Animal growth and development / Developmental biology
1/115
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
116 Terms
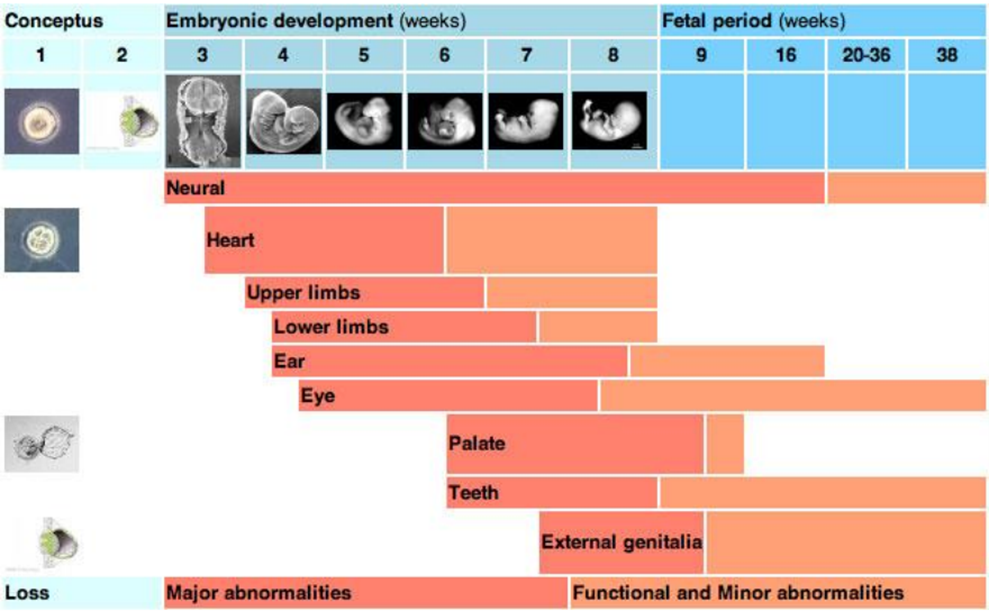
Examples of consequences of errors in normal embryo/foetus
embryonic loss > cannot be noticed or seen, no major signs, indication may be longer oestrus
fetal death > any stage of pregnancy
fetal mummification > dies inside the uterus, may be osmotic pressure, foetus loses liquid & becomes “liquid”
abortion > at any stage of pregnancy
stillbirth
birth of nonviable neonates
birth of viable offsrping with defects
Embryogensis is a?
Multifactorial process
Define congenital
A developmental disruption results in a deviation from normal that is present or apparent at birth. Genetic, environmental (nutritional), physical and infectious agents have all been defined as etiologic, determinants. Can be multi-factorial
Define Carcinogen
Agents or factors that initiate or induce neoplasia = excessive and abnormal growth of tissue: neoplasm / carcinogensis = initiates cancer
Define Mutagen
Agents or factors that produce a change in the genetic code of an organism
Define Tetragens
Agents or factors that cause the dev of physical defects in the embyro or foetus, has windows of susceptibility:
early embyrogenesis > primarily cause effects on DNA - mutations at a genomic or chrmosomal level
mid embryogensis / early foetal > effects on cell proliferation, differentiation or cell death / affects cell growth
late foetal > most tissues relatively protected, only highly proliferating tissues still susceptible (e.g. palate, eye, cerebellum)
What can affect the critical periods of sensitivty to external / internal factors affecting development
factors include: teratogens, envi, genetic, mechanical.
the longer the time of dev the higher the chance of being affected
Common congentical defects of domesticated animals, not a question
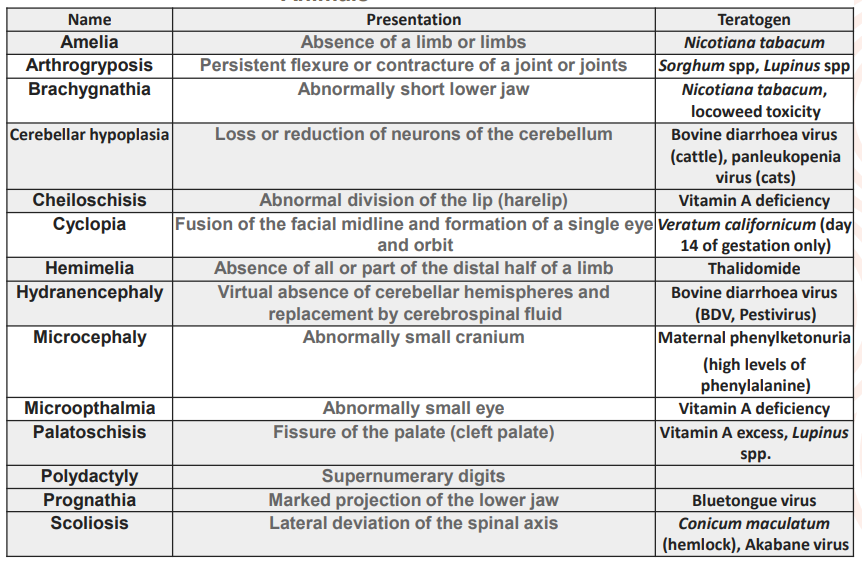
Physical causes of embryonic abnormalities?
physical trauma during gestation / on mother
congenital joint contracture by in-utero crowding > when animal gives birth to many offspring, crowding in uterus
spinal & limb deformities in foals following transverese or caudal presentaton > position of the foetus in utero
aggressive palpation cause either limb deformities or disruption of vascular supply to intestinal tract = no faecal matter exiting, atresia coli
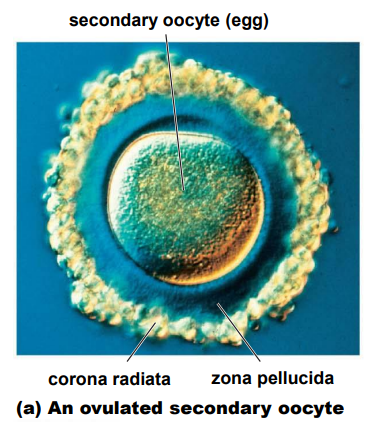
Describe Fertilisation
The process whereby a spermatozoon and a ovum fuse to form a single celled zygote in uterine tube (exact location dependent on species)
The 2 accessory follicle cells surrounding the egg once it leaves the ovary
Corona radiata - form barrier between sperm and egg
Zona pellucida - “clear area”, lies between the CR & egg
How does the sperm fuse its genetic material with the egg & what are the consequences if there isn’t enough sperm?
sperm must transit through the thick outer coating of the egg (ZP)
to acheive this each sperm releases enzymes from its acrosome
enzymes weaken both CR & ZP, allowing 1 sperm to wiggle through to egg
if there aren’t enough sperm, not enough enzyme is released & none of the sperm will reach the egg
whent he first sperm finally contatcs the egg’s surface, the plasma membrane of egg & sperm fuse, & sperm’s head is drawn into the egg cytoplasm> egg has vesicles that secrete a chemical to fuse the sperm to it
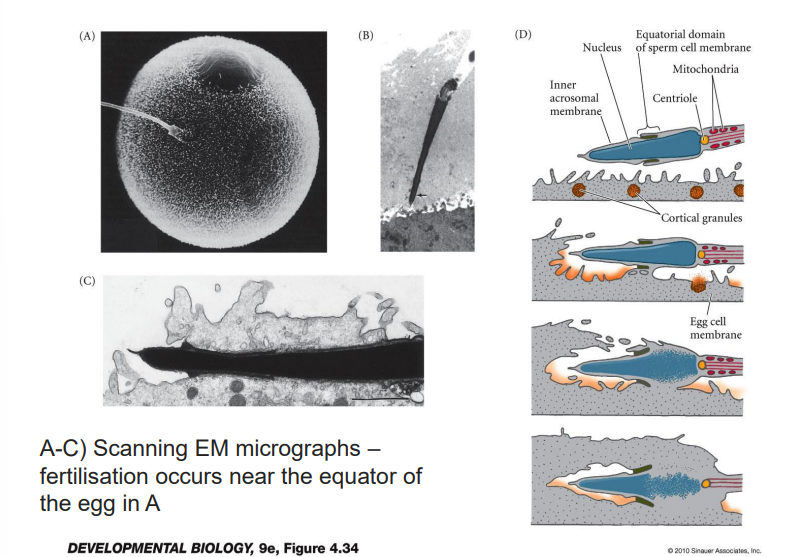
Detail fertilisation
once sperm enters, triggers 2 critical changes:
1 > vesicles near the surface of the egg release chemicals into the ZP that reinforce it & prevent additonal sperm from entering
2 > the egg has been in a dormant state until fertilisation ( no cell division or replication of genetic material has occured since the germ cell was laid down in the embryo)
in order for embryogeneis to proceed the fertilised ovum must be “activated” > calcium waves
occurs after fusion of sperm head to cell membrane of the ova
What do calcium waves result in?
reactivation of the genetic material of the ova
resumption of profilferation
release of inhibition of the material genome
exocytosis of M & F pronuclei (genetic material that gives rise to future organism)
Sumarise the 5 steps of fertilisation
1. Sperm is attracted to the egg by secretion of soluble molecules from the egg itself
2. Exocytosis occurs from the sperm acrosomal vesicle to release degrading enzymes
3. Sperm binds to extracellular matrix = ZP in mammals
4. Sperm passages through the extracellular matrix
5. Cell membranes of egg & sperm fuse – fertilisation & a zygote is produced
Embryos start from?
Fertilised zygote
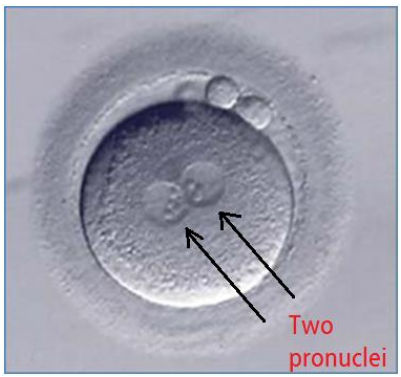
What are pronuclei?
genetic material of the fertilised zygote, one of maternal & one of paternal origin > in humans each contain 23 chromosones that give rise to 46 total
What is a structural similarity between species and what drives them to be different?
