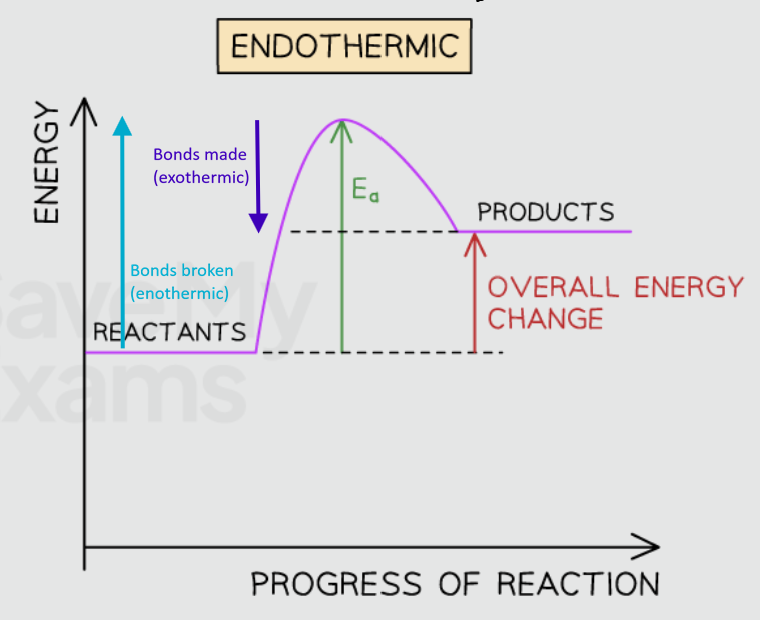
Draw the energy profile diagram for an endothermic reaction
Products have more energy than the reactants (products are less stable than the reactants), so the change in energy is positive. This is represented with a upwards arrow
Overall energy is absorbed in this reaction
Activation energy is needed to start the reaction
Key point
What does the term stability mean?
term used to describe the relative energies of the reactants and the products in a chemical reaction. The more energy a chemical has, the less stable it is
What type of process is bond making? What about bond breaking?
Bond making is an exothermic process
Bond breaking is an endothermic process
A reaction is exothermic if the energy needed to break the bonds in the reactants is ____ than the energy released to the surroundings on making new bonds in the products
LESS (sería more if we were talking about endothermic reaction)
What is meant by the term ‘bond energy'?
The amount of energy needed to break 1 mole of covalent bonds in gaseous molecules
Different chemical bonds have different bond energies (e.g a bond between H—Cl might require 428kJ to break but 420 kJ to make whereas a H—H bond only requires 300kJ to break and 390 kJ to make)
END OF TRIPLE: How can we calculate the total energy change in a reaction to find whether it is endo or exo?
Energy in - energy out
Energy required for bond breaking - energy required for bond making
If it is negative = exo
If positive = endo
What is meant by the term specific heat capacity? Unit?
Amount of heat energy needed to raise the temperature of 1 gram of a substance by 1°C
J/g/°C or Jg-1°C-1
What is the specific heat capacity of water?
4.18 J/g/°C
4.18 J of heat energy is needed to increase 1g of water by 1°C
If we want to increase it by 2°C then 2 × 4.18
If we want to increase 2g of water by 2°C then 4 × 4.18
What is the equation to find the heat energy change?
Q = m x c x ΔT
Q = the heat energy change, J
m = the mass of the substance being heated, g (solution 1 + solution 2 or solution 1 if solid is dissolved)
c = the specific heat capacity, J/g/°C
ΔT = the temperature change, °C
If they don’t tell you the density OR the specific heat capacity of the mixture/solution, assume that:
its density is the same as that of water so 1 cm³ of solution also has a mass of 1g
its specific heat capacity is the same as that of water (4.18 J/g/°C)
What is the equation to find molar enthalpy change (ΔH)?
ΔH = Q/n
Q = heat energy change and it has no direction
n = number of moles (n=mass/Mr OR n=vol x conc)
Energy released per mole = energy released/number of moles
ΔH positive = endo
ΔH negative = exo
We can compare the amount of energy released per gram and per mole (this is for the experiment comparing different fuels). A fuel might be very good when we calculate per gram but not per mole
energy released per gram = energy released (Q)/mass of fuel burnedç
energy released per mole OR molar enthalpy change = energy released (Q)/ number of moles
REMEMBER: units for ΔH is kJ/mol (KILOJOULES) so remember to change it to kJ/mol by dividing by 1000
Key point
What is a calorimeter? Examples?
