17. Hemoglobin and gas transport through the circulatory system
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
in erthyrocytes, O2 and CO2 are -?
bound to hemoglobin
what happens when O2 binds to hemoglobin and how much of the O2 that gets into our body is bound to hemoglobin?
once O2 is bound to hemoglobin, it no longer contributes to the partial pressure of that gas in solution (now, only the freely dissolved gas molecules contribute to partial pressure)
since the O2 that entered is bound and it no longer contributes to partial pressure, more O2 can enter from the atmosphere into the fluid by going down its partial pressure gradient
respiratory pigments such as hemoglobins greatly increase the total amount of O2 that can be carried by a unit of blood (oxygen-carrying capacity)
almost all of the oxygen is bound to hemoglobin
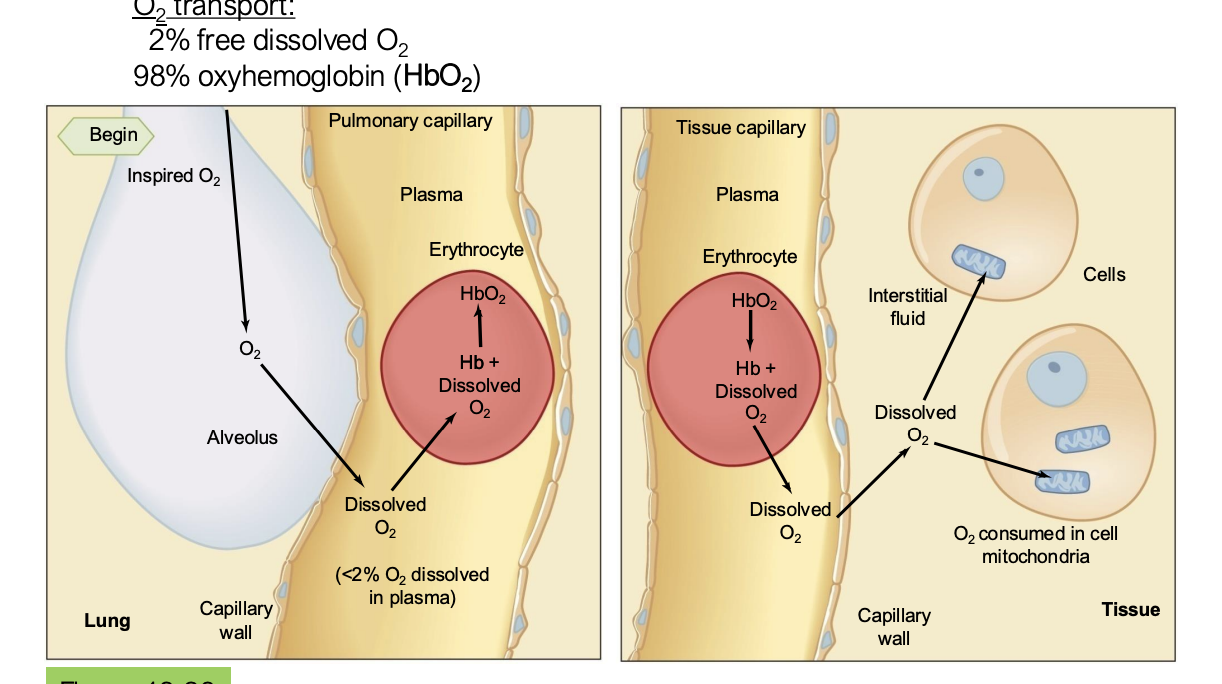
hemoglobin have what groups that allow them to interact with O2 molecules? what are the different states and their names? what is the equivalent of hemoglobin but found in muscle cells instead of the blood?
hemoglobin have iron-containing heme groups that interact with O2 molecules
when it is bound to up to 4 oxygen molecules, it is referred to as oxygenated (oxyhemoglobin)
when it is unbound to oxygen molecules, it is referred to as deoxygenated (deoxyhemoglobin)
where does oxygen bind and unbind from hemoglobin?