> in early embryogenesis, all embryos have the same number of pharyngeal arches
> evolution, environment & genetics drive species specific differences in development during gestation & beyond
What are the events that occur from fertilisation to implantation?
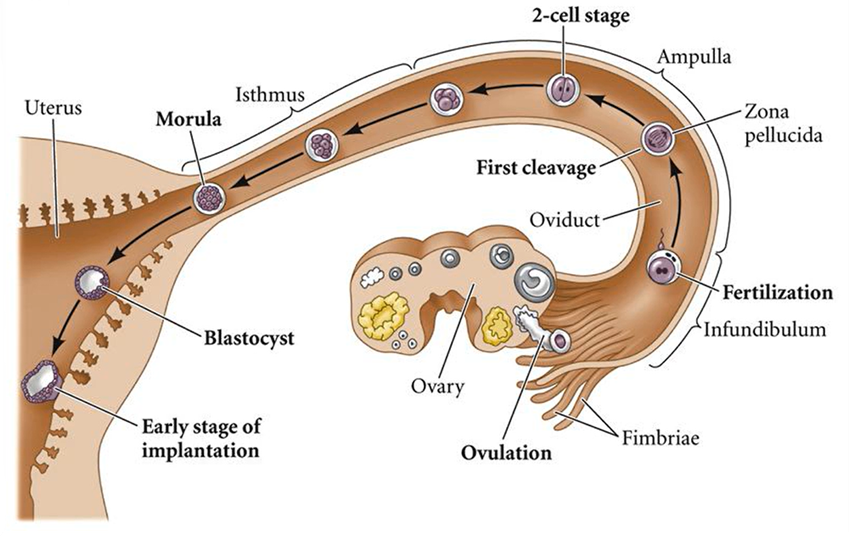
Summary of stages involved in embryonic cleavage & implantation 1
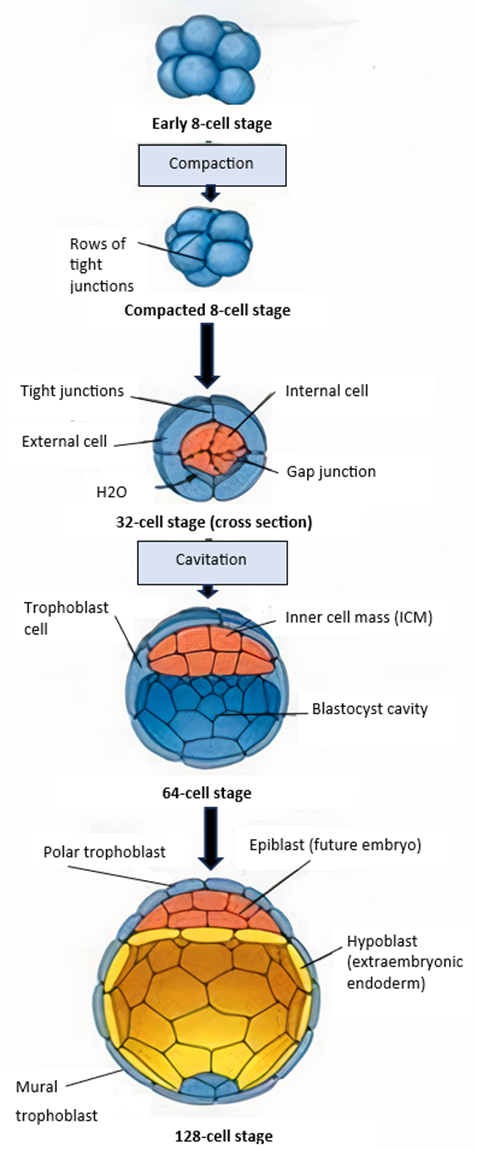
Summary of stages involved in embryonic cleavage & implantation 2
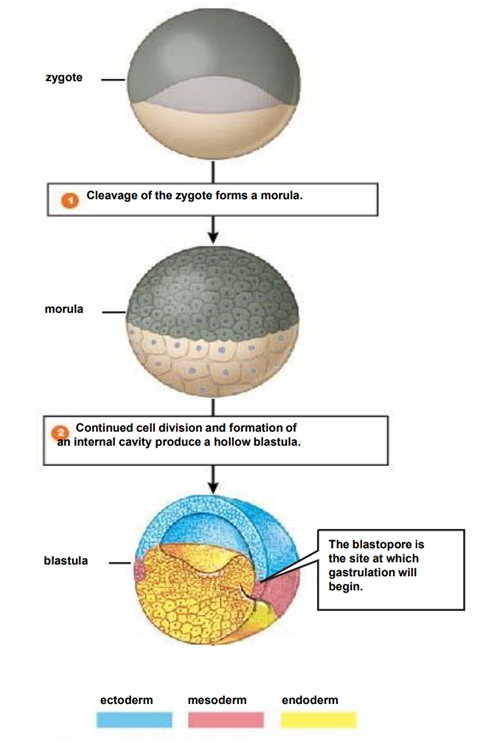
What is cleavage?
a series of rapid (mitotic) division following fertilisation
cell size progressively diminishes from that of the zygote
absence of cell growth phase between each division
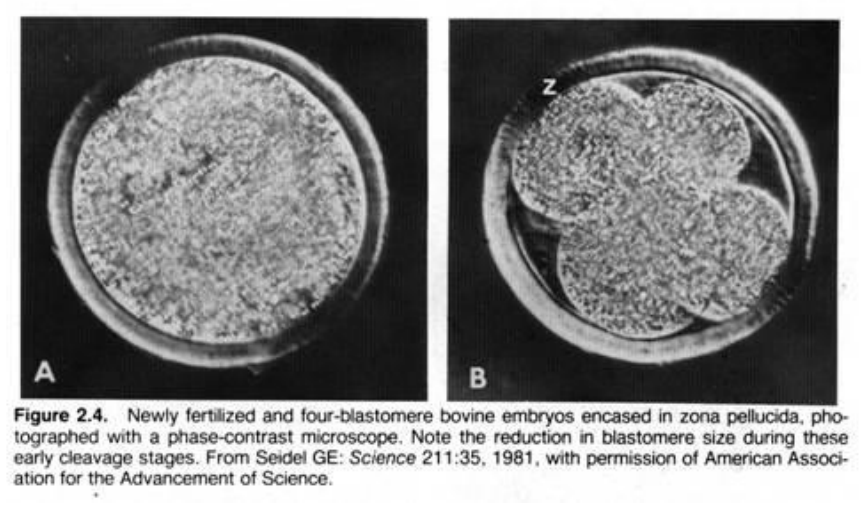
How does cleavage in mammals compare to other animals?
slower > 12-24 hrs between divisions
asynchronous > all blastomeres don’t divide at the same time
produces compact ball cells encircled by ZP
outermost > extraembryonic tissue only
central > foetus & extraembryonic tissue
= morula
What are the cells resulting from the cleavage process
Blastomeres > each individual cell is called a blastomere
How and where does cleavage occur?
occurs within isthmus
contractions propel embyro forward
produces compact ball cells encircled by ZP
outermost > outerembryonic tissue only
central > foetus & extraembryonic tissue
4-5 days in most domestics to uterus
secretions from epithelial lining
provide nutrients
specific proteins contribute to development
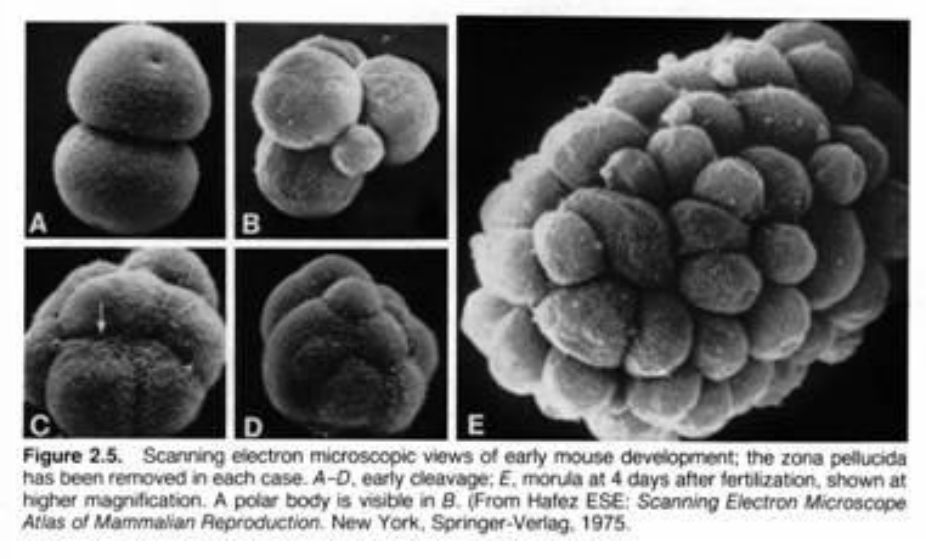
What is a morula?
solid ball of cells
16-64 blastomeres
formed near the end of cleavage processes
surroounded by the ZP
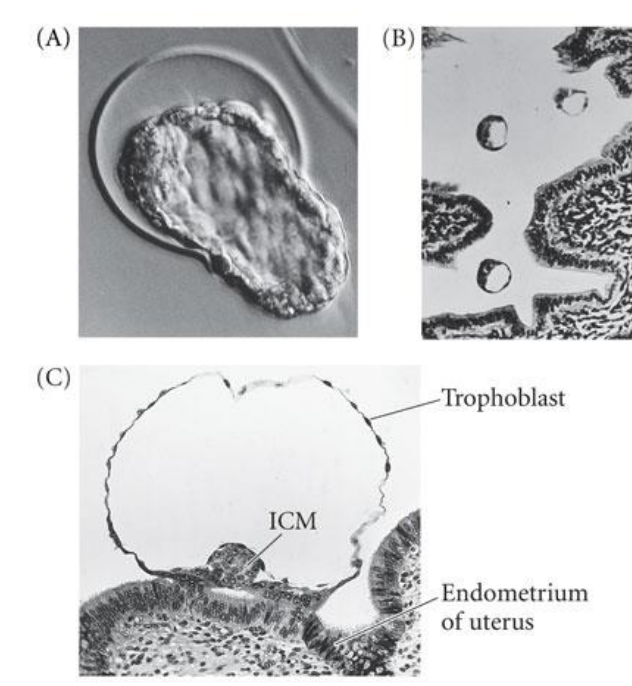
What needs to happen for implantation to occur?
