Amino Acids (for BSCI 2520)
1/23
Earn XP
Description and Tags
From Table 4-1 in the Voet textbook, 5th ed. All structures here are predominant at pH = 7 (except for His).
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
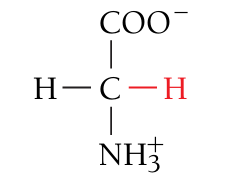
Glycine, Gly, G
Nonpolar
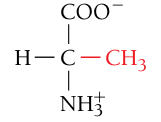
Alanine, Ala, A
Nonpolar
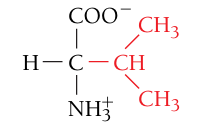
Valine, Val, V
Nonpolar
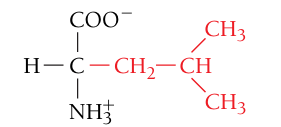
Leucine, Leu, L
Nonpolar
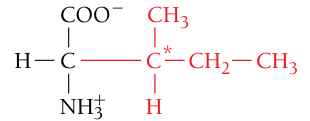
Isoleucine, Ile, I
Nonpolar
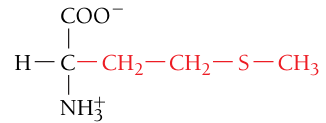
Methionine, Met, M
Nonpolar
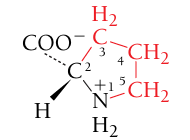
Proline, Pro, P
Nonpolar
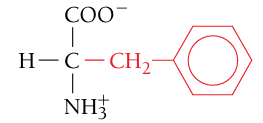
Phenylalanine, Phe, F
Nonpolar
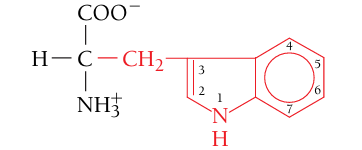
Tryptophan, Trp, W
Nonpolar
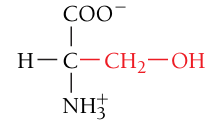
Serine, Ser, S
Polar, uncharged
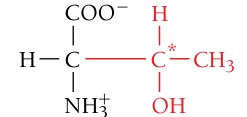
Threonine, Thr, T
Polar, uncharged
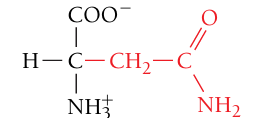
Asparagine, Asn, N
Polar, uncharged
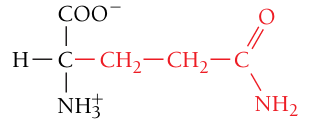
Glutamine, Gln, Q
Polar, uncharged
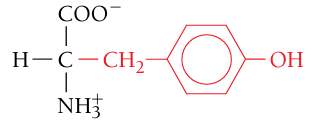
Tyrosine, Tyr, Y
Polar, uncharged
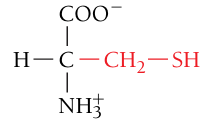
Cysteine, Cys, C
Polar, uncharged
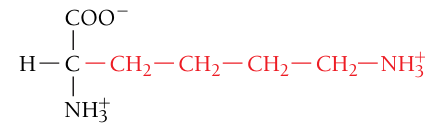
Lysine, Lys, K
Positively charged (basic residue)
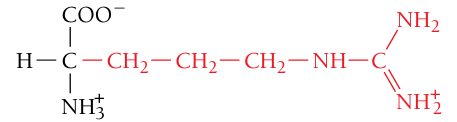
Arginine, Arg, R
Positively charged (basic residue)
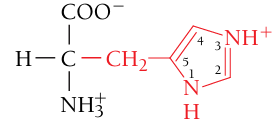
Histidine, His, H
Positively charged (basic residue)
The structures for all the other amino acids in this set depict what the amino acids look at pH = 7. However, at pH = 7, histidine’s neutral and protonated (positively charged) forms are BOTH present. This is because the pK of histidine’s R group is 6.04 (close to pH = 7)
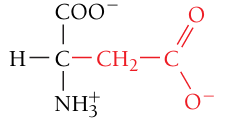
Aspartate / Aspartic acid, Asp, D
Negatively charged (acidic residue)
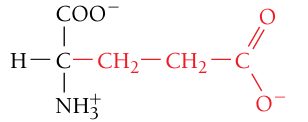
Glutamate / Glutamic acid, Glu, E
Negatively charged (acidic residue)
Asparagine OR aspartic acid (aspartate)
Asx, B
Glutamine OR glutamic acid (glutamate)
Glx, Z
Undetermined or nonstandard amino acid
X
Significance of pH = 7 on charge of amino acids
The pK of the carboxylic acid group is usually around pH = 2. The pK of the amino group is usually around pH = 9.
Thus, at pH = 7, the carboxylic acid group is predominately deprotonated. The amino group is predominately protonated.
If pH < pK of R group: protonated form of R group predominates (considered positively charged AA residues)
If pH > pK of R group: deprotonated form of R group predominates (considered negatively charged AA residues)