Something we do the calorimetry experiment in
copper can
polystyrene cup
vacuum flask
Method to calculate the enthalpy of combustion experiments
Method?
Measure 100cm³ of cold water and add it to a copper can
Take initial temp of water
Weigh spirit burner with the lid on contaning the first fuel (e.g ethanol)
Light the wick to heat the water
Wait until the temp has risen by 20oC and extinguish the flame by putting lid back on
Stir the water
Weigh the spirit burner again with its lid on
Repeat experiment with the rest of alcohols
Why should the lid be kept on when the wick is not lit?
To prevent the alcohol from evaporating
Key point
There are many versions of this experiment. There is no ideal version.
The higher the temperature of the water reaches, the ______
If you increase the temp of the water by a small amount ______
The higher the temperature of the water reaches, the greater the heat losses during the experiment
If you increase the temp of the water by a small amount, errors in reading the thermometer or in finding the mass change of the alcohol become too significant
Why is the value we obtained for the amount of heat released lower than what it should be?
Main source of error: large amounts of heat loss
Not all heat produced by the combustion reaction is transferred to the water (some to surroundings, absorbed by the calorimeter can and the thermometer)
Warm water gives out heat to the air
Main source of error: incomplete combustion
Flame moves around because of draughts
Evaporation of water
When does incomplete combustion occur? How will we know if it it complete or incomplete?
When there is not enough oxygen present
(incomplete combustion releases less heat energy than complete combustion)
Incomplete
flame of the wick is often yellow/orange rather than blue
soot (carbon) is produced at the bottom of the copper can instead of carbon dioxide being produced
How can we minimise sources of error?
Copper calorimeter should not be placed too far above the flame
A lid should be placed over the calorimeter
Shielding can be used to reduce draughts
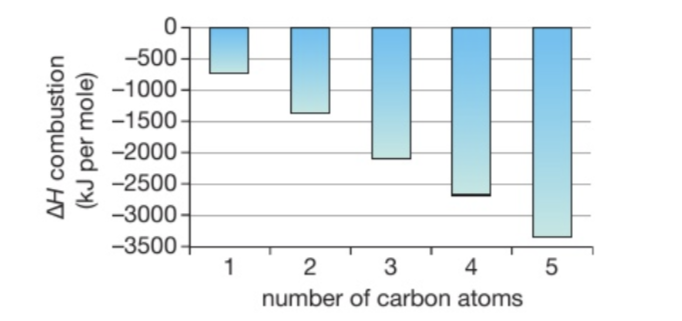
ESTUDIATE ESO DESPUÉS DE ORGANIC CHEMISTRY: This shows the acohols with increasing number of carbon atoms
Explain the trend in this graph
Combustion reaction gets more exothermic (more energy is released) as the alcohol chain becomes longer
Longer alcohols give out more heat energy per mole when they burn than shorter ones
The difference between one alcohol and the next is always an extra CH2, and so the number of extra bonds broken and made increases in a regular way. This means that the heat evolved will also increase in a regular way
Key point about the graph
Why can’t these results be plottled as smooth graphs (smooth curve)?
A smooth curve should only be used for a continuous independent variable, one which can take any value
However, an alcohol with 0.5 or 1.64 carbon atoms does not exits. The number of carbon atoms is a non-continuous variable beacause it can only take whole number values
Method to measure the enthalpy changes for displacement, dissolving and neutralisation reactions
In this case we will be investigating the enthalpy change for the displacement reaction between zinc and copper(II) sulfate
Place a polysterene cup in a beaker
Add a fixed volume of copper(II) sulfate solution to the calorimeter and measure initial temperature
Add excess amount of zinc
Stir the solution vigorously/as quickly as possible
Record the maximum temperature reached
For this reaction, 1.20g of zinc was added in excess to 50cm³ of 0.200mol/dm³ of copper(II) sulfate solution.
These were the results:
Initial temp: 17.0 degrees C
Final temp: 27.3 degrees C
Use this data to calculate the enthalpy change for this displacement reaction, when 1 mole of copper(II) sulfate reacts with zinc.
