BIOL311 Exam 4: Tissues, Stem Cells, and Cell Movement
1/112
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
113 Terms
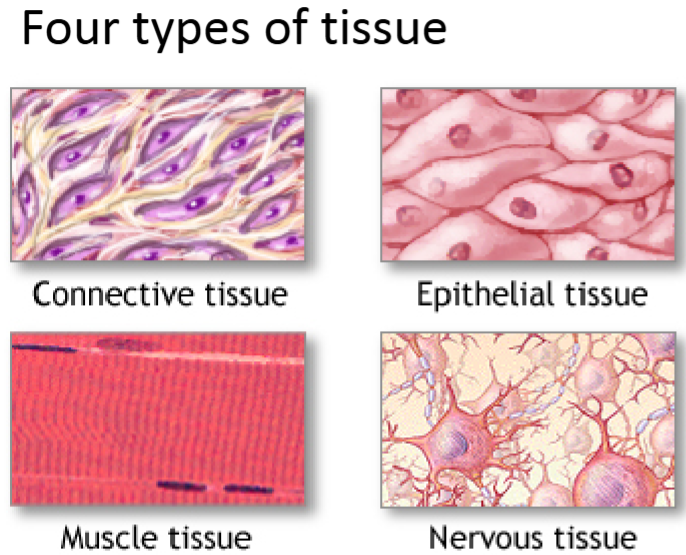
What are the 4 tissue types? Give an example of the cell type that fits into each category.
Of all the cell types, which tissue type is represented the most?
Epithelial (most represented): epithelial cells.
Connective: blood vessels, bone, fibroblasts, endothelial cells, fatty tissue, etc.
Nervous: neurons, glia, astrocytes, etc.
Muscle: smooth muscle cells, cardiac muscle cells, striated muscle cells.
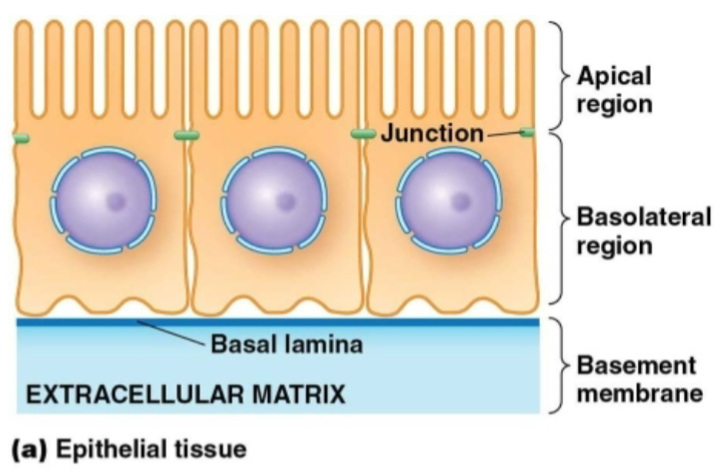
Epithelial cells
Part of epithelial tissue.
Packed together with lots of cell-cell contacts.
Connected to the ECM on the basolateral side.
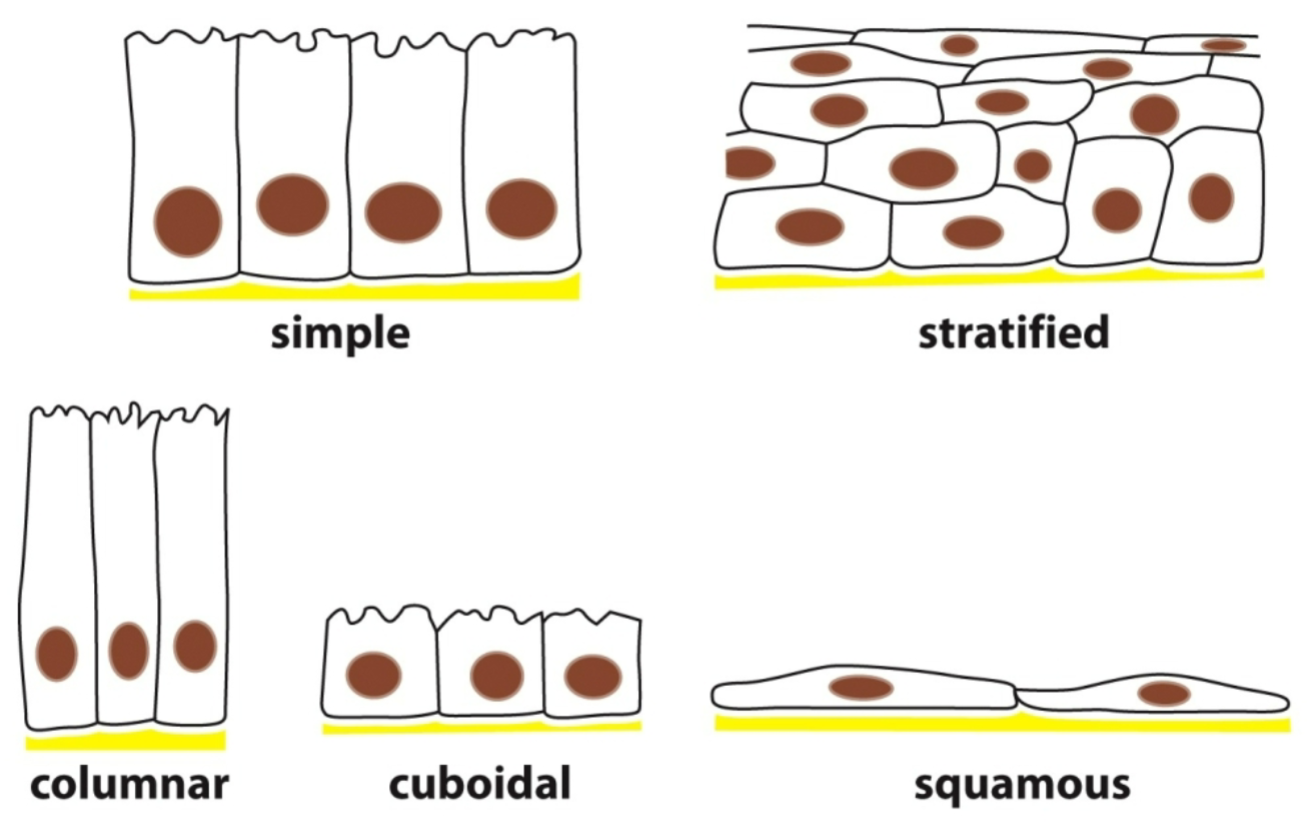
What are the different ways to pack epithelial cells?
Simple: single cell layer, basal lateral.