when oxygen diffuses from the alveoli to the pulmonary capillary, it enters the red blood cell where it binds to hemoglobin
then, in the systemic capillary, the oxygen unbinds to hemoglobin and diffuses into the tissues
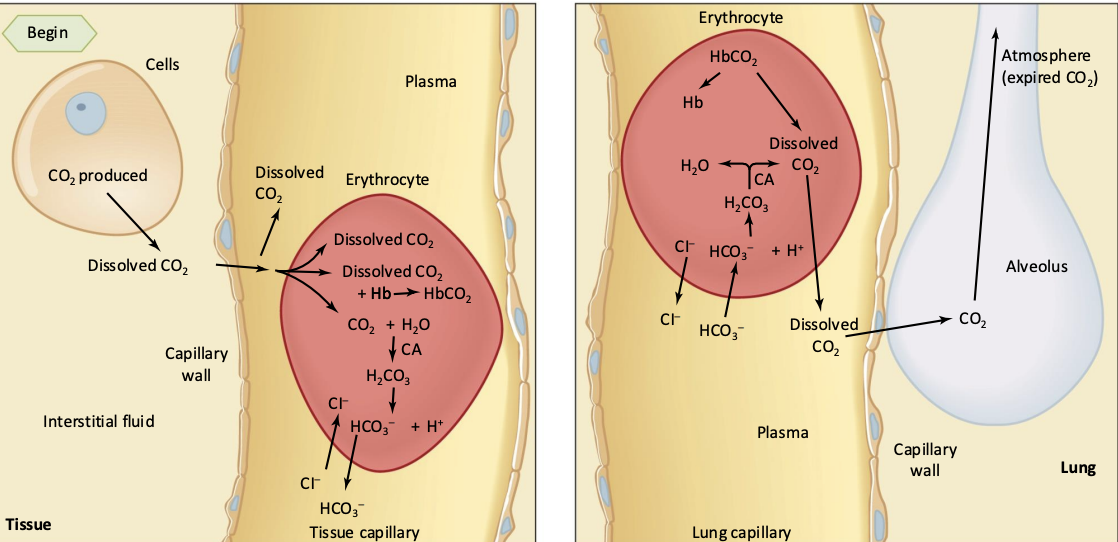
what are the three things that can happen to CO2?
freely dissolve
bind to hemoglobin forming carbaminohemoglobin
chemically converted to bicarbonate ions
enzyme carbonic anhydrase facilitates rapid CO2 equilibrium in circulating blood
for the sake of this class, you can think of bicarbonate as -?
a molecule that is transporting CO2 and it reasonable to think this because the reaction from CO2 to bicarbonate is reversible in certain parts of our body
map out the three ways CO2 mentioned above
cells produce CO2 and it travels to the systemic capillary where it can either be freely dissolved, enter the red blood cell to either become bound to hemoglobin or be converted to bicarbonate
then, in the pulmonary capillary, the very little freely dissolved CO2 diffuses out into the lungs, and in the red blood cells, the CO2 unbind and the bicarbonate reverts back into CO2 and they also leave the red blood cell to diffuse into the lungs to be exhaled out
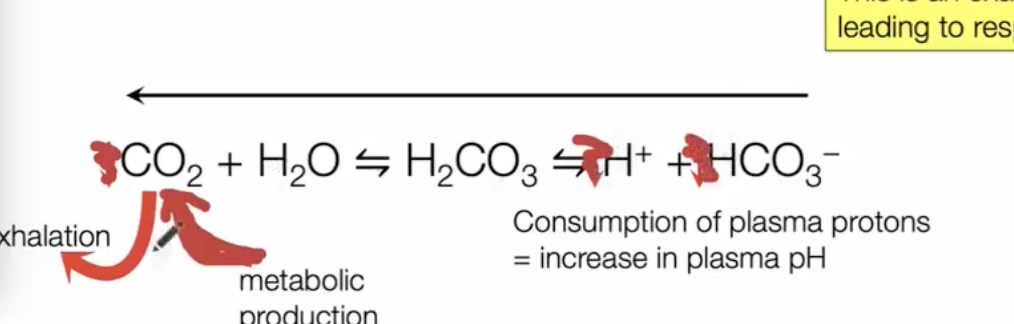
Bicarbonate and blood buffering
-
CO2 is not just a waste product, but is also a critical player in what?
body acid-base homeostasis
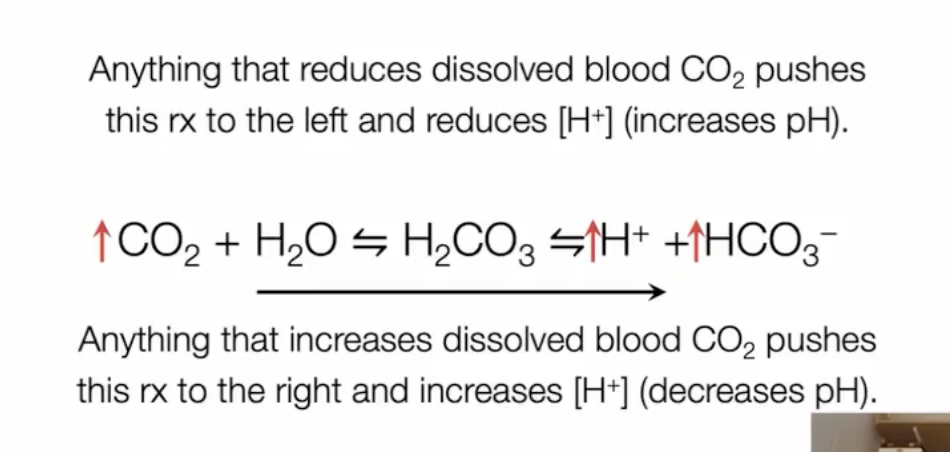
anything that reduces dissolved blood CO2 pushes reaction to the left and reduces H+ (increases pH)
anything that increases dissolved blood CO2 pushes reaction to the right and increases H+ (decreases pH)
what happens to the bicarbonate formed in the systemic capillaries?