The blastocyst (comes after the blastula) must shed the ZP > hatching
What happens during implantation?
commences once blastocyst is free of ZP - delayed in horse
polytocous species (multiple) blastocyts evenly distributed
some monotocous species have preferred implantation site e.g. horse base of uterine horn
uterine endothelium catches the blastocyst on a sticky extracellular matrix
blastocyst secretes proteases (enzymes that break down protein) > embed
Timing from ovulation to implantation in different species
Sheep, cat > day 14-18
Pig > day 12-16
Dog > day 14-18
Cattle > day 17-35
Horse day > 17-56
Human day > 6-7
partly due to different types of implantation & their mechanisms for embedding of the embryo into or on the uterine lining
What are the different types of implantation?
classified according to relationship between blastocyst & uterine lumen
central
blastocyst remains within uterine wall
ungulates, carnivores & lower primates
wide variation in timing
eccentric
blastocyst lies within uterine crypt or recess
mouse rat, hamster, rabbit and some bats
quick process
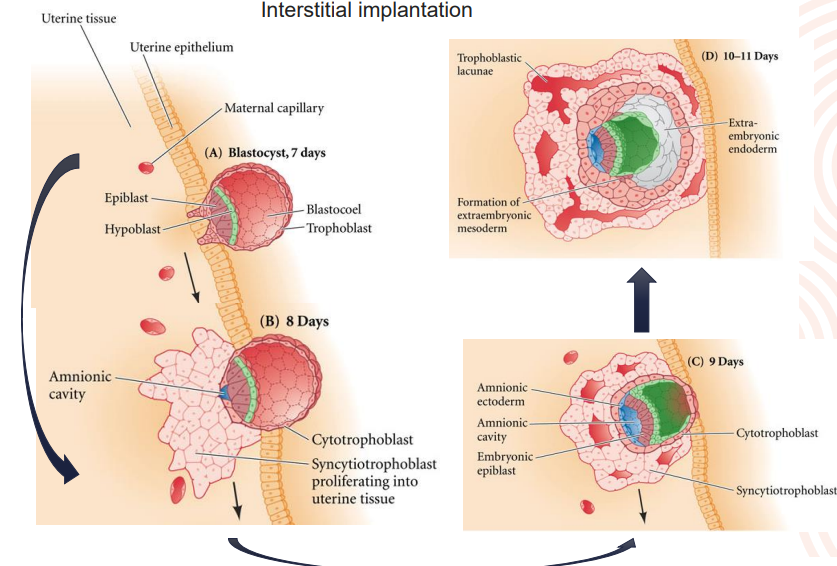
interstitial
conceptus invades the uterine wall
guinea-pig, chimpanzee & man
quickest process
Explain Interstitial implantation
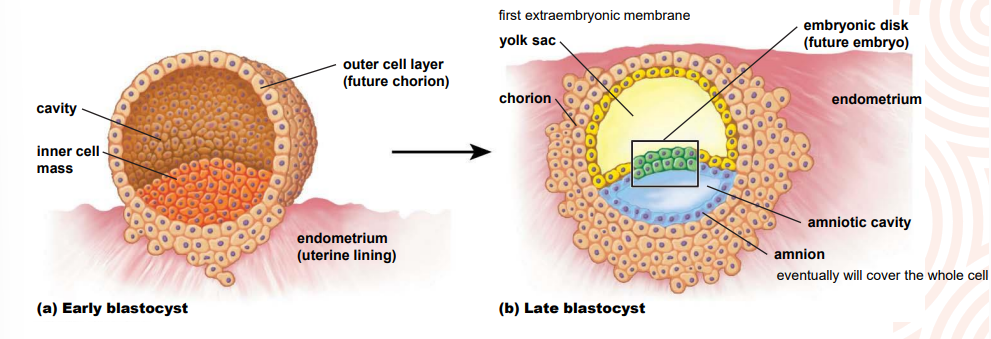
What are the foetal membranes, extraembryonic membranes and what is their purpose?
provide protection & nutritional & excretory requirements for the dev embryo
four major membranes
yolk sac
amnion
chorion
allantois
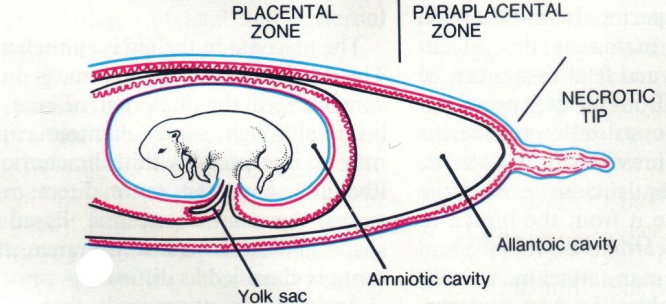
Yolk sac
first membrane formed
involved in early haematopoiesis > formation of blood cellular components & angiogenesis > formation of new blood vessels
vitelline blood vessels - gut vessels
cranial mesenteric artery
hepatic portal vein > blood vessel that carries blood from the gastrointestinal tract, gallbladder, pancreas and spleen to the liver
Amnion
surrounds foetus
covers umbilical cord & continuous with body surface at umbilicus
fluid filled
filtration from superficial blood vessels of embryo
secretions from alimentary & respiratory tracts
urine from kidneys > from urethra until bladder sphincter is patent
allantoic fluid which may be transported across the allantoamnion > fused membrane
Amniotic fluid
cushions the foetus
allow unrestricted mvt
important in later stages of dev
prevents pressure related growth abnormalities
at birth when ruptures acts as lubricant for the birth canal
Amnion at birth
horse, dog & cat raphe (> the line of fusion of the amnionic folds over the embryo in reptiles, birds, and certain mammals) degenerates
may be born in amniotic sac
ruminants & pigs raphe retained
amniochorion formed above dorsal aspect of embryo ruptures at birth
foetus born without surrounding amniotic sac
Chorion
established at the same time as amnion
chorionic sac surrounds other embryonic membranes & foetus
composed of
trophoblast > outer layer
extraembryonic mesoderm > inner layer
lies in apposition to uterine lining
participates in formation of foetal component of placenta
Allantois
dev as diverticulum of the hindgut
varies considerably in size between species
humans / primates : residual structure
pigs : v large
reptiles & birds : v large waste sac
stores urinary waste
in birds also mediates gas exchange & calcium transport from shell to embryo by fusion with the chorion (called the chorioallantoic membrane)
Placenta
placentation is the structural organisation & mode of attachement of the placenta
organ of metabolic interchange between mother & foetus
large surface area
nutritional
excretory
immunological
endocrine
What are the components that undergo modification for the placenta
maternal
endometrial lining of uterus
foetal
chorion
Provision of the embryos nutritional requirements
number of sources provide the embryo/foetus with nutrition