Stratified: stacks of cells on top of each other/basement membrane.
Columnar: columned.
Cuboidal: cubed.
Squamous: squashed/flattened.
Although epithelial cells/tissue have a variety of shapes and layers, what are some common features among all of them?
Lay on top of thin strip of ECM (the basal lamina).
Are in direct contact with each other.
Have cell-cell junctions to act as a barrier, hold the cells together or allow communication between them.
A lot of them have polarity as well.
What types of junctions are in epithelial cells?
Tight junctions
Adherens junctions
Gap junctions
Desmosomes
Hemidesmosomes
#1-4 are connections that occur between 2 epithelial cells.
#5 are connections that occur between an epithelial cell and the ECM.
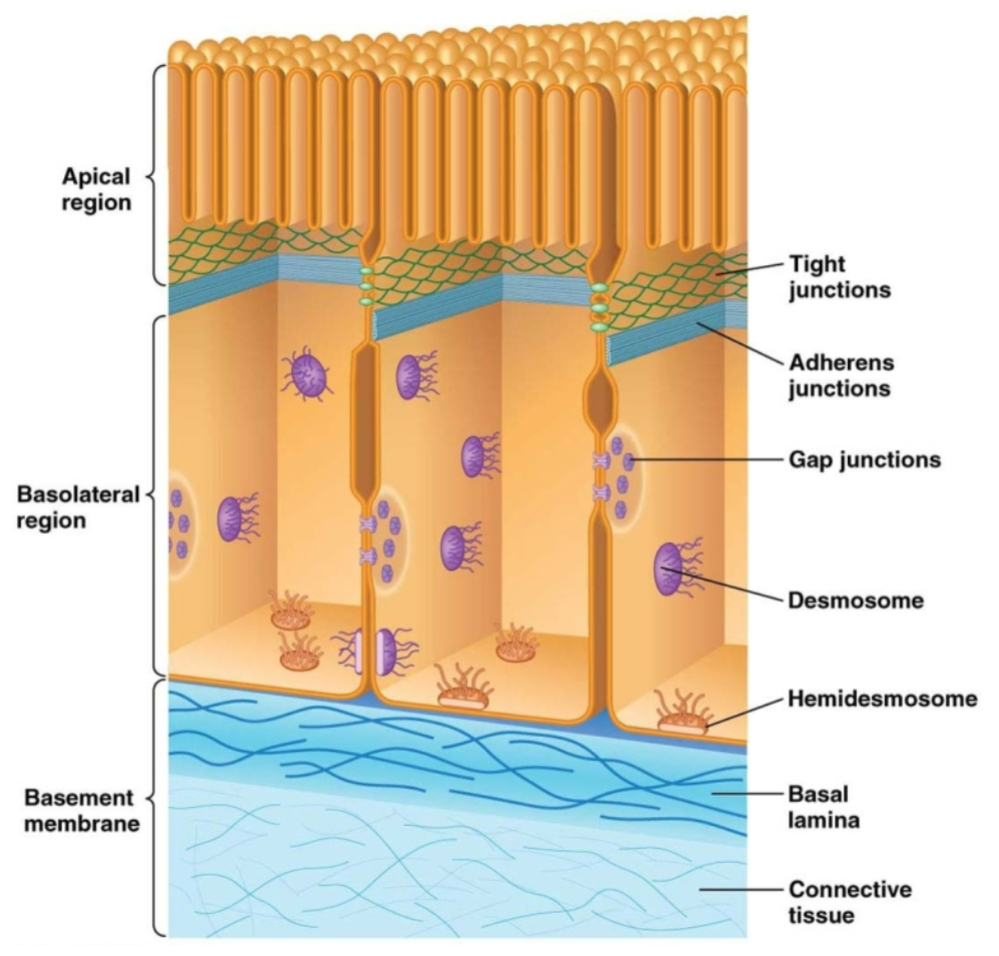
Which types of junctions are “adhesive junctions?”
Adherens junctions
Desmosomes
Hemidesmosomes
How is the cornea an extension of the skin?
