Topic 2 Energy Intermediary Metabolism
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
3 ways to consider energy metabolism
whole body (short term)
whole body (long term) → calorie counting
cellular (chemistry of chemical reactions/”energy metabolism”)
can be aerobic and anaerobic
energy transferring molecules (define)
molecules that bind our energy and put them in bonds
short-term fuel molecules
long-term fuel molecules
ionic bonds (low energy)
covalent bonds (higher energy)
energy transferring molecules (list): used in oxidation/reduction reactions and in electrron and proton-transport pathways
Nicotinamide Adenine Dinucleotide (NAD+/NADH + H+)
Flavin Adenine Dinucleotide (FAD/FADH2)
Coenzyme A (oxidized, reduced)
short-term fuel molecules
molecules tend to have covalently bound phosphate groups attached to a carrier molecule
relatively unstable, not able to accumulate large amounts in cells
adenine triphosphate (ATP), GTP, and UTP
phosphagens (creatine phosphate, etc.): anything we slap the Pi on for energy
adenine triphosphate (ATP)
made of adenosine (adenine + ribose) and three phosphate groups
the energy “currency” of the cells
almost all metabolic activities requiring energy get energy by breaking this down to ADP
also used for cell to cell signaling in the body + substrate for creating cAMP
Delta G (change in Gibbs free energy): -7kcal energy/mol ATP (standard cond.)
1 kcal = 1 Cal = 1000 cal
in cells, real conditions yield ~11 kcal/mol
creatine phosphate (CrP, CP, or PCr) aka phosphocreatine
not stable; more stable than ATP, GTP, UTP
Delta G (change in Gibbs free energy): -10.3 kcal/mol under standard conditions
CrP regenerates ATP by substrate-level phosphorylation when total metabolism exceeds aerobic limit
phosphagens
physiologically produced organic molecules that store energy in phosphate bonds, ATP/CrP are the main phosphagens used in the human body
long-term fuel molecules
C-H bonds NOT phosphate bonds