haemotropic source
maternal blood stream > primary source
histotropic source
coiled endometrial galnds roduce histotrophe > uterine milk > fat & glycogen
The different classifications of placenta
based on gross appearance of the definitive placental zone
diffuse
placental zone covers almost entire surface of chorionic sac
horse & pig
cotyledonary
placental zone restricted to specialised cotyledons
cotyledons develop in respponse to chorionic contact > caruncles
caruncles permanent arranged in rows
70-140 in cows
88-110 in sheep
160-180 in goats
cotyledon & caruncle > placentome
zonary
placental zone in band around central region
complete in dogs & cats incomplete bears & mustelids (ferrets, skunk, weasels)
trophoblast modified & invades endometrium
discoid
placental area 1 or 2 disc shaped area
man, rodents 1 disc
monkeys 2 discs
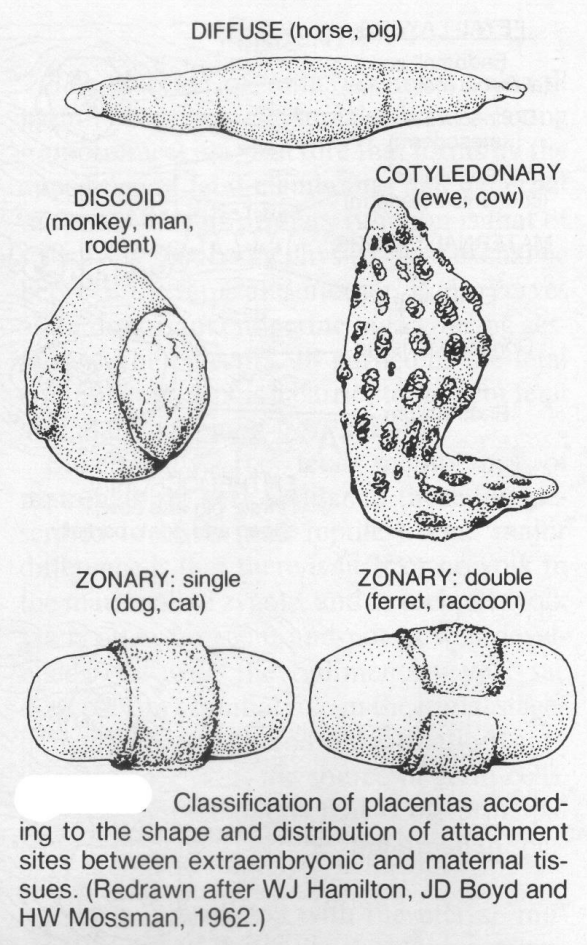
What happens to the placenta at birth and how are the diff placentas classified
loss of maternal tissue at parturition
deciduate
invasion & destruction of maternal tissue resulats in shedding of maternal tissue
decidua > modified mucosal lining of the uterus (that is, modified endometrium) that forms every month, in preparation for pregnancy, forms the maternal part of the placenta and remains for the duration of the pregnancy. After birth the decidua is shed together with the placenta
maternal haemorrhage may occur > carnivores, primates & rodents
non-deciduate
virutally no loss of maternal tissue at partiturition
ruminants, horses, pigs
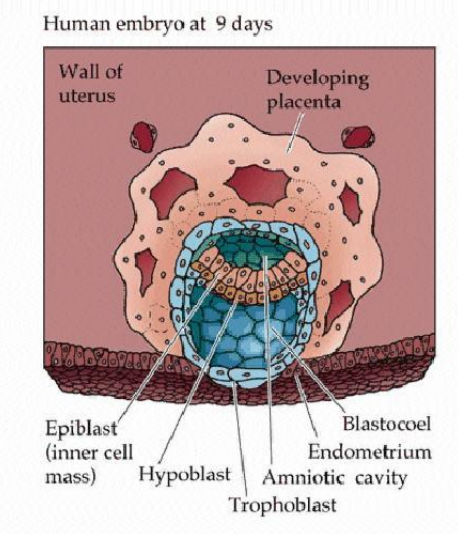
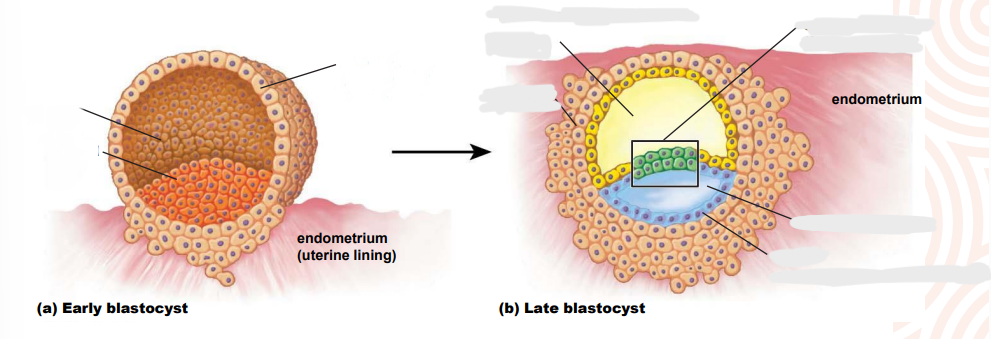
Briefly describe the formation of the Inncer cell mass (ICM)
cellular division creates morula
cavitation occurs due to sodium being pumped actively into central area
osmotic pressure (osmotic pressure imbalance) causes the cavity within the blastocyst to expand and form blastocoel > migration
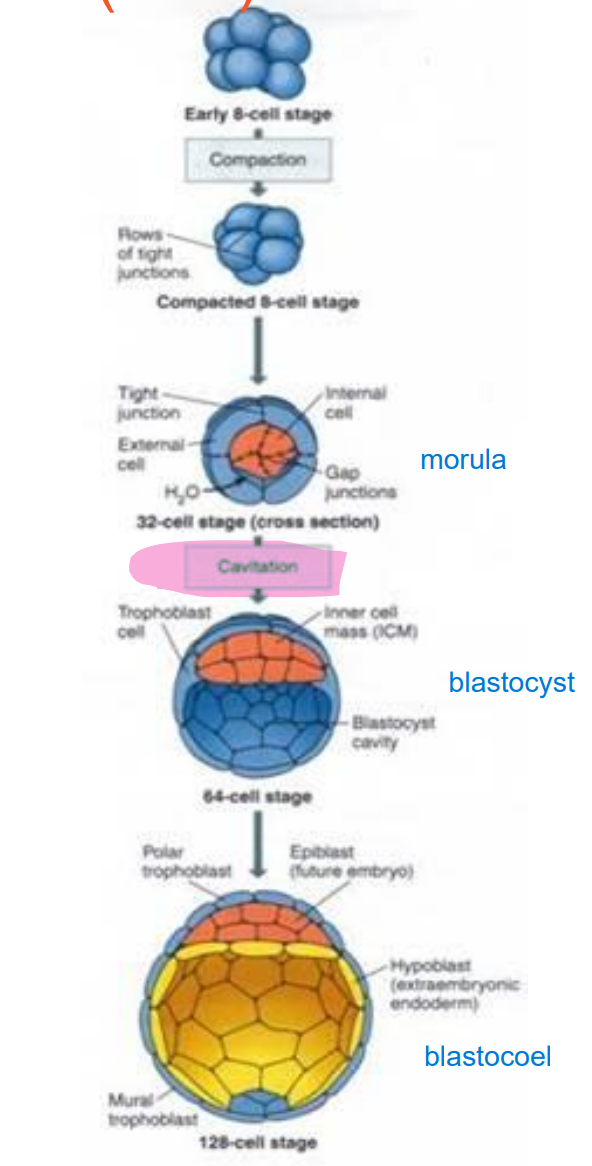
What is the blastocoel?
the fluid filled cavity, or space, in the developmental stage known as the blastocyst
List the steps of animal embryonic dev
zygote > morula > blastocyst > gastrula > embryo
fertilisation > cleavage > blastulation > gastrulation > organogenesis
Embryonic disc formation
further fluid & cellular migration creates disc from the ICM which is a flattened pear shape
disc initally bilaminar but becomes trilaminar > GASTRULATION
What makes up the bilaminar disc
epiblast
superficial layer > embryonic body
hypoblast
deep cell layer form extraembryonic membranes
cells migrate - delaminate - from second layer under trophoblast - blastoceol now covered by two layers > the yolk sac
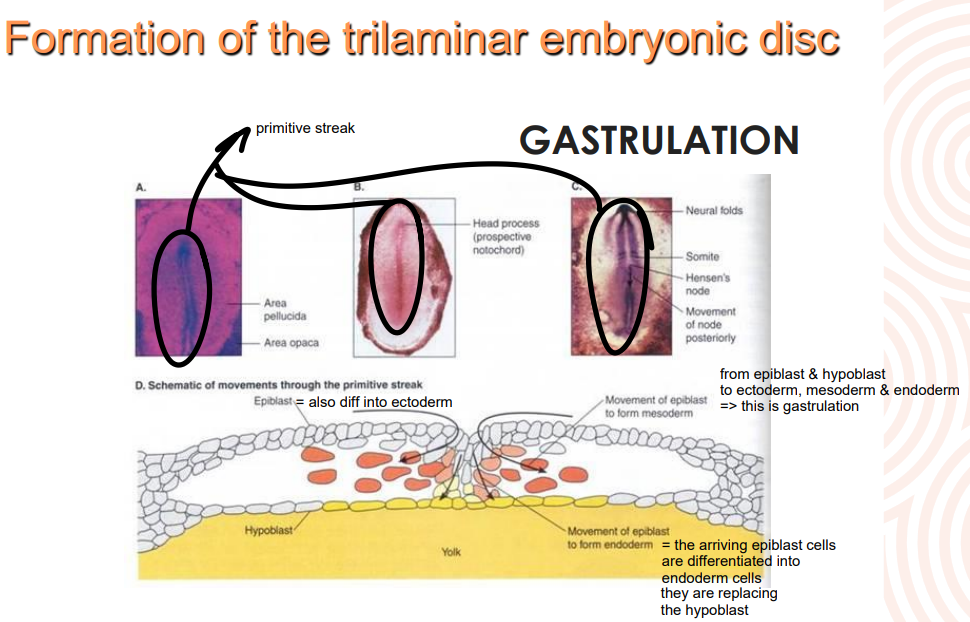
What is gastrulation
also called germ layer formation is the stage of embryological dev where disc becomes trilaminar
due to cellular migrations from surface to interior
mvt creates depression known as primitive streak
layers of the trilaminar disc gives rise to specific organ systems
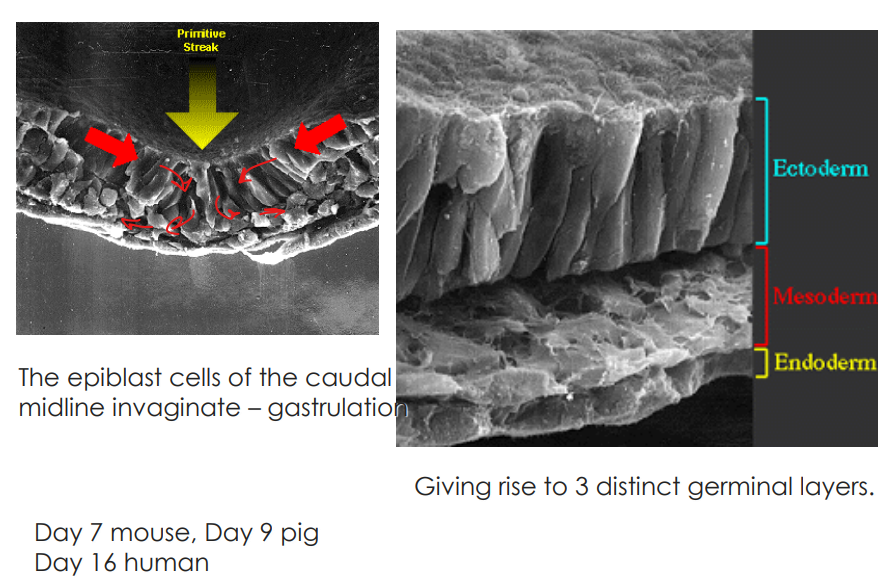
What causes the primitive streak to form?
devs in the epiblast cell layer bc of the weight of the cellular migration
What is the node at the top of the primitve streak called
Hensen’s node
not a question, need to know this
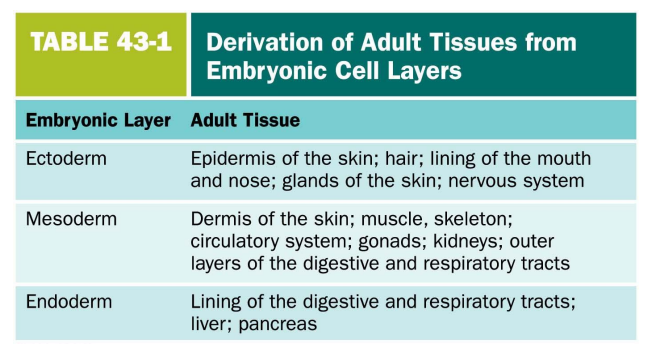
Detail the triminar layers & what they form in the adult tissues
What is patterning
the mechanism by which initially equivalent cells in a developing tissue in an embryo assume complex forms and functions
What is the first patterning event
the creation of a “head” from a “tail” > polarisation
anterior > cranial to posterior > caudal regions
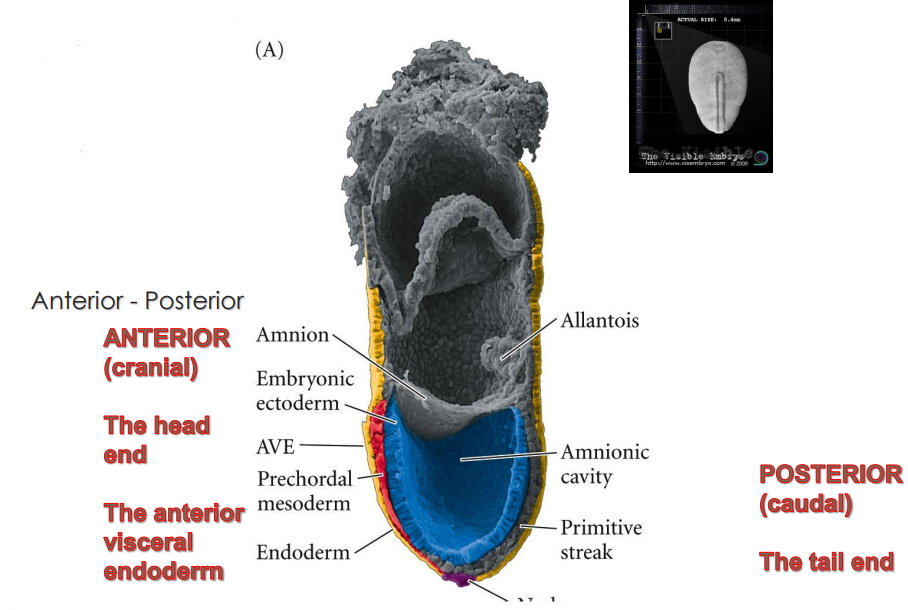
What are the two major signalling centres that pattern the embryo and what are their functions during the gastrulation/primitive streak stage?