It has both an epithelial layer and fibroblast-like cells (keratocytes) in its connective tissue.
Corneal nerves, the most innervated part of the body, have a swirl pattern and prevent eye damage by recognizing if something is in the eye.
Microvilli, the outermost part of the cornea with finger-like extensions, have glycocalyx sugars that absorb tear fluid to keep the eyes wet.
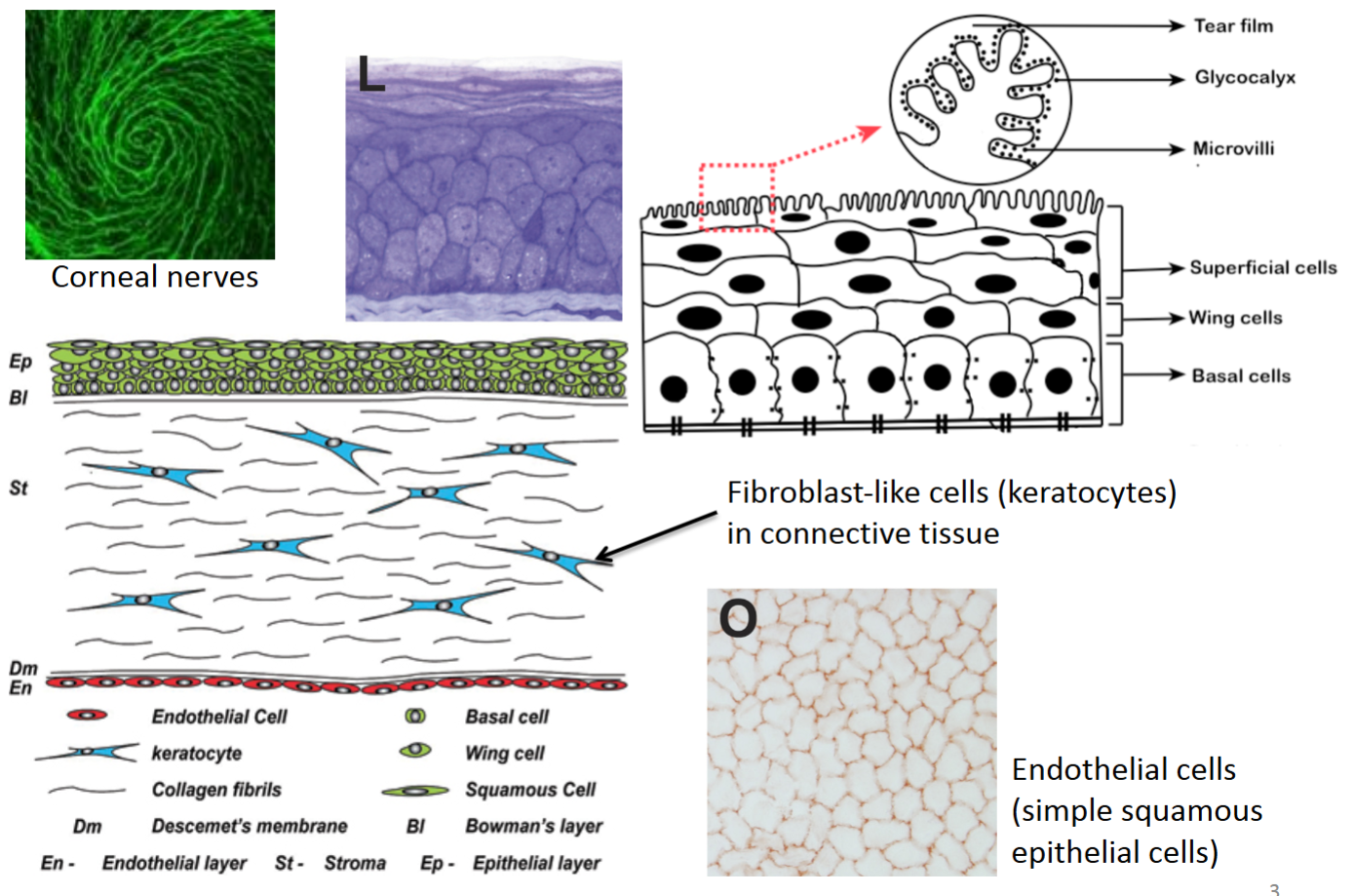
True or false? Each epithelial cell within stratified epithelium will have all 5 types of cell junctions.
False.
Tight junction:
Purpose?
Key proteins involved?
Prevents leakage between cells of a tissue. Especially important in cells that line body cavities and maintain different environments on either side of the lining.
Tight junction proteins (transmembrane proteins with intracellular and extracellular domains)—large extracellular loops of proteins on adjacent cells interact with each other.