carbohydrates exist as monosaccharides, disaccharides and polysaccharides
glucose → glycogen (polysaccharide)
glucose is the principle monosaccharide used as fuel; ~4.2 kcal/g
the body stores ~24 hrs worth of glycogen in liver and skeletal muscle
fats (triglycerides)
long-term fuel molecules
one glycerol molecule, three fatty acids (14-18 C long and ALWAYS an even # of C)
fat provides ~9.4kcal/g
most fat is stored in ADIPOSE (fat) cells, the body has no limit to how much fat can be stored
excess free amino acids (fuels) → long-term fuel molecules
proteins are not typically synthesized for the purpose of “fuel storage” in the human body
proteins can be degraded for fuel providing ~4.2-4.3 kcal/g
specific proteins are made on an as needed basis by cells in order to accomplish some function
preference of fuels (calories)
most calories from carbohydrates (glucose when fed)
some calories from fats
fewest calories from protein turnover and amino acids
under starvation conditions: body resorts to long-term fuel molecules (in this order)
glycogen
fats
proteins
metabolism
all reactions of the body; what your cells are doing OR how much energy you’re using to do it
anabolism (anabolic)
building reactions (smaller pieces put together)
catabolism (catabolic)
breakdown reactions (bigger things taken apart to smaller pieces)
pathways
sets of chemical reactions that begin with a specific set of reactants and sequentially lead to a specific products
energy metabolism aka respiration
the catabolic pathways used to generate ATP
anaerobic respiration
doesn’t use oxygen
e.g. glycolysis is a catabolic pathway that is anaerobic
aerobic respiration
uses oxygen
e.g. krebs cycle and electron transport chain (aka oxidative phosphorylation) are two catabolic pathways that are aerobic
though aerboic respiration is aerobic it begins with glycolysis
aerobic metabolism (examples)
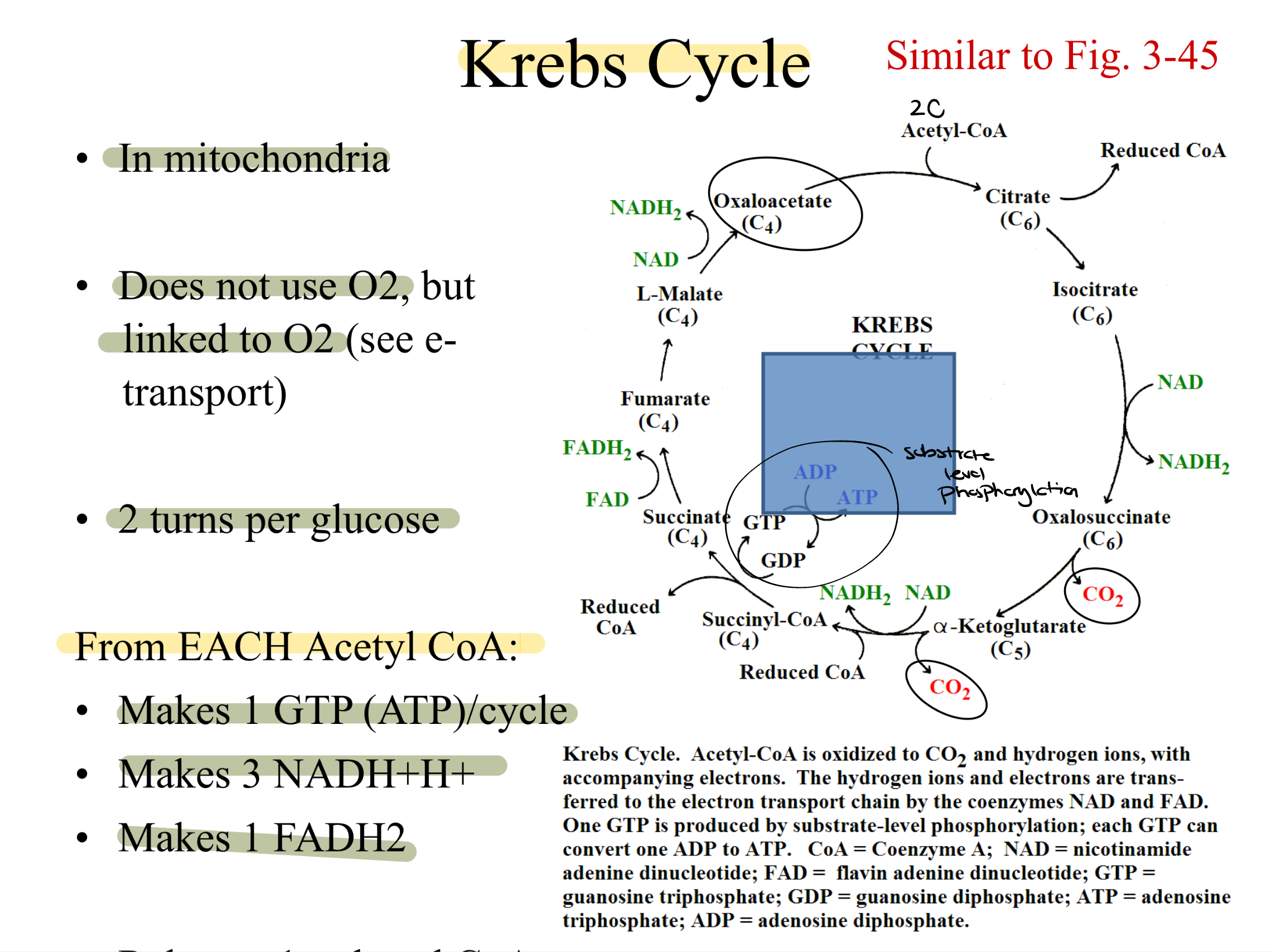
krebs cycle
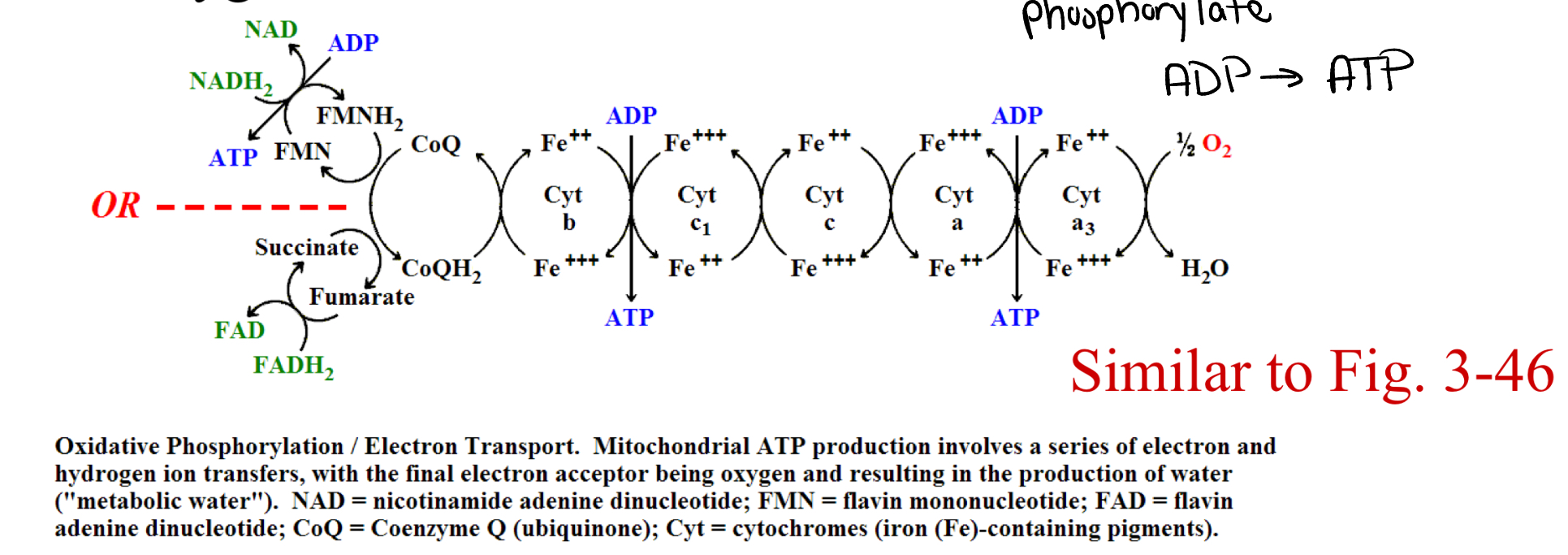
