chemistry - chemical changes: electrolytic processes (3.22 - 3.31)
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
3.22 electrolytes definition
ionic compounds molten/dissolved in water
3.23 electrolysis
energy from (2 electrodes connected to) d.c. supply decomposes electrolytes
3.24 movement of cations in electrolysis
+ cations attracted to & migrate to - cathode
3.24 movement of anions in electrolysis
- anions attracted to & migrate to + anode
3.25 rules for formation of products in electrolysis
anode:
anions → anode: oxidation (e- lost)
halide ions discharged as halogen gases (e.g. Cl- → Cl2)
hydroxide ions discharged as oxygen gas (OH- → O2)
cathode:
cations → cathode: reduction (e- gained)
copper/silver/gold/platinum discharged as solid (e.g. Ag+ → Ag)
hydrogen gas discharged
(anything less reactive than hydrogen discharged, otherwise hydrogen discharged)
3.25 formation of products in electrolysis (inert electrodes) of copper chloride solution
anions → anode: chlorine & hydroxide
chlorine gas produced
cations → cathode: copper & hydrogen
solid copper produced
3.25 formation of products in electrolysis (inert electrodes) of sodium chloride solution
anions → anode: chlorine & hydroxide
chlorine gas produced
cations → cathode: sodium & hydrogen
hydrogen gas produced
3.25 formation of products in electrolysis (inert electrodes) of sodium sulfate solution
anions → anode: sulfate & hydroxide
oxygen gas produced
cations → cathode: sodium & hydrogen
hydrogen gas produced
3.25 formation of products in electrolysis (inert electrodes) of water acidified with sulfuric acid
(acidified water: water that has had acid added to it)
anions → anode: sulfate & hydroxide
oxygen gas produced
cations → cathode: hydrogen & hydrogen
hydrogen gas produced
3.25 formation of products in electrolysis (inert electrodes) of molten lead bromide
anions → anode: bromine
bromine gas produced
cations → cathode: lead
solid lead produced
3.26 predict products of electrolysis of other binary, molten ionic compounds
identify elements in ionic compound
+ metal ion produced at cathode
- non-metal ion produced at anode
3.27 half equations for reactions at anode & cathode in electrolysis
e.g. electrolysis of molten zinc chloride
2Cl- → Cl2 + 2e-
Cl- → anode: lose electrons, become chlorine molecules (oxidation)
Zn2+ + 2e- → Zn
Zn2+ → cathode: gain electrons, become zinc atoms (reduction)
3.28 oxidation & reduction
Oxidation Is Loss of electrons
Reduction Is Gain of electrons
OILRIG
3.29 which electrodes do oxidation & reduction occur at in electrolysis?
OXidation at ANode (oxidation = loss of e-: - anions → + anode, lose e-)
REDuction at CAThode (reduction = gain of e-: + cations → - cathode, gain e-)
RED CAT, AN OX
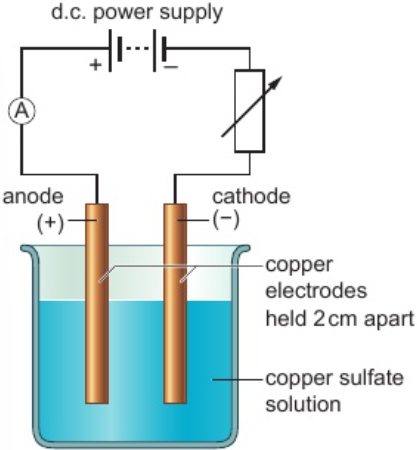
3.30 formation of products in electrolysis (copper electrodes) of copper sulfate solution - purifying copper
copper atoms in anode —lose e-→ copper ions
dissolve in solution, migrate to cathode - deposited as pure copper
impurities from anode don’t form ions, collect below anode as ‘sludge’
3.31 core practical: investigate electrolysis of copper sulfate solution with inert electrodes & copper electrodes
select 2 clean pieces of copper foil, label one ‘anode’ & other ‘cathode’
measure & record masses of 2 electrodes
set up electrolysis circuit as in diagram
turn on power & adjust variable resistor to give current about 0.2A, record current & adjust variable resistor to keep it constant, leave power on for 20 mins
turn off power & remove electrodes from beaker
wash electrodes with distilled water, dip into propanone, lift out & shake off propanone, let rest of propanone evaporate
measure & record masses of dry electrodes
repeat experiment using currents 0.3A, 0.4A & 0.5A