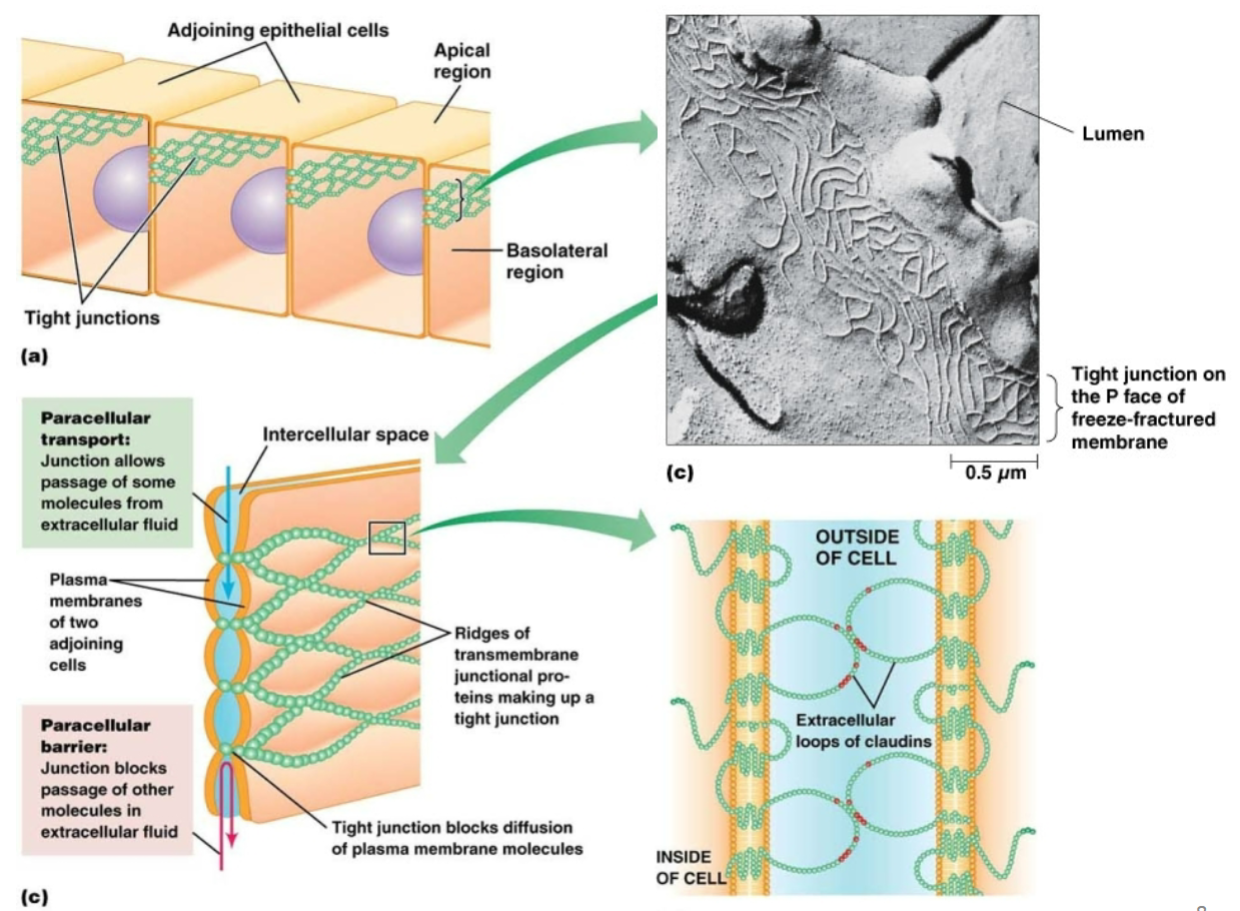
Tight junctions between epithelial cells serve to…
A. bind the cells strongly together so that they cannot be torn apart.
B. keep ions from diffusing out from the cell into the extracellular medium.
C. transport small molecules from the apical to the basal side of the epithelium.
D. allow small molecules and ions to pass from one epithelial cell to its neighbor.
E. seal cells together so that water-soluble molecules will not leak across the epithelium between the cells.