electron transport
glycolysis (with glycogenolysis)
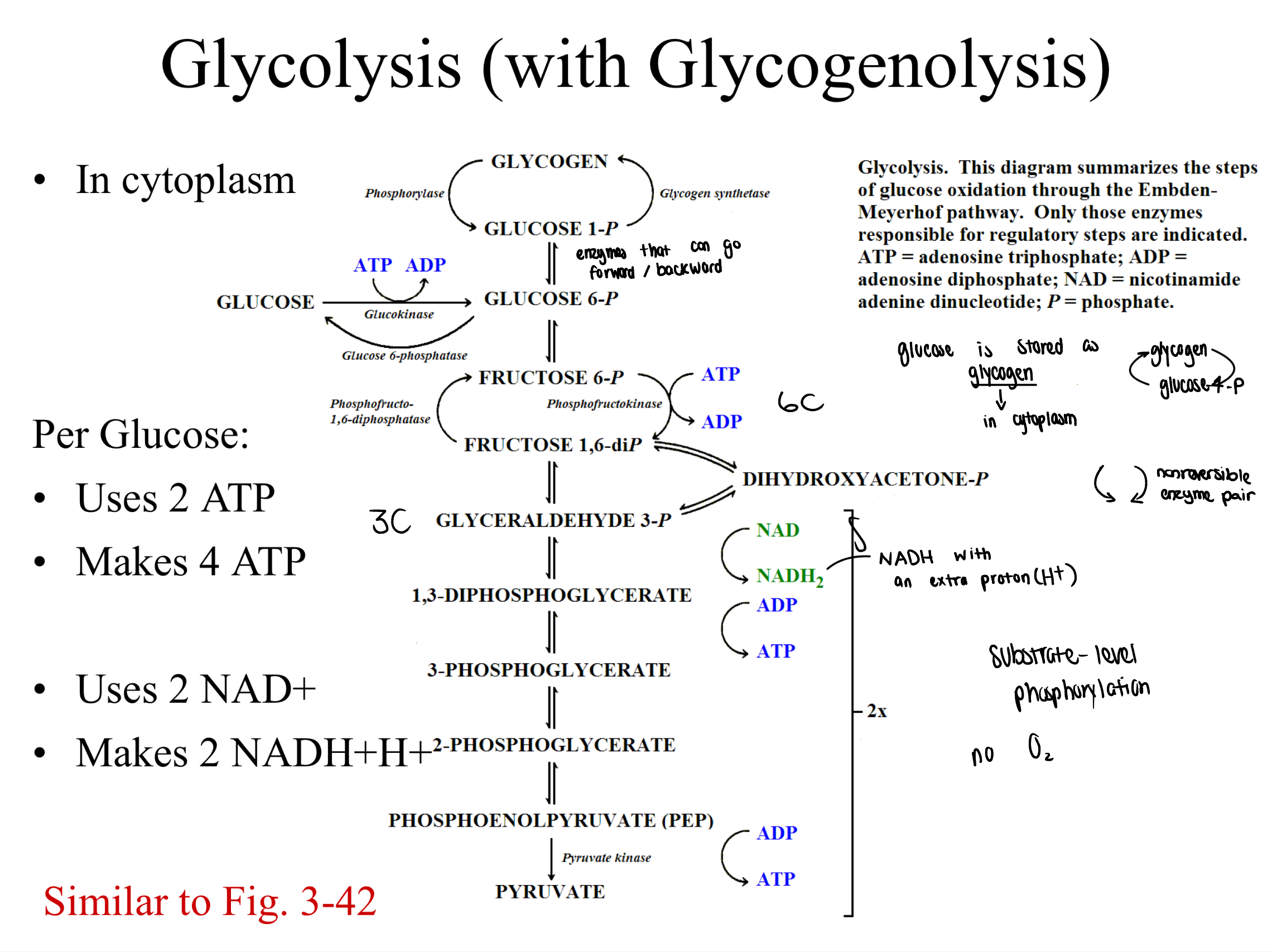
what happens to pyruvate in glycolysis?
depends on whether oxygen is available
pyruvate enters the mitochondria
per pyruvate
makes 0 ATP
uses 2 NAD+ (acts as coenzymes)
makes 2 NADH+H+
enzymes
proteins that act as catalysts in chemical reactions
have ACTIVE SITES to bind to their substrates
most are highly specific
coenzymes
shuttle electrons/protons from fuel reactions to oxidative phosphorylation (electron transport) for ATP synthesis
(vitamin derivatives)
low specificity
organic (not proteins)
catalysts
used in oxidation-reduction (REDOX) reactions
vitamin
organic molecule we need for life that we cannot make or synthesize
cofactors (minerals)
catalysts that help enzymes work
oxidize
removes e- or H
reduce
adds e- or H
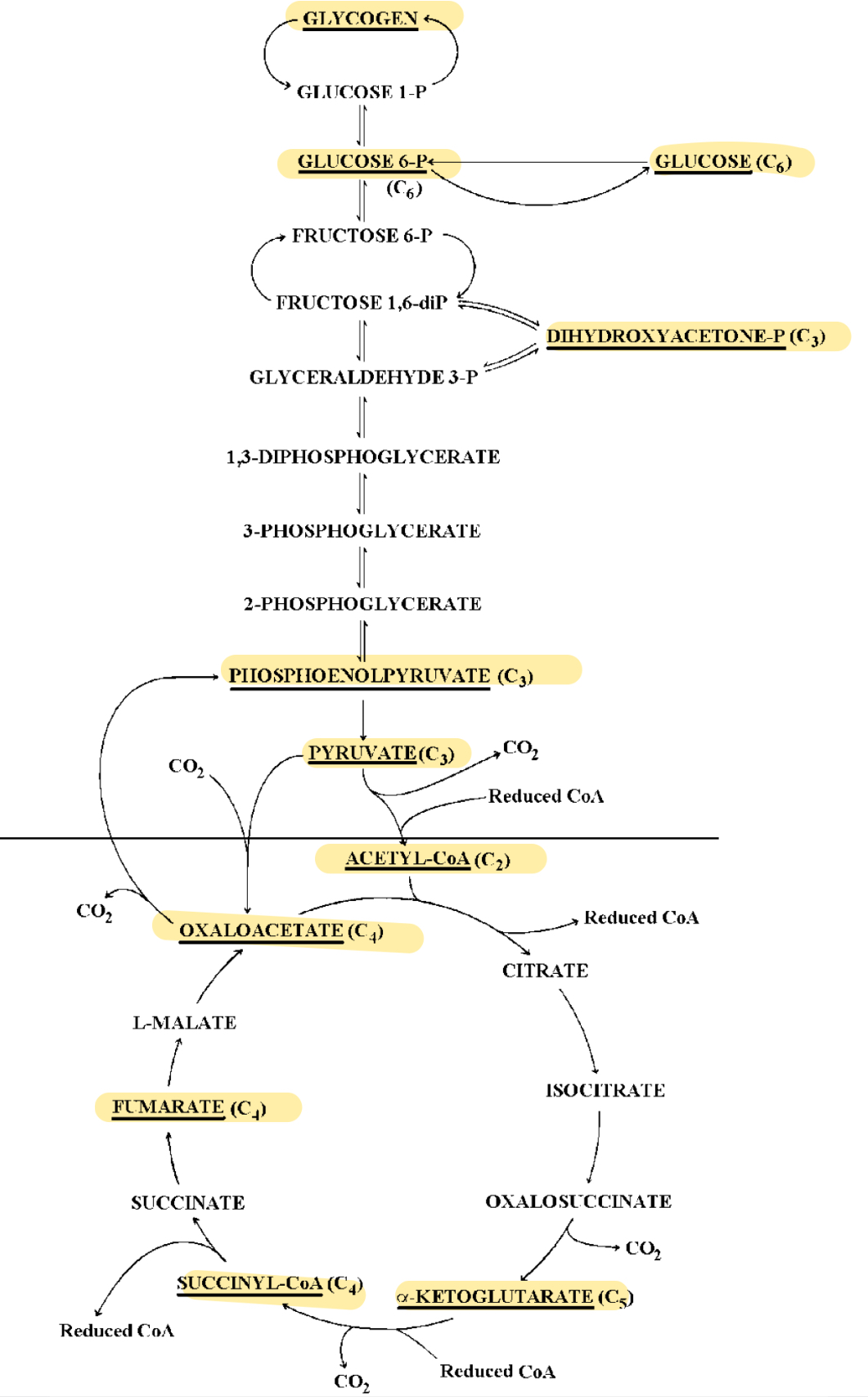
krebs cycle