two main possibilities:
breakdown of bicarbonate to make CO2 (reaction is driven to the left and makes fluid more basic with less H+)
bicarbonate can be reabsorbed into the plasma by the kidneys and be voided into urine as part of homeostatic control of blood pH
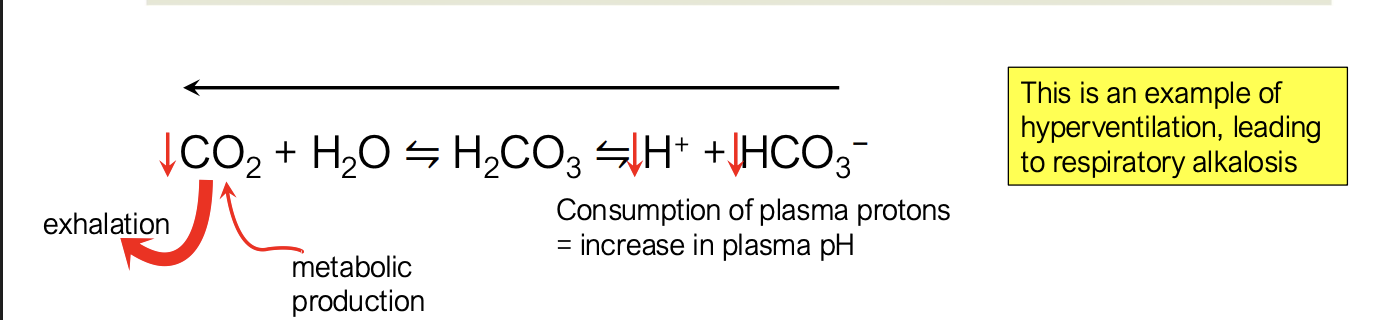
what happens when you have increased alveolar ventilation but unchanged metabolism?
you are exhaling a lot more CO2 than CO2 is created from metabolic production → hyperventilation
drives reaction to the left
this leads to a consumption of protons and bicarbonate which increases plasma pH, making it more basic → respiratory alkalosis
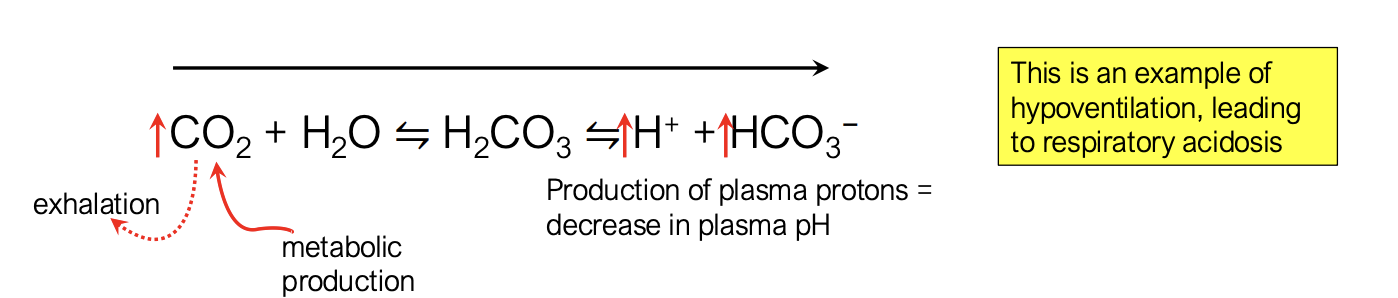
if you have decreased alveolar ventilation and unchanged metabolism, what happens?
more build up of CO2 in the body from lack of CO2 leaving the body
drives reaction to the right
CO2 → more protons and more bicarbonate → more acidic and leading to respiratory acidosis
if alveolar ventilation goes up and metabolism goes up, are you hyperventilating?
no, because they are both increasing due to homeostatic regulations