E. seal cells together so that water-soluble molecules will not leak across the epithelium between the cells.
Tight junctions…
A. function to allow passage of small hydrophilic molecules.
B. function to link the inside of the cell to the outside.
C. function as a barrier.
D. function to strengthen cell-cell contacts.
C. function as a barrier.
Cadherin proteins
transmembrane proteins with intracellular and extracellular domains.
Important components of adherens junctions and desmosomes (and other types of adhesive junctions)
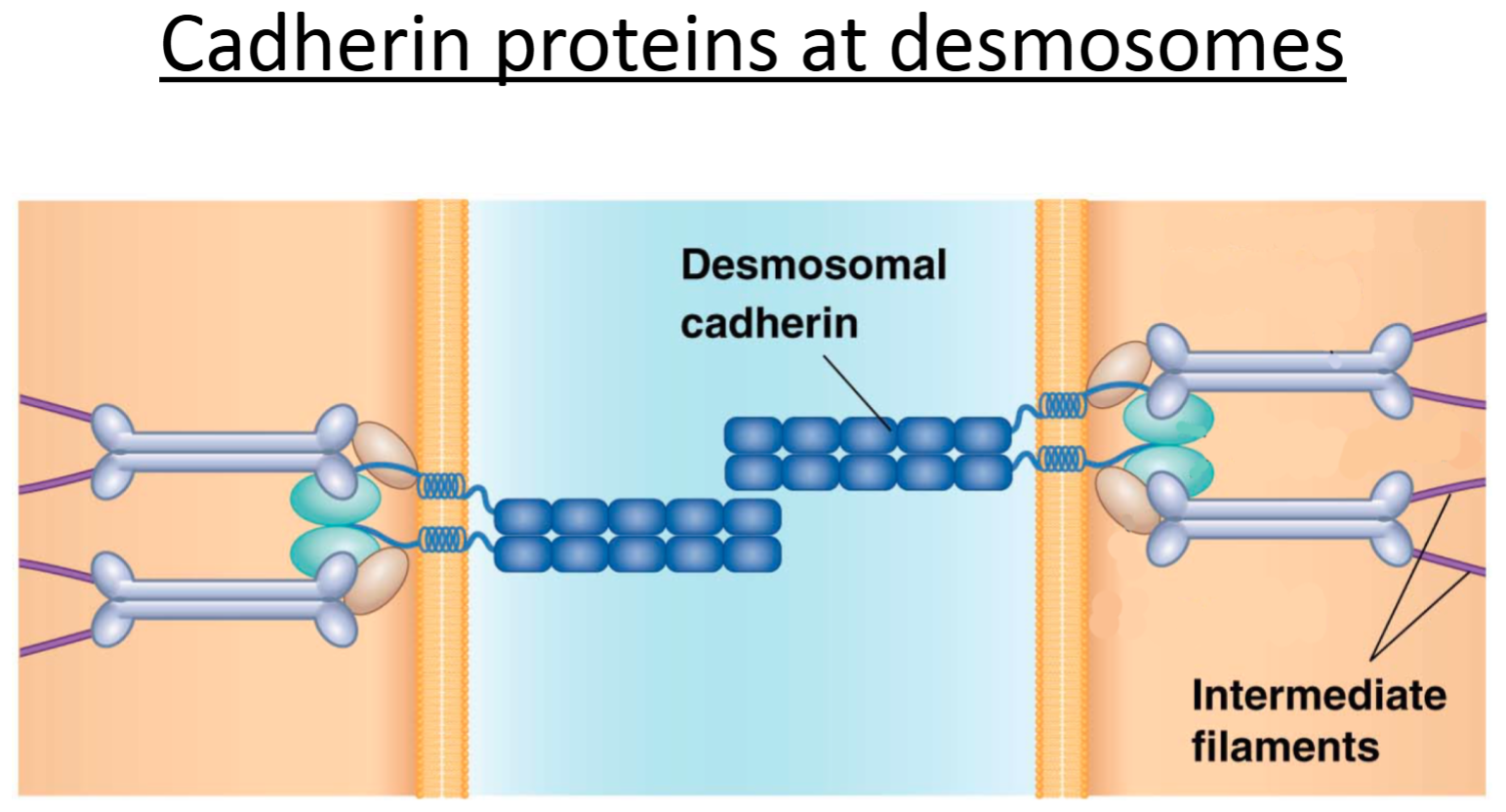
Adherens junction:
Purpose?