Acetyl CoA oxidized to CO2 and hydrogen ions, with accompanying electrons. Hydrogen ions and electrons are transferred to the ETC by the coenzymes NAD and FAD. one GTP is produced by substrate-level phosphorylation; each GTP can convert one ADP to ATP.
requires a lot of oxidized coenzymes
doesn’t use O2, synchronized with electron transport
oxidative phosphorylation (e- transport)
occurs in mitochondria
recycles 3 ATP per NADH+H+
recycles 2 ATP per FADH2
oxidizes the original coenzymes
NADH+H+ → NAD+
FADH → FAD+
reduces oxygen to create water
All the ATP made per glucose
glycolysis = 2 NADH+H+
pyruvate→acetyl CoA (x2) = 2 NADH+H+
K.C. (x2) = 6 NADH+H+ and 2FADH2
2 NADH+H+ X 3 ATP/NADH+H+ = 6 ATP
2 NADH+H+ X 3 ATP/NADH+H+ = 6 ATP
6 NADH+H+ X 3 ATP/NADH+H+ = 18 ATP
2 FADH2 X 2 ATP/NADH+H+ = 4 ATP
Total ATP produced by oxid. phosphorylation = 34 ATP
plus 2 ATP by substrate level phosphorylation in glycolysis
plus 2 ATP (as GTP) by substrate level phosphorylation in krebs cycle
= 38 ATP per glucose by aerobic metabolism
anaerobic metabolism (accelerated glycolysis)