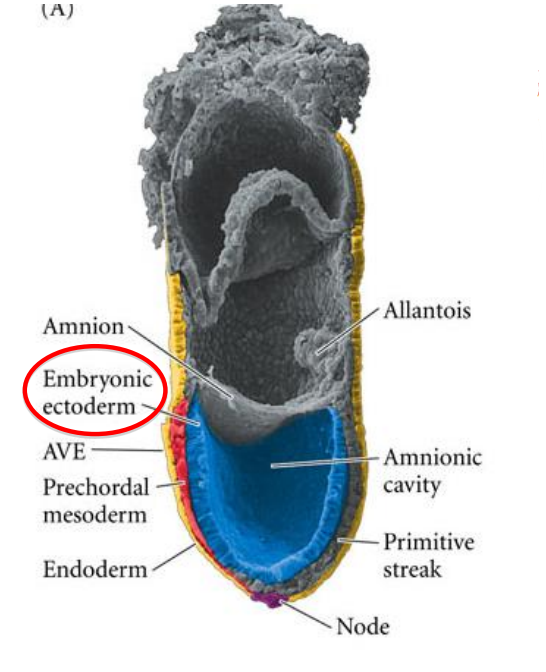
anterior visceral endoderm AVE > head organiser
node > rest of body organiser
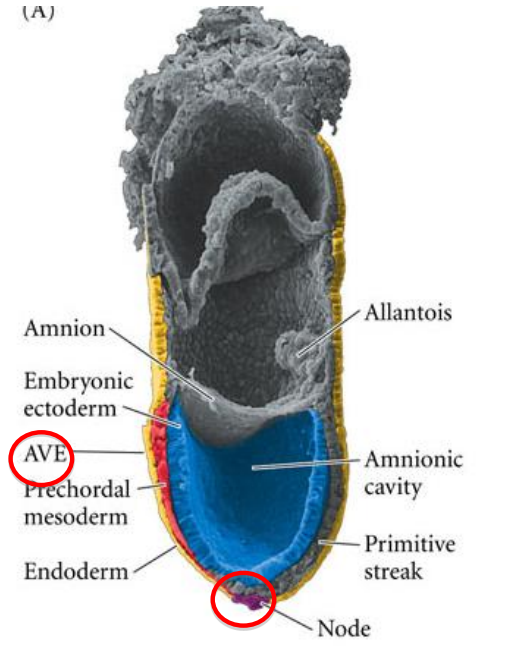
What covers the dorsal surface during the gastrulation/primitive streak stage?
the embryonic ectoderm > in contact with the amniotic cavity
How is the anterior posterior formation formed in avian species?
gravity
rotations in the shell results in the light components of the yolk pushing up one side of the blastoderm
the higher side becomes the posterior region of the embryo
List the steps in establishing asymmetry in the early chick embryo - making a left and right
to the left hand side of the Hensen’s node, sonic hedgehog protein (encoded by the Shh gene) activates cerberus which causes expression of nodal
nodal in turn activates the expression of Pitx2 > gene that confers “leftness” to that side of the body (also expressed in the head region but plays a different role)
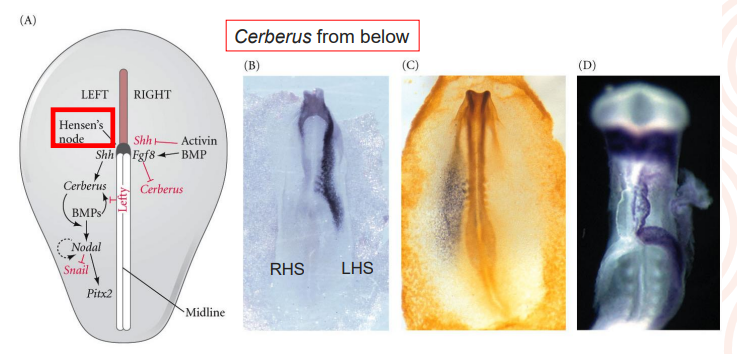
Left right asymmetry in the dev mammal, not a question
Why is patterning so important?
assymetry in the morulae contributes to normal dev of the blastocoel cavity
loss of assymetry in the early embryo can (cleavage, morula, early blastocyst) give rise to twins (also implantation of more than one embryo)
What are the two types of twins?
dizygotic > twins arise from 2 ova from 2 ovarion follicles fertilised by separate spermatazoa during a single breeding cycle (fraternal twins / littermates)
monozygotic > arise at the primitive streak stage, observed in humans, sheep & pigs
What is the rate of twinning
cattle > dizygotic : 2-3% & monozygotic : 0.1%
sheep > dizygotic 2-5% (lowland > highland)
horses > multiple ovulation <30%, twins <2% → due to innate physiological mechanisms inhibiting twin implantation in mare
monozygotic incidence <1%
Timing of separation of monozygotic twins, not a question
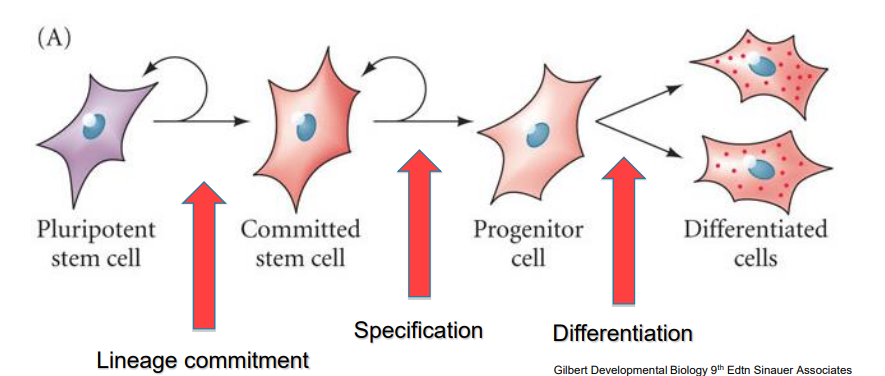
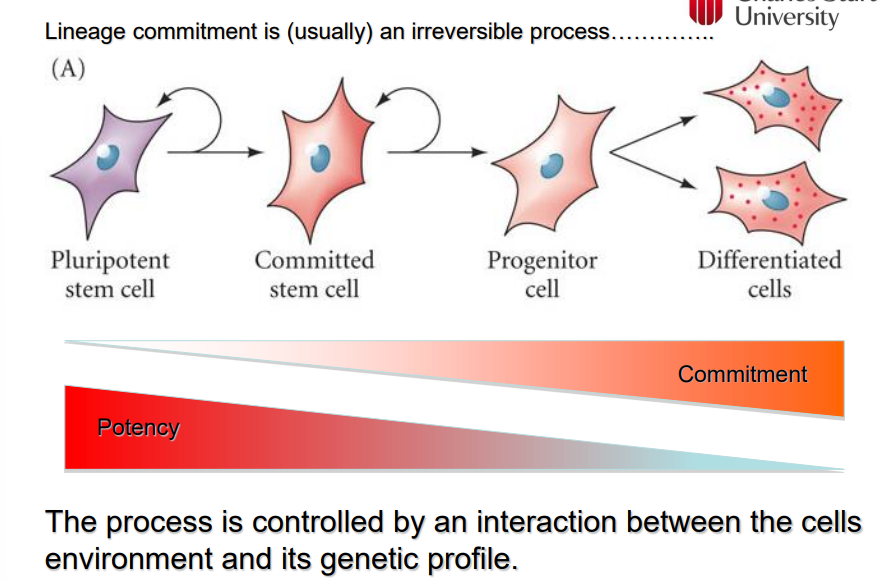
What is the definition of a stem cell?