Key proteins involved?
To create an adhesive site between 2 cells (anchor adjacent cells together). Allows cytoskeleton (actin) to span across cells—provides structure and support to epithelial sheets; allows movement of cells.
Extracellular portions of cadherin proteins attach to each other on adjacent cells; actin (indirectly, through linker proteins) binds to the intracellular portion of cadherins.
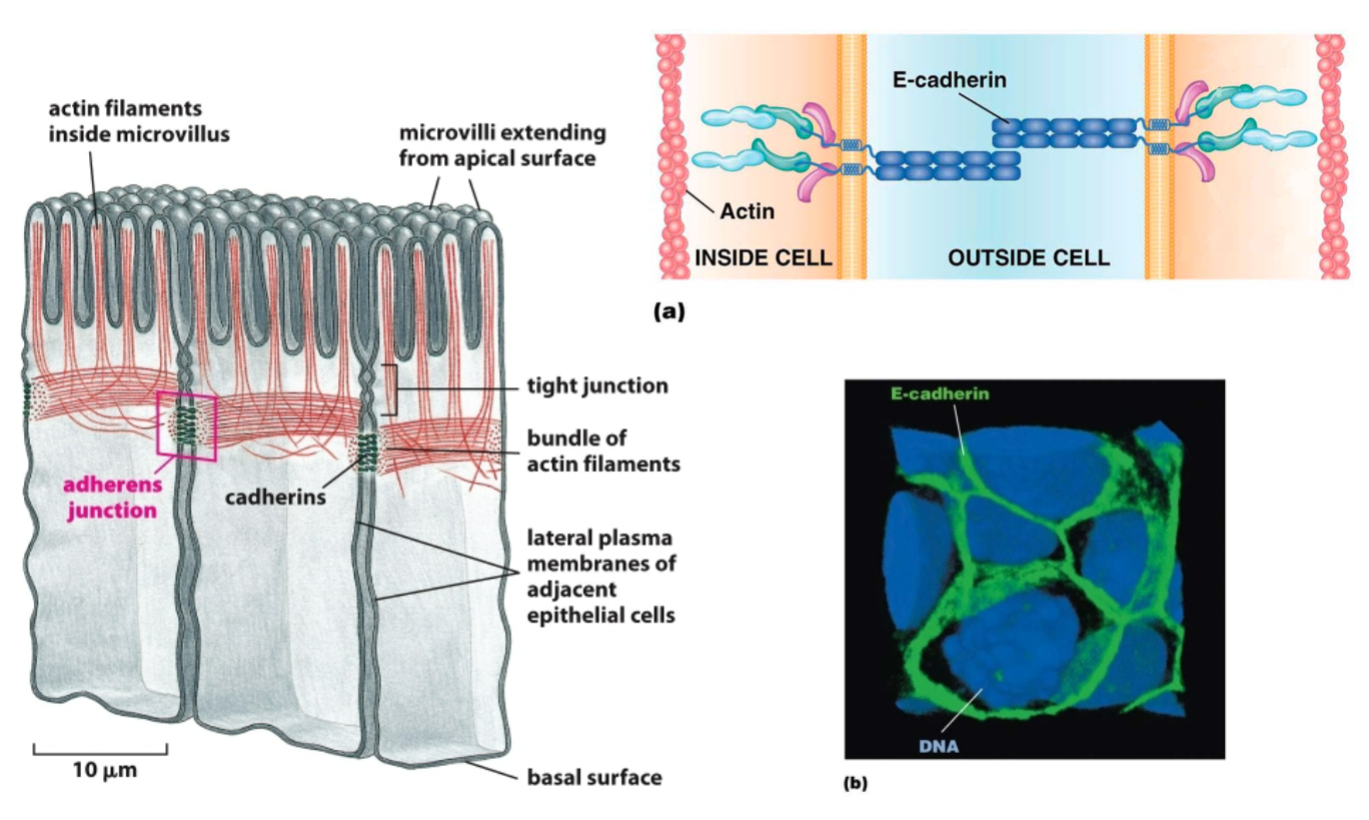
Adherens junctions… Choose all that apply.