never runs out of coenzyme (runs as fast as it wants to)
can run as fast as it wants to
aerobic metabolism of other fuels
proteins: deamination of amino acids
fats: beta oxidation of fatty acids
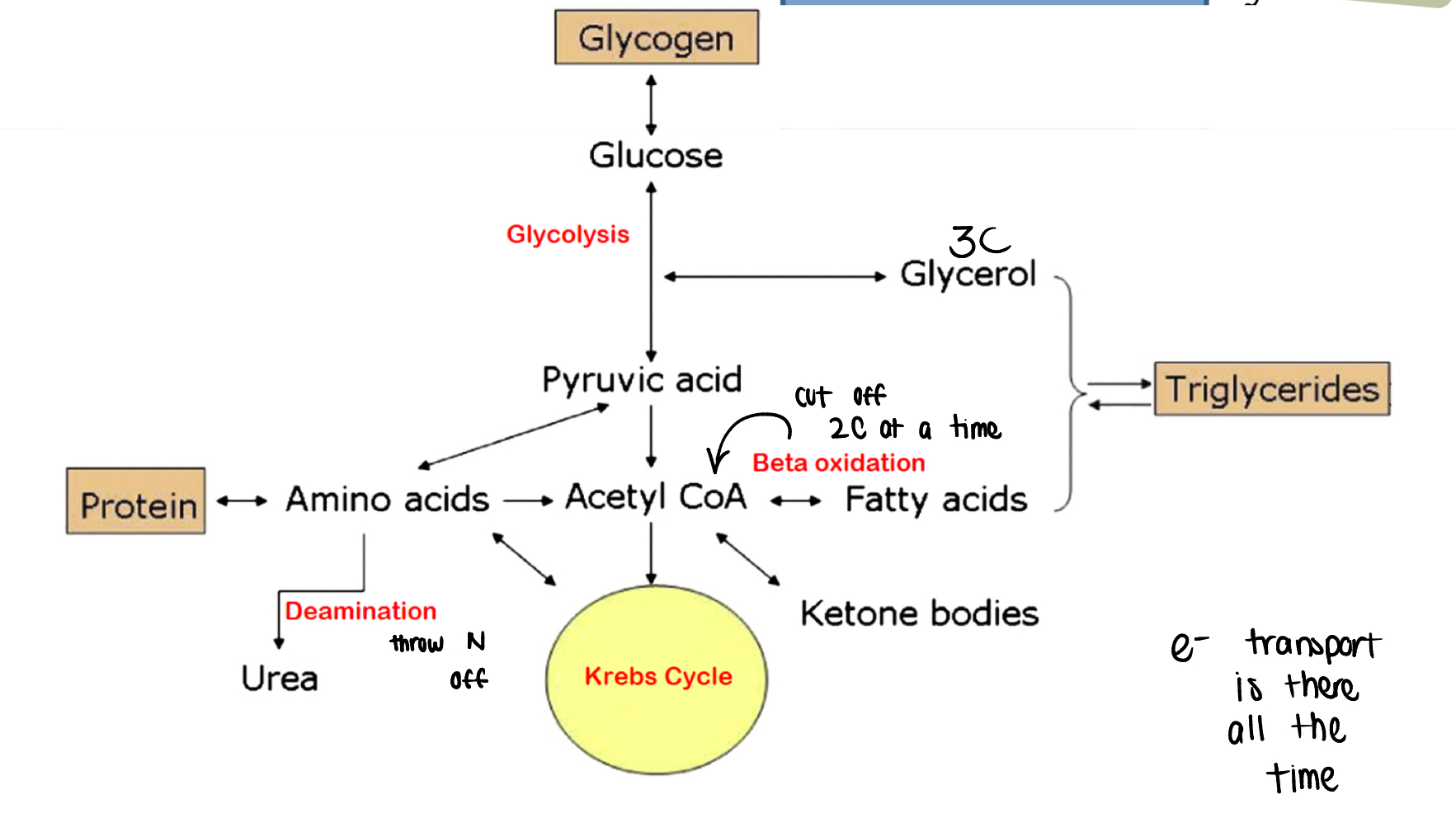
lipolysis (process)
fats are hydrolyzed to glycerol and three fatty acids
β-oxidation
fatty acids degraded to many Acetyl CoA
β-oxidation of fatty acids
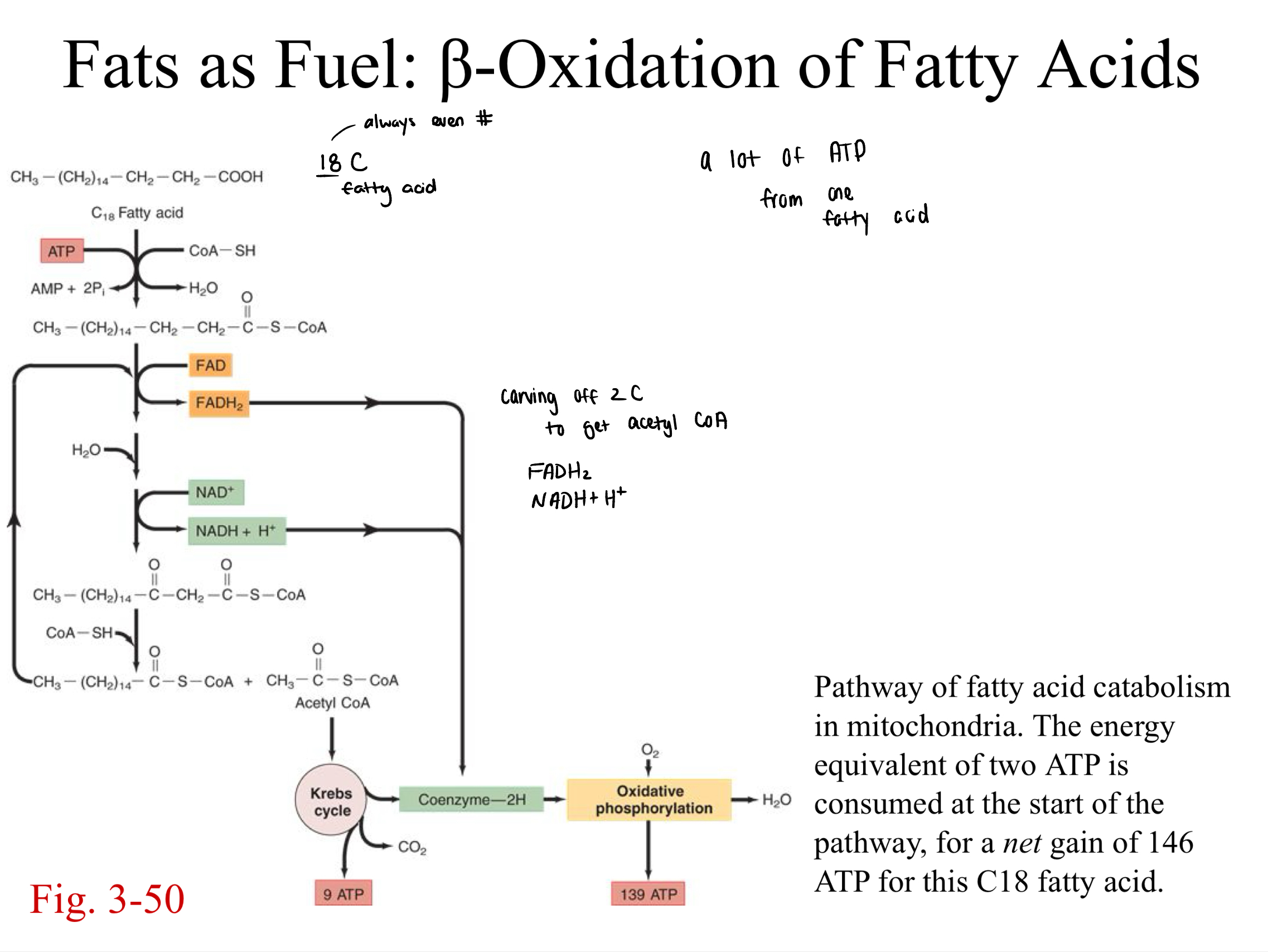
fats as fuel
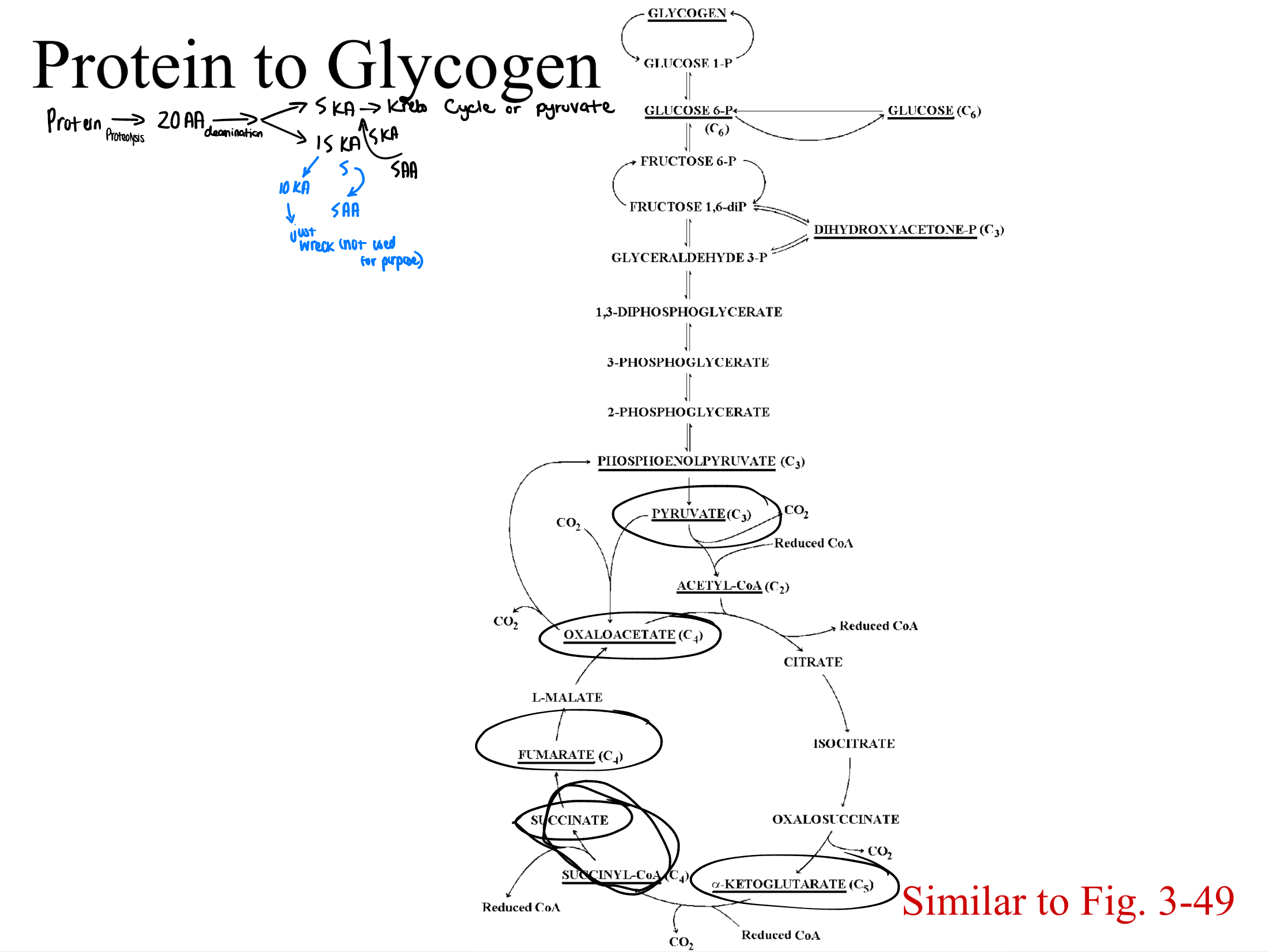
proteolysis
proteins are hydrolyzed to amino acids
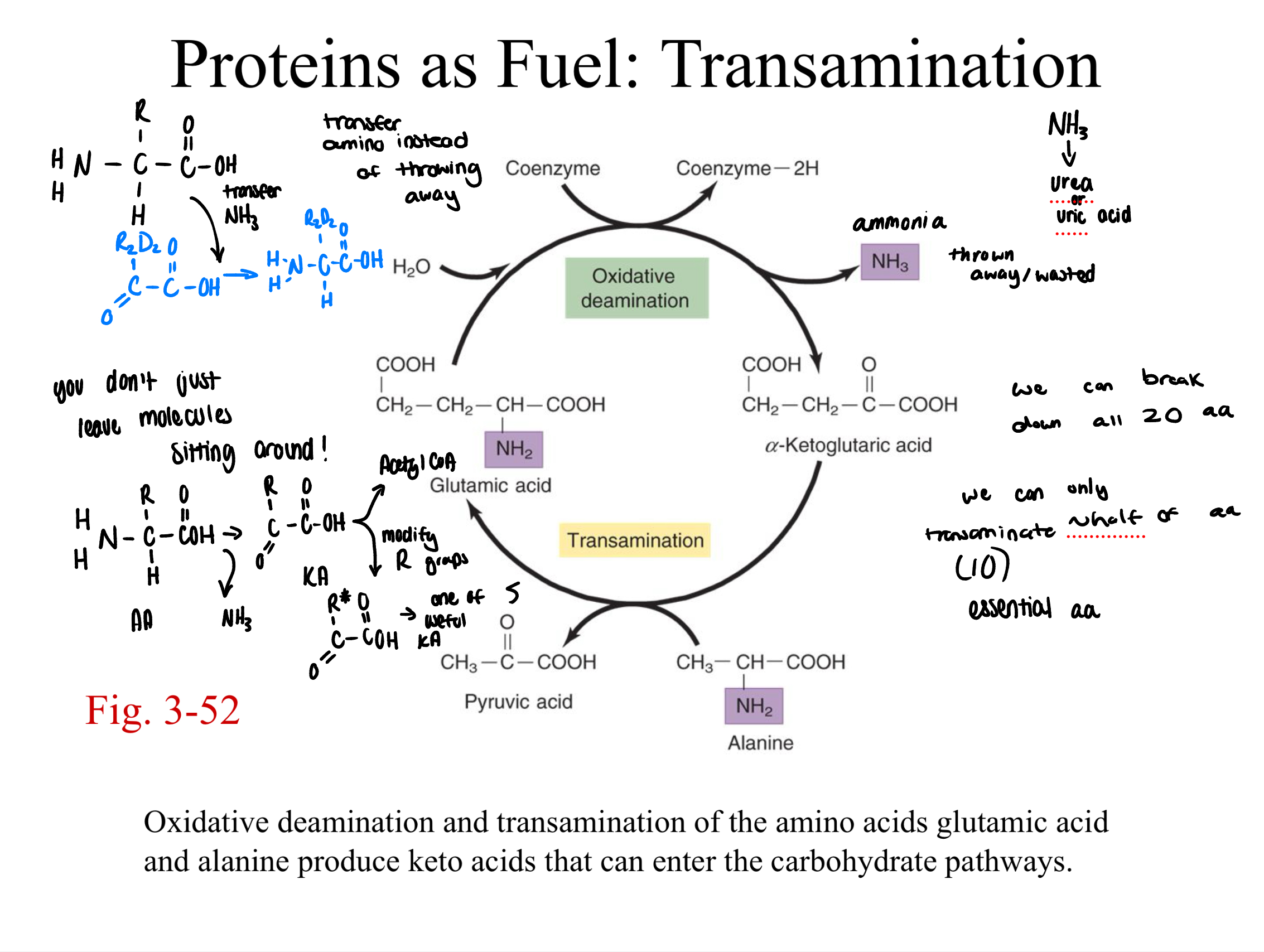
deaminated
all amino acids must go through this process to form keto acids (released amino groups → ammonia/converted to urea)
5 keto acids produced by amino acids that can be directly used in the glycolytic and KC pathways
pyruvate
alpha-ketoglutarate
succinate (succinyl-CoA)
fumarate
oxaloacetate
most amino acids…