a cell that can produce identical copies of itself (self-renewal) indefinitely (true stem cell vs progenitor cell)
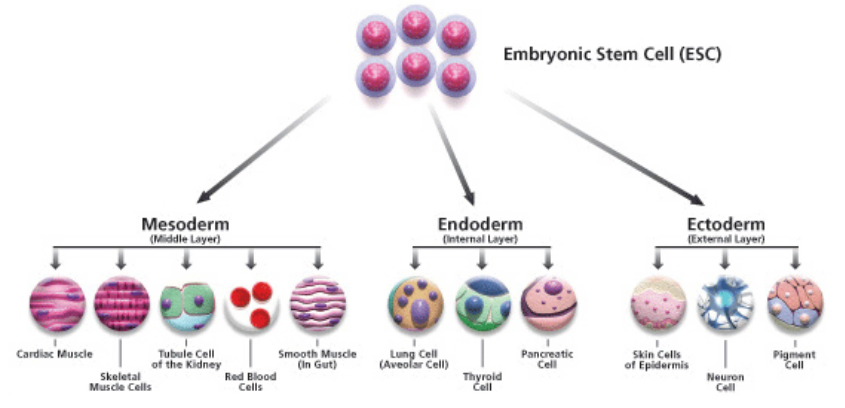
What do stem cells do in an embryo
building blocks of the embryo and all its tissues
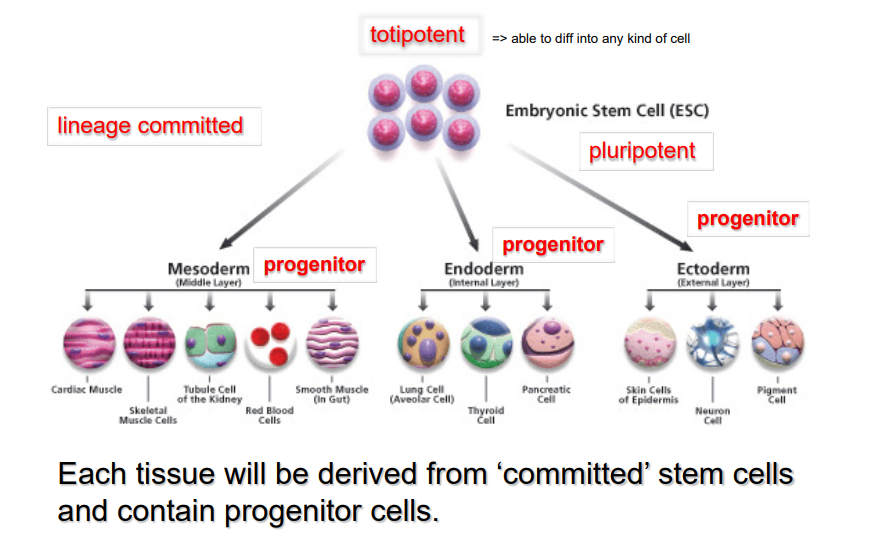
What is potency and how are the potency and the commitment of a stem cell linked
potency is the ability of a stem cell to become any type of cell
potency reduces once a stem cell becomes commited
once it has committed it has a reduced ability to come back to a pluripotent stem cell
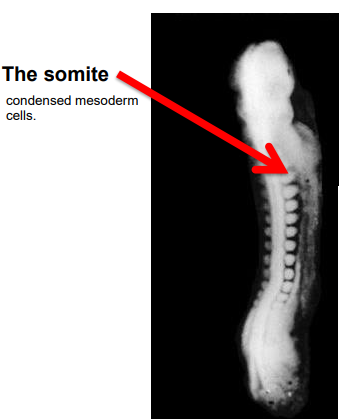
What are the earliest visible structures in the embryo
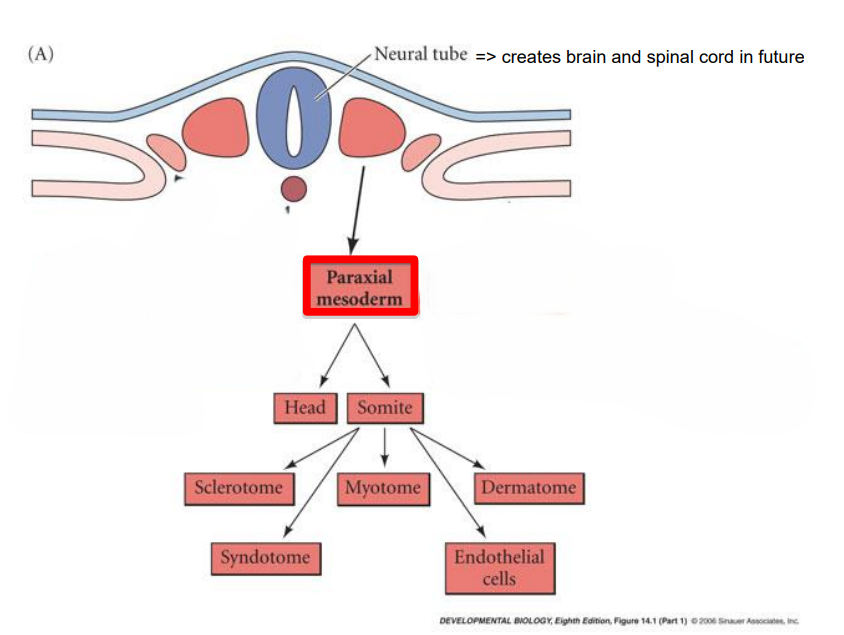
somites > condensed mesodern cells
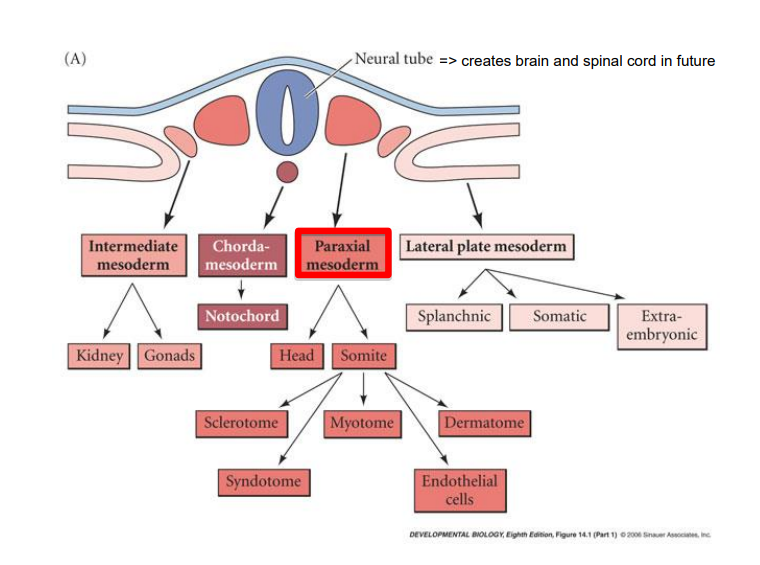
What is the trunk of the embryo at neural tube stage comprised of?
4 types of specific mesodermal cells
chordamesoderm (notochord)
paraxial (somitic) mesoderm (connective tissue, bone, muscle, cartilage) > make up the somites
intermediate mesoderm (urogenital system & adrenals)
lateral plate mesoderm (heart, blood vessels & blood cells, body cavities, non-muscular components of the limbs)
mnemonic > cabbages partake in lesbianism
Where do somites form as the neural folds rise into apposition?
pairs of somites form on either side of the neural tube
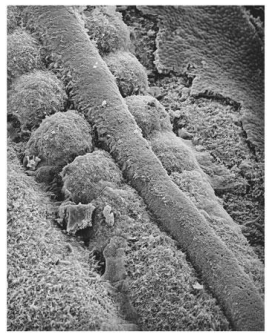
How are these somites derived / how do they start out?
condensation of the paraxial mesoderm
What are the 4 cell types of mature somites
sclerotome > vertebrae & rib cartilage
myotome > musculature of back ribs & limbs
dermatome > dermis of the back
syndetome > tendons & blood vessels
mnemotic => sexting my dermatologist sexy
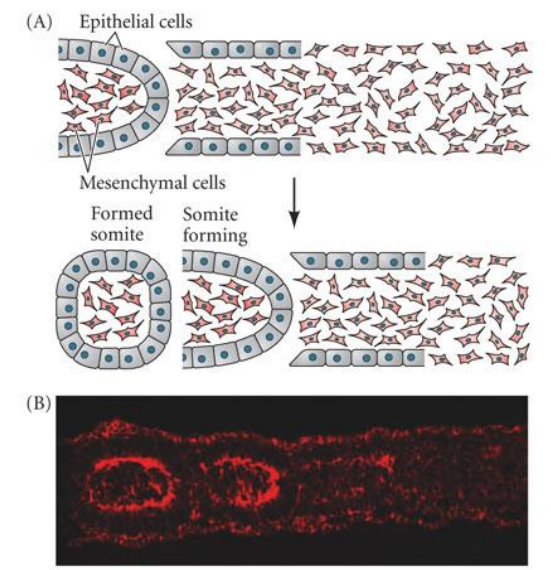
What does “epithelialisation of somites” refer to?
after separation, expression of fibronectin & N-cadhrein organise “inner” mesenchymal” & “epithelial” components
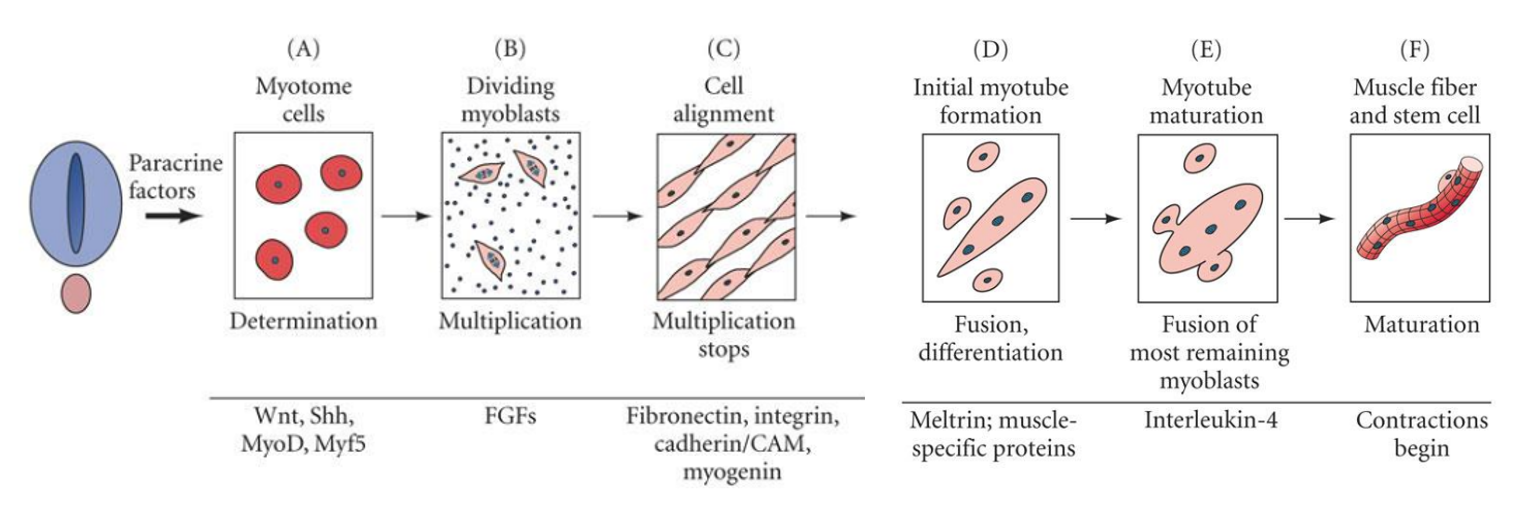
What are the steps in the generation of muscle tissues?