A. are most similar to tight junctions.
B. connect epithelial cells to the basal lamina.
C. join adjacent epithelial cells to each other via cadherins.
D. bind intermediate filaments on the intracellular side of the membrane.
E. allow epithelial sheets to be strengthened through passage of actin filaments across cells.
C. join adjacent epithelial cells to each other via cadherins.
E. allow epithelial sheets to be strengthened through passage of actin filaments across cells.
Desmosome:
Purpose?
Key proteins involved?
To create an adhesive site between 2 cells (anchor adjacent cells together). Allows cytoskeleton (intermediate filaments) to span across cells. Provides strong structural support.
Extracellular portions of desmosomal cadherin proteins attach to each other on adjacent cells; intermediate filaments (indirectly, through linker proteins) bind to the intracellular portion of cadherins.
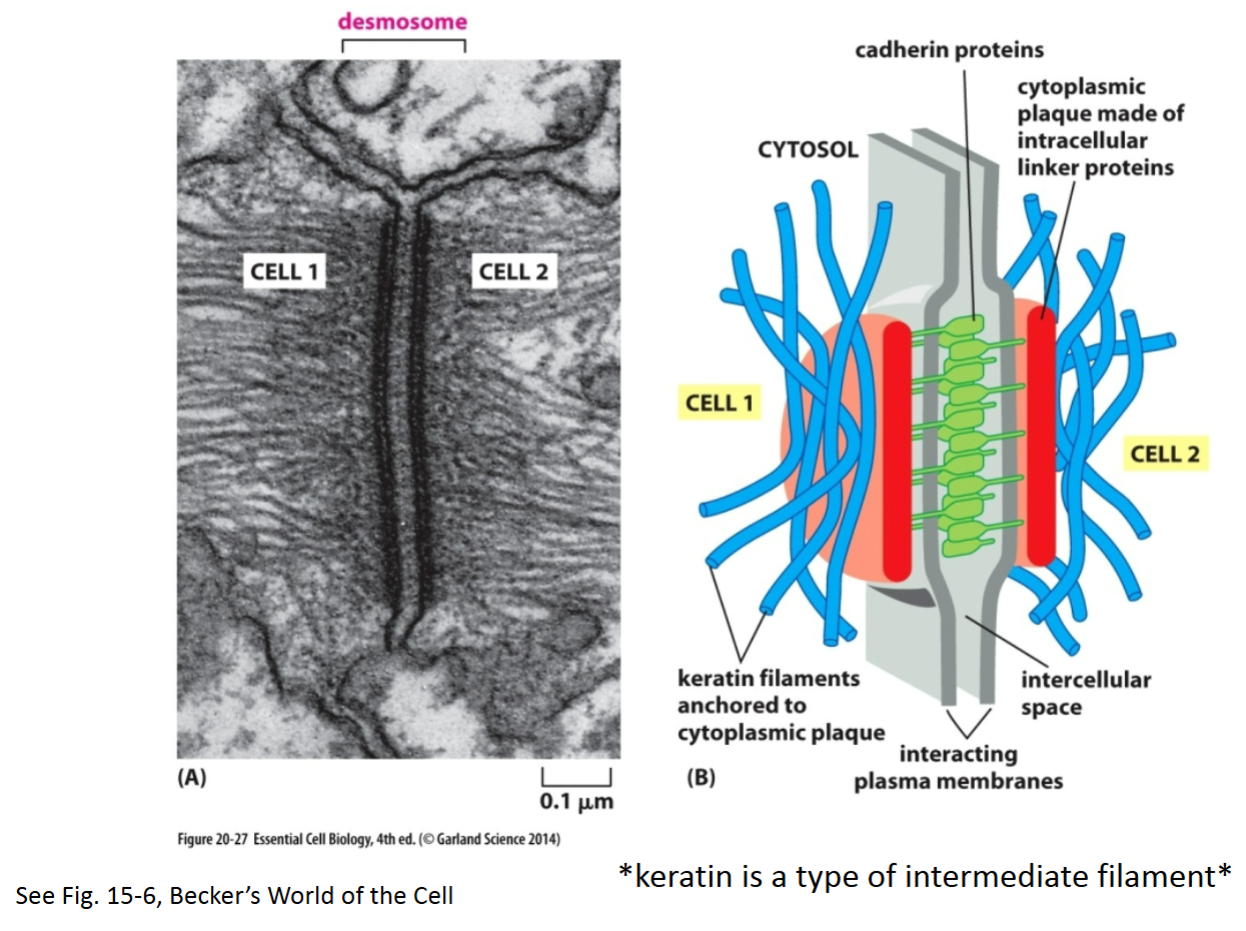
How are cadherin proteins attached at desmosomes?