create keto acids that don’t directly fit into glycolysis or krebs cycle. these must be converted further or used in TRANSAMINATION reactions (to create one of the five keto acids)
proteins as fuel: transamination
intermediary metabolism
metabolism of a common pool of short chain (~2-4, can go up to 6 carbon) organic molecules that can be used to produce carbohydrates, proteins, or lipids
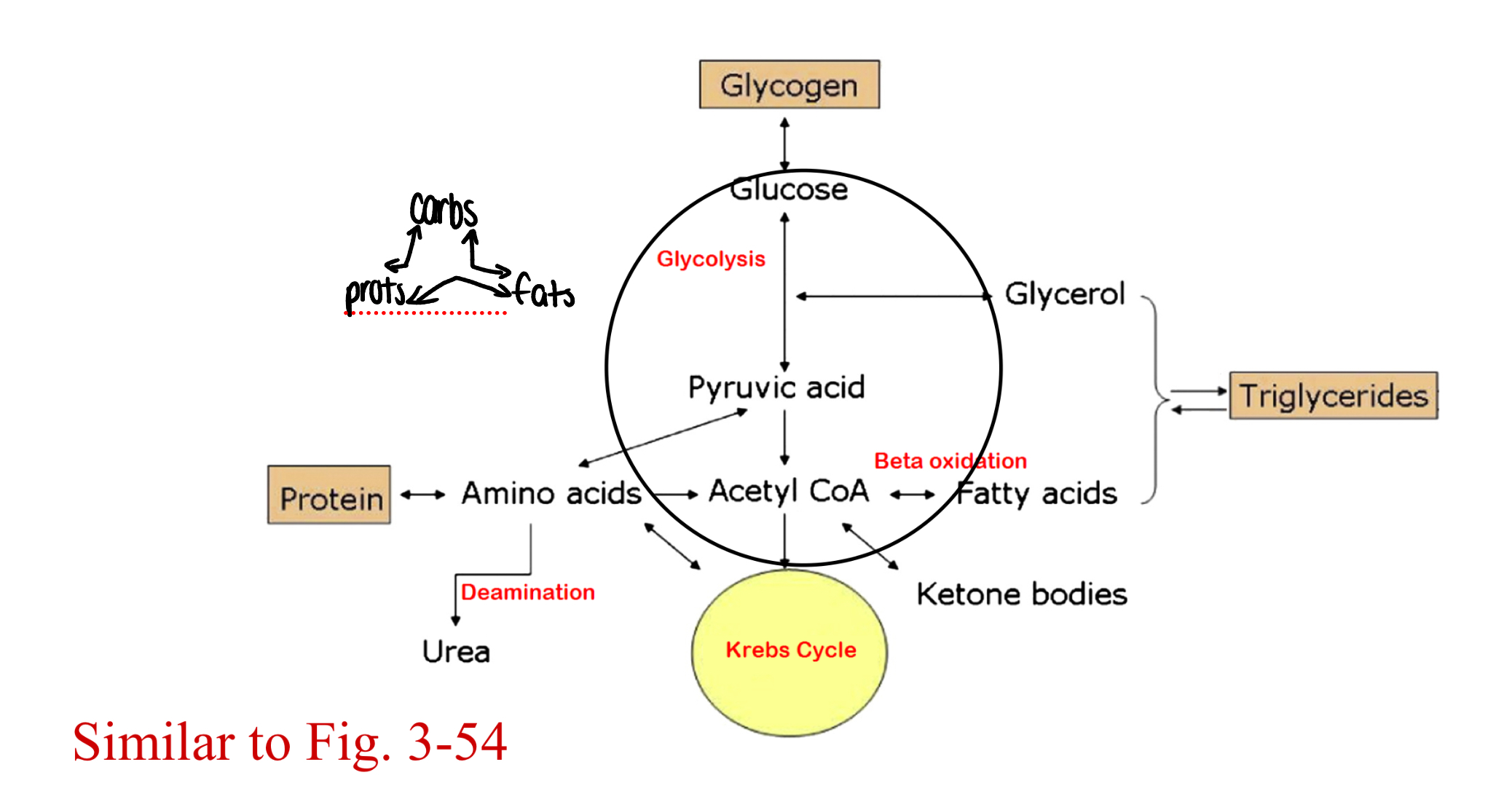
anabolism (pathway)
“building” pathways
catabolism (pathway)
“degrading” pathways
glycogenesis
anabolism of glycogen
gluconeogenesis (the -neo distinguishes creation vs. release)
anabolism of glucose
protein anabolism
anabolism of protein
lipogenesis OR lipid anabolism
anabolism of triglyceride
glycogenolysis
catabolism of glycogen, gives glucose
glycolysis
catabolism of glucose, gives pyruvate
proteolysis
protein catabolism, gives amino acids
lipolysis
catabolism of triglyceride, gives glycerol + 3 fatty acids
fat to glycogen (Study diagram)
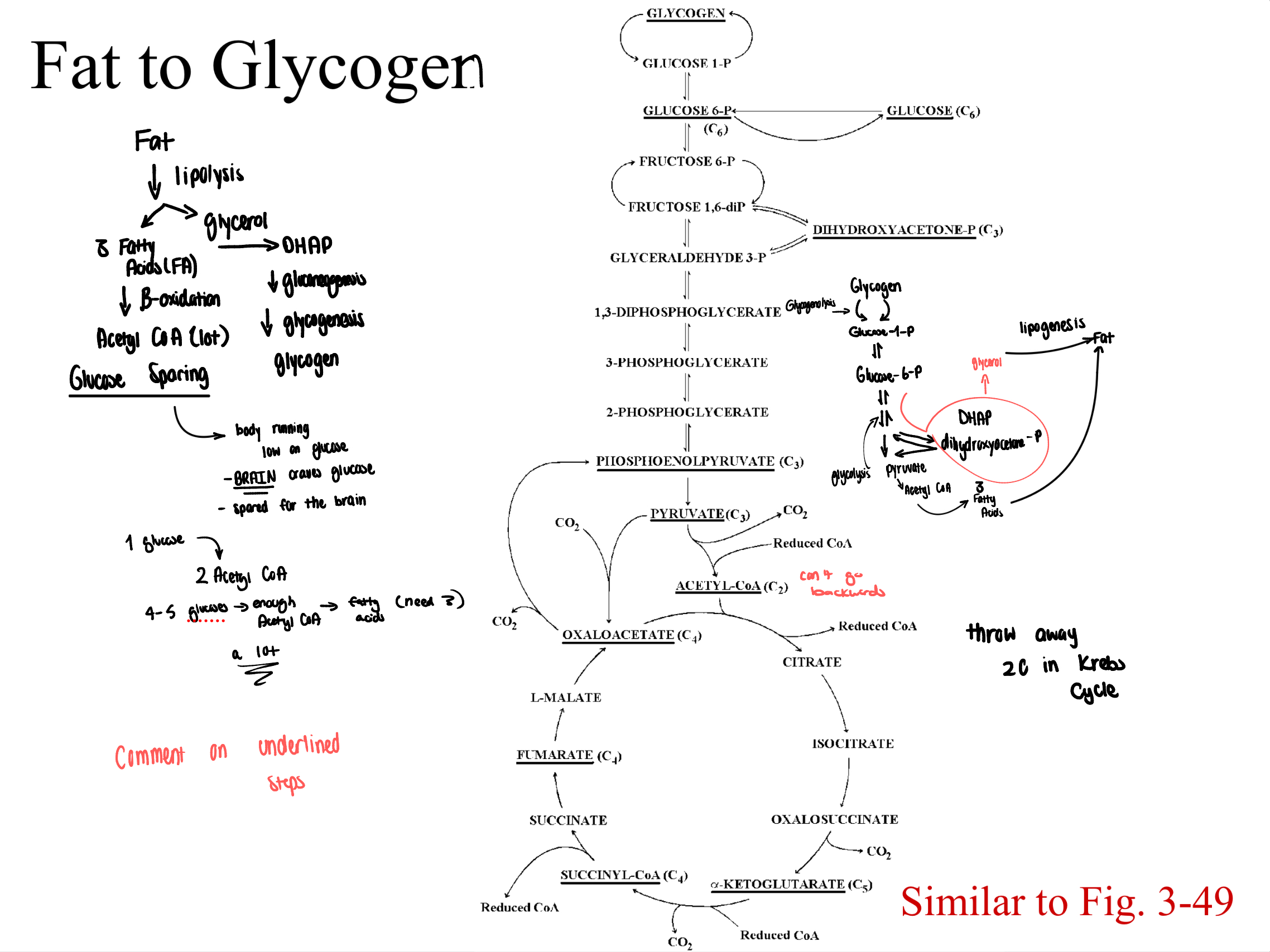
protein to glycogen (Study image)