Zn(s) + CuSO4 → ZnSO4(aq) + Cu(s) ΔH = -215kJ
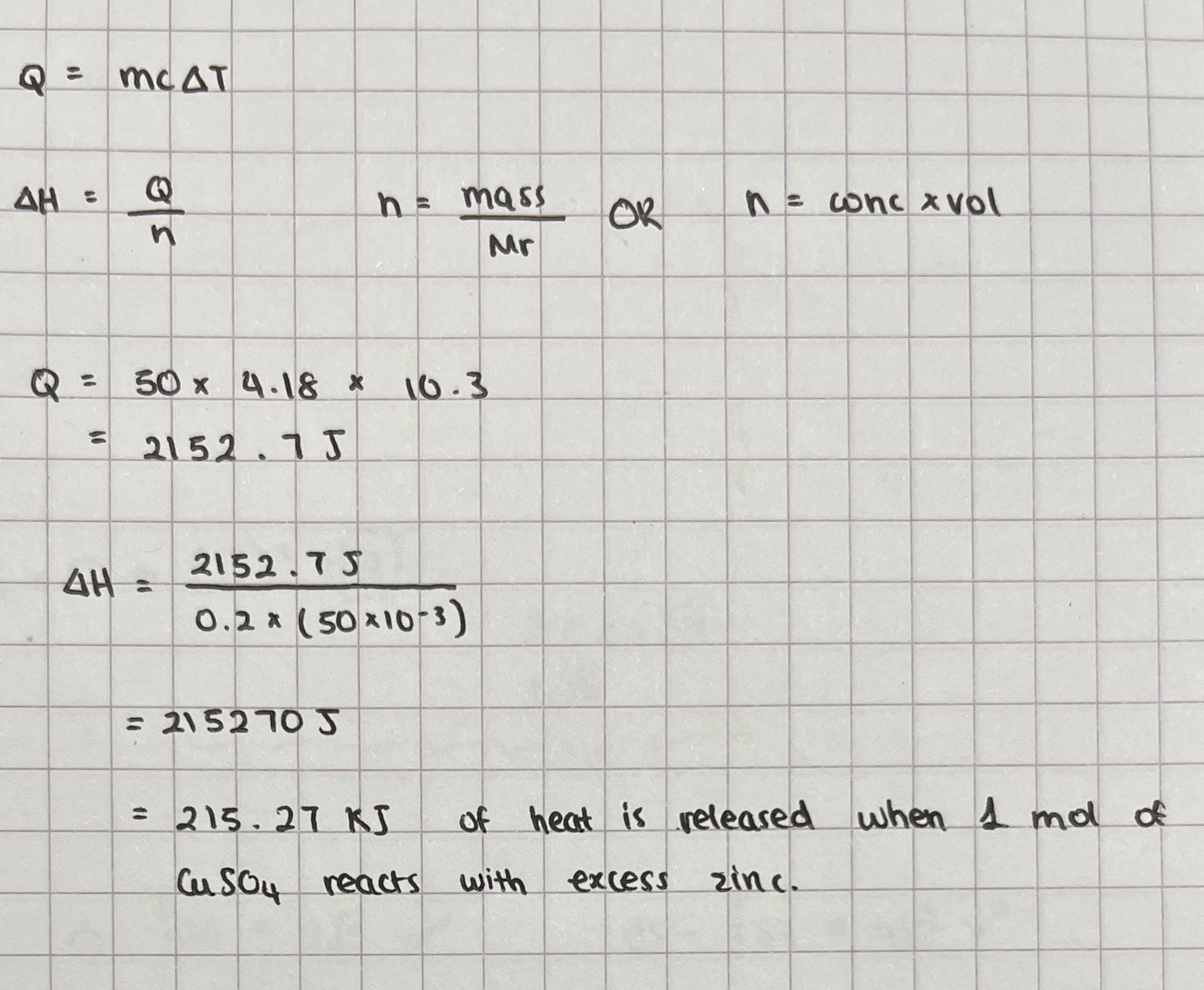
Why would the specific heat capacity of this solution being 4.18 J/g/°C be a reasonable assumption?
The reaction mixture is mostly water
Describe a method to measure the enthalpy changes of neutralisation between an alkali and an acid
Potassium hydroxide and HCl
Place a polystyrene cup in a beaker
Add 25cm³ of potassium hydroxide into the cup
Record initial temp
Fill a burette with 50.00cm³ of HCl
Use the burette to add 5.00cm³ of HCl to the KOH
Stir vigorously and record max temp reached
Continue adding further 5.00cm³ of HCl to the cup, stirring, and recording max temp reached each time until a total vol of 50.00cm³ has been added
How do you find the maximum temperature reached during the neutralisation reaction? How can we use this graph to find out how much HCl is needed to neutralise the volume of KOH added?