A. myotome cells are specified to become myoblasts by induction of MyoD (protein regulator of muscle differentiation)
B. myoblast numbers are expanded under the influence of FGFs > fibroblast growth factors
C. cell adhesion molecules control muscle cell alignement
D. myoblasts begin to fuse together to create myotubes, this occurs as myoblasts exit the cell cycle due to depletion of fibroblast growth factors (FGFs)
E. F. Myotubes complete fusion & coordinated contraction can be initiated
myotome cells become the myoblast cells > when there are enough myoblast cells they line up and become the muscle cells
What does sclerotome need for cartilage differentiation?
expression of Pax1 gene > required for cartilage differentiation
List the lineages for osteogenesis and the major modes of bone formation
3 distinct lineages generate the skeleton
somites > axial (vertebral skeleton)
lateral plate mesoderm > limb skeleton
cranial neural crest > craniofacial bones & cartilage
2 major modes of bone formation
direct conversion of mesenchyme to bone > intramembronous ossification
indirect conversion via cartilage to bone > endochondral ossification
Explain intramembranous ossification
mesenchymal cells condense to form osteoblasts which lay down an (osteoid) collagen proteoglycan matrix that is able to bind calcium
osteoblasts then become embedded in the matrix & become osteocytes
process involves BMP’s (bone morphogenetic proteins) & a transcription factor CBFA1 (core binding factor alpha 1)
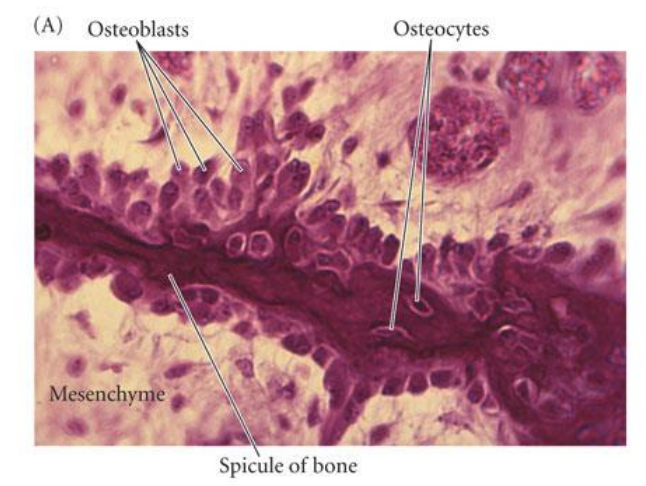
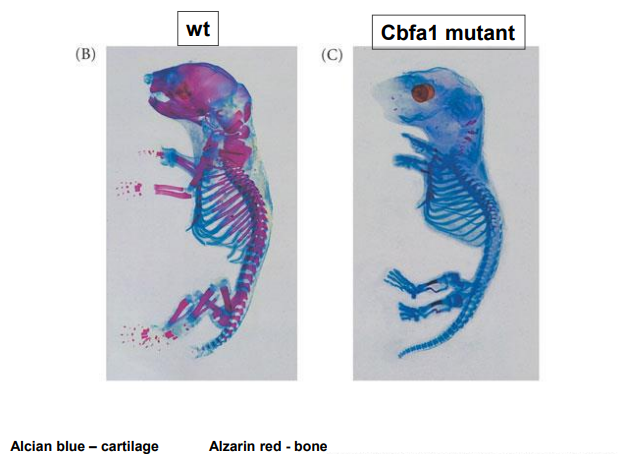
not a question
What is endochondral ossification
occurs primarily in the vertebral column, ribs, pelvis, & limbs (somitic & lateral plate mesoderm-derived bones)
involves formation of cartilage from aggregations of mesenchymal cells
replacement of cartilage with bone
How are limbs zones of polarising activity
the limb has polarity > finger / toes at one end & humerus / femur at the other end
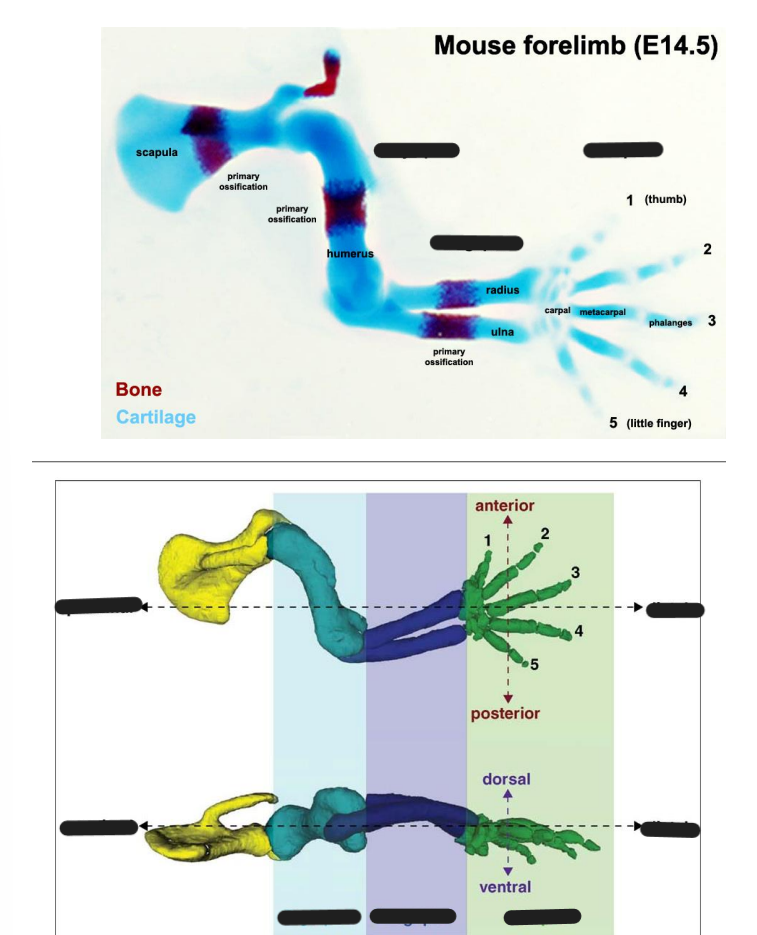
What segments are tetrapod limbs created as
3 segments
proximal stylopod (humerus / femur)
middle zeugopod (radius, ulna / tibia, fibula)
distal autopod (carpals / tarsals)
mnotic : see zebras autopilot
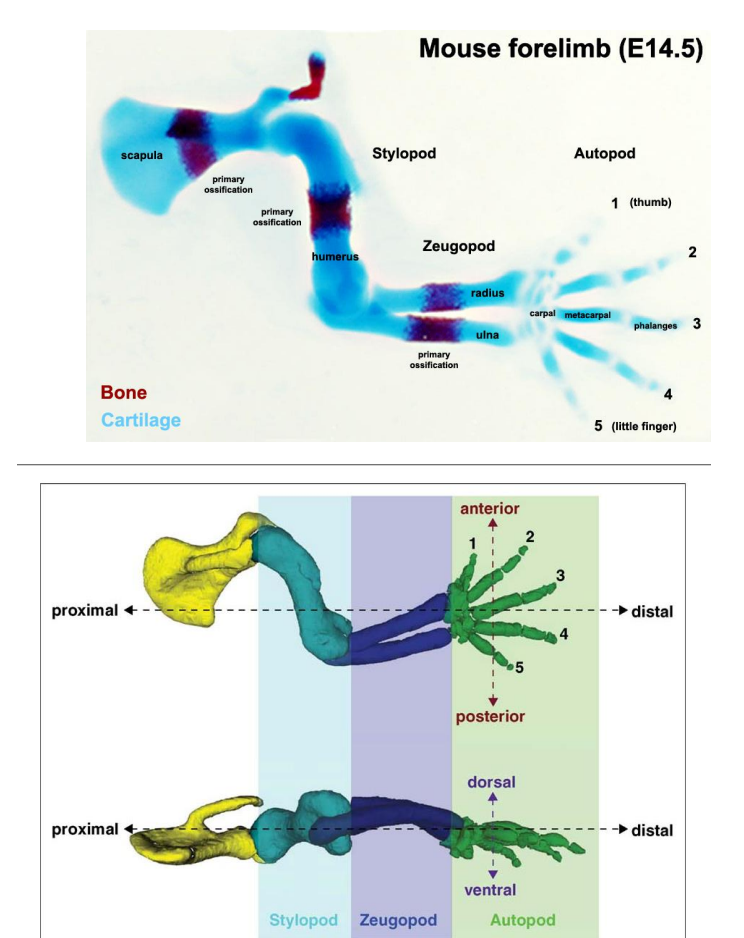
How does the limb develop?
limb bud
in sheep, pigs & cats limb bud development commences at the end of the of the 3rd week of gestation
in humans, cattle & dogs, occurs in 4th week
limb buds form as mesenchymal condensation of the later plate mesoderm
What are limb buds and at what levels do they form?
begin as small elevation on dorsolateral body wall
aggregation of underlying somatic mesoderm
form at 2 lvls
forelimb - C5-8
hindlimb - L3-5
What precedes, the forelimb or the hindlimb?
the forelimb precedes the hindlimb
What are limb inducing signals?
lateral plate mesoderm destined to become limb skeleton
secrete paracrine factor FGF10
FGF10 initiates limb bud formation - interaction between mesoderm & overlying ectoderm
formation of the apical ectodermal ridge (AER)
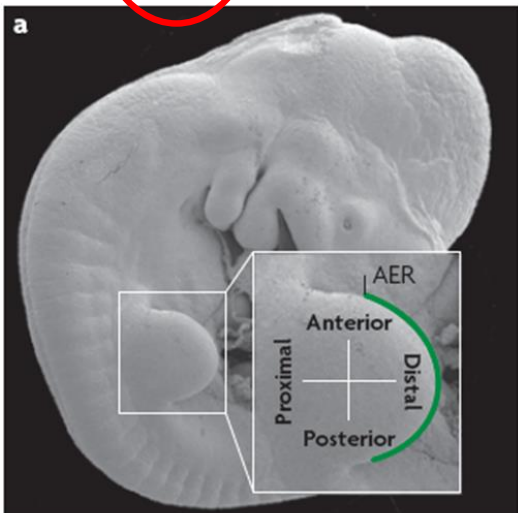
What are the roles of the AER
the AER keeps the mass of underlying mesenchyme (the progress zone (PZ)) in a “plastic” proliferative state to ensure limb outgrowth
maintains expression of molecules that specify the A/P (thumb pinkie) axis
interacts with proteins that specify the D/V (knuckle-palm) axis of the distal limb
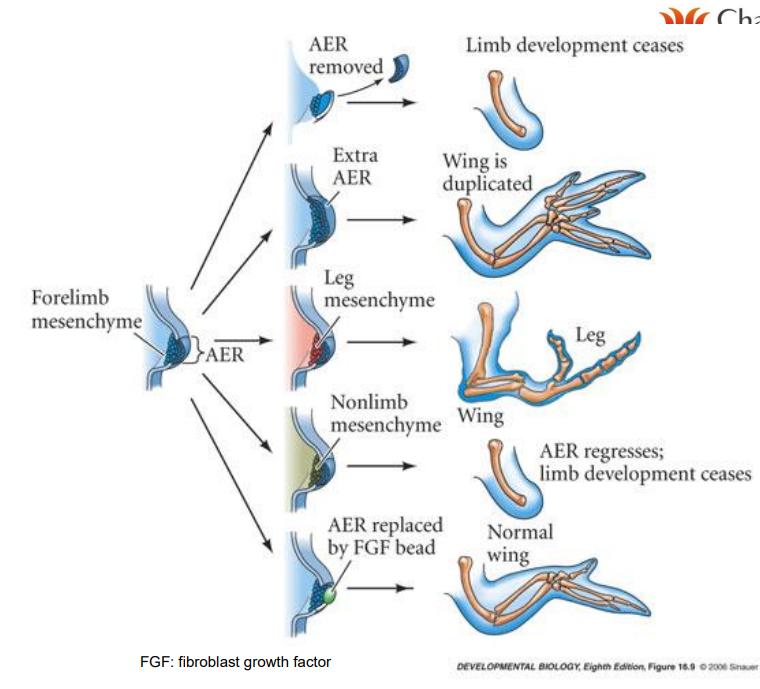
What is the relation between FGF and AER
FGF is involved in initiation & maintenance of the AER
disrupt FGF signalling, affect limb outgrowth, digit formation but not bone differentiation
How do digits form?
increase mesenchymal density in distal extremity
tissue between rays programmed to degenerate
digits 4-10 days after limb bud appears
if no mesenchymal cell death - soft tissue web
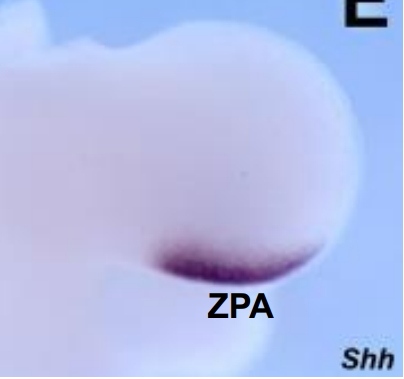
What is the ZPA and briefly explain ZPA and AP axis specification
A/P axis specified very early
singal emanating from mesodermal cells located at posterior junction between limb bud and body wall
called the zone of polarising activity => ZPA
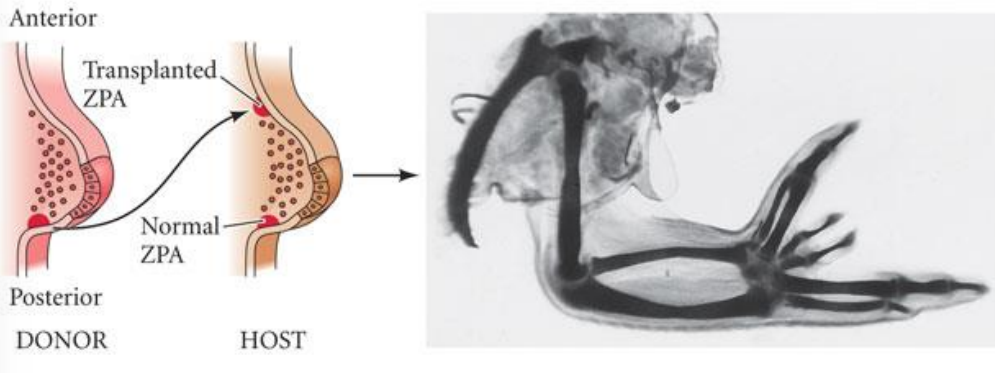
What happens if there is a duplication of the ZPA?
distal limb duplication
What is the polarising signal expressed in the ZPA?
Sonic hedgehog > SHH
overexpression of SHH in the limb bud results in mirror-duplication of digits > polydactyly
List examples of postnatal limb growth and development
achondroplasia (hereditary dwarfism) > initiates premature differentiation of epiphysial growth plates of the long bones
if high levels of GH are produced prior to puberty > failure of grwoth plate closure - giantism
if high levels of GH after puberty > acromegaly enlargement of the extremities
Why is it important to know the dev of the cardiovascular system
cardiovas. defects represent the most common class of congenital defects presenting in animals
frequently encountered in dogs and cattle
classified as either cyanotic (insufficient oxygen provided to peripheral vasculature) or acyanotic (sufficient oxygen - but other issues)
List examples of acyanotic & cyanotic abnormalities
acyanotic abnormalities
aortic stenosis (obstruction of the L ventricular outflow) > frequently detected in large breeds of dogs
pulmonary stenosis (narrowing of the pulmonary outflow)
ventricular septal defects
atrial septal defects
cyanotic abnormalities
large ventircular septal defects including a syndrome called Tetralogy of Fallot (multiple defects)
transposition of the outflow vessels (reversal of pulmonary & systemic flows)
What rank of the embryo at neural tube stage is involved in cardiovascular dev
lateral plate mesoderm
What does specification of the heart primordia mean?
heart is 1st functional organ to dev, evident at 18 days
future cardiogenic mesoderm (near Hensen’s node) migrates through the primitive streak
intiated through the FGF (fibroblast growth factor) & BMP (bone morphogenic protein) pathways via adjacent endoderm
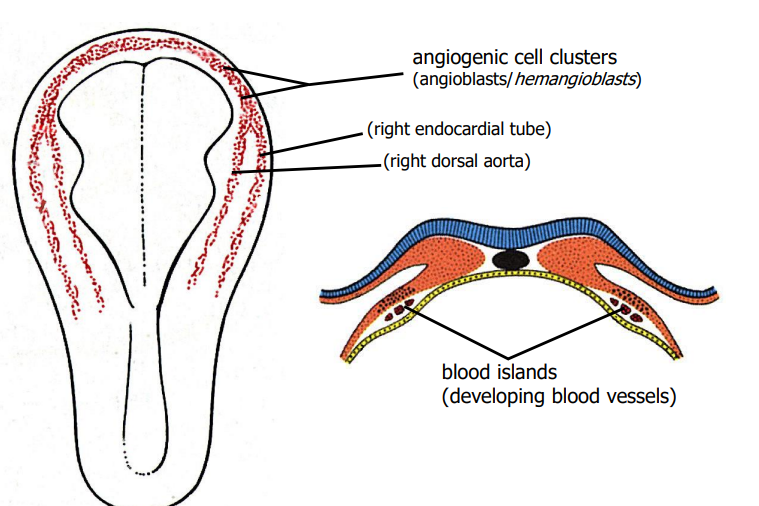
Explain the formation of the heart tube
develops from splanchnic mesoderm near the head of the embryo in a region known as cardiogenic area
the cardiogenic area begins to form 2 strands called cardiogenic cord
lumen develops rapdly in the cardiogenic cord & referred to as endocardial tubes
the part of the intra-embryonic cavity (space between the somatic & splanchnic mesoderm) close to spetum transversum at the cranial end of the embryo reorganises to give a distinct cavity known as pericardial cavity
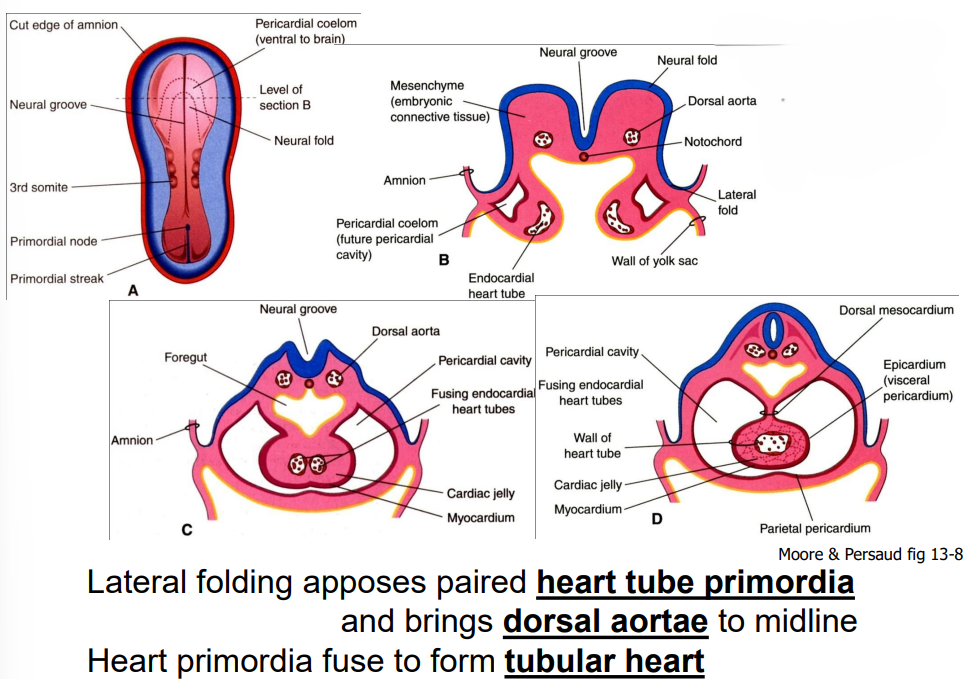
lateral folding, not a question