Plot a graph of the temp of the mixture in y-axis versus the volume of acid added x-axis
Draw 2 lines of best fit
The point where they meet show the max temp reached
This point also represents the point of complete neutralisation (vol of HCl to neutralise KOH)
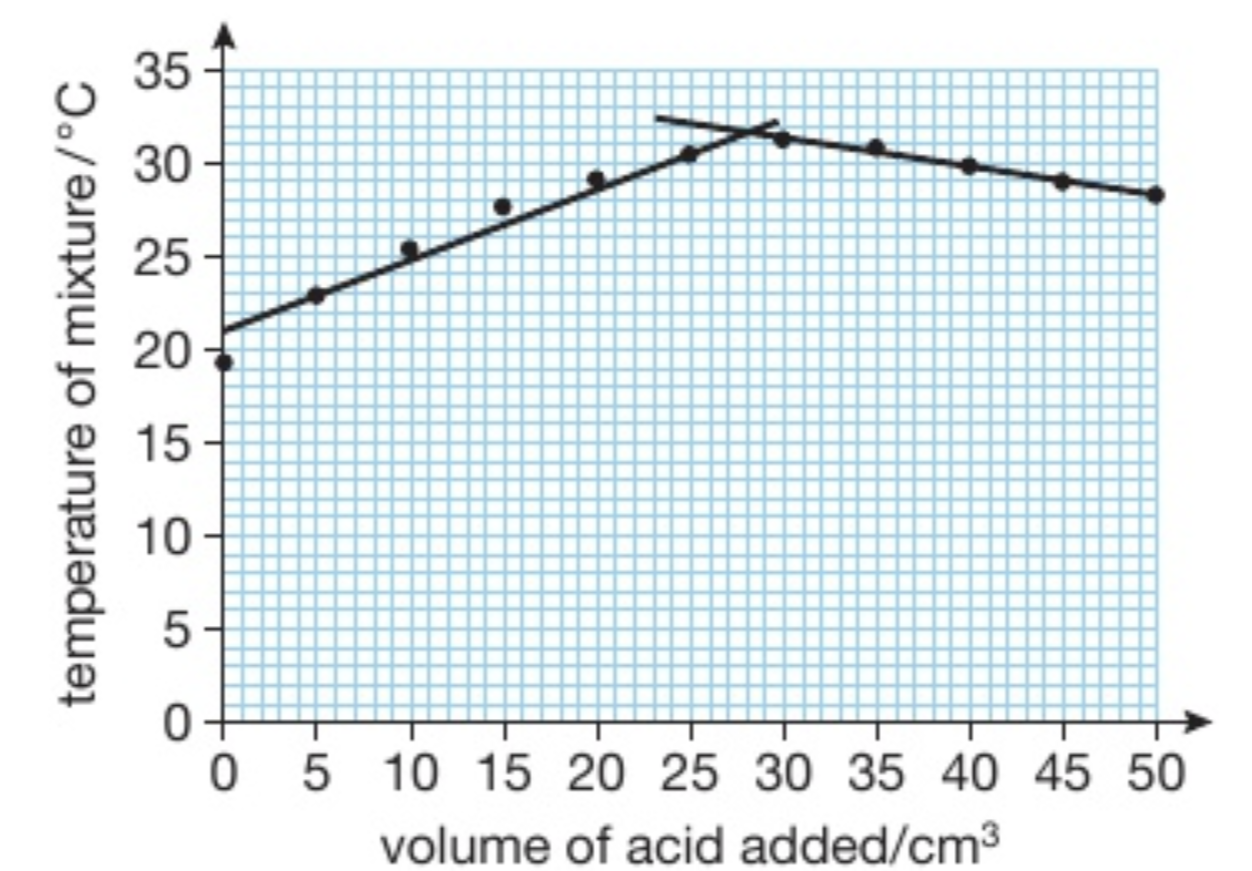
Explain the graph
Temp increases at first because the reaction between the acid and the alkali is exothermic
Temp starts to decrease after because all the alkali has been used up so we are just adding cold acid to our warm solution (there is no reaction so no heat is released)
Why can we assume that the mass and the specific heat capacity of this solution is the same as the water’s?
Density of the reaction mixture is the same as that of water (1cm³ = 1g)
Neutralised solution is mostly water (OH-ions from alkali react with H+ ions from acid to form water)