Linker proteins attach intermediate filaments indirectly to cytoskeletal proteins—cadherins.
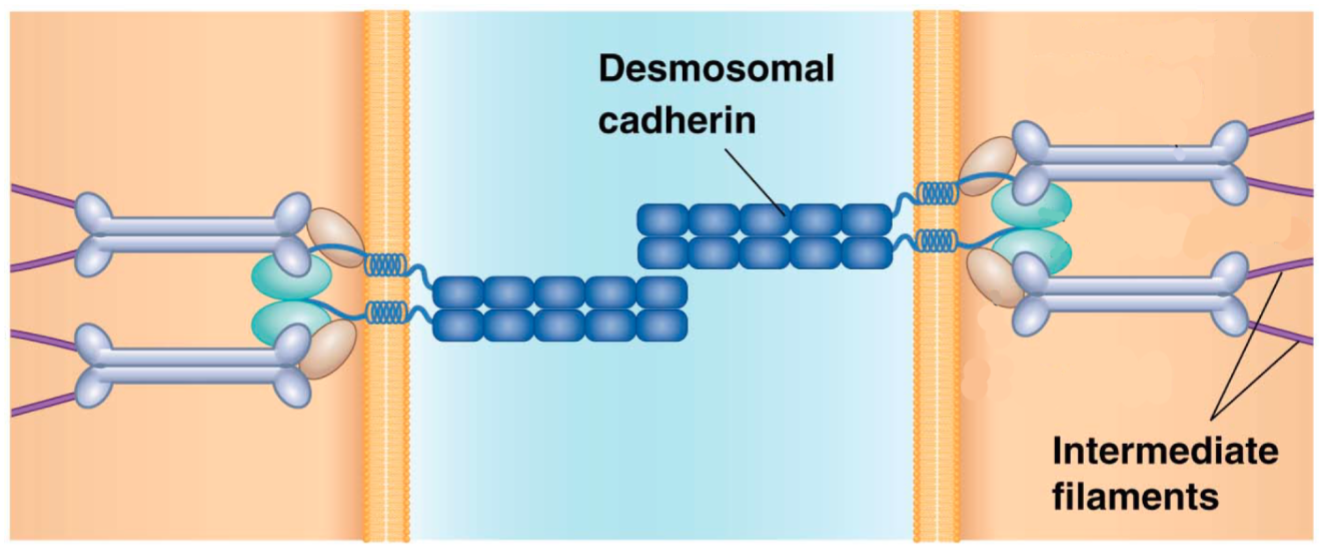
How are adherens junctions and desmosomes similar? Choose all that apply.
A. Both are a type of adhesive junction.
B. Both are composed of proteins that bind to actin.
C. Both are composed of cadheren proteins.
D. Both are composed of proteins that bind to microtubules.
A. Both are a type of adhesive junction.
C. Both are composed of cadherin proteins.
How are desmosomes similar to adherens junctions? How are they different?
Similar:
Both are adhesive junctions, which link cells together, enabling them to function as a unit.
Both use cadherin-type proteins at their junctions and interact with cytoskeletal components.
Different:
Adherens junctions bind to the cytoskeletal component actin, whereas desmosomes bind to the cytoskeletal intermediate filaments. Also, there are differences in the specific types of cadherins present at each of the junctions.
Gap junction:
Purpose?
Key proteins involved?
Allows communication between adjacent cells and passage of small hydrophilic substances between cytosol of cells. Important in tissues/cells that have to act as a coordinated unit, such as those of the heart.
6 connexin subunits make up a connexon on one cell; two connexons on adjacent cells make a single gap junction.
Very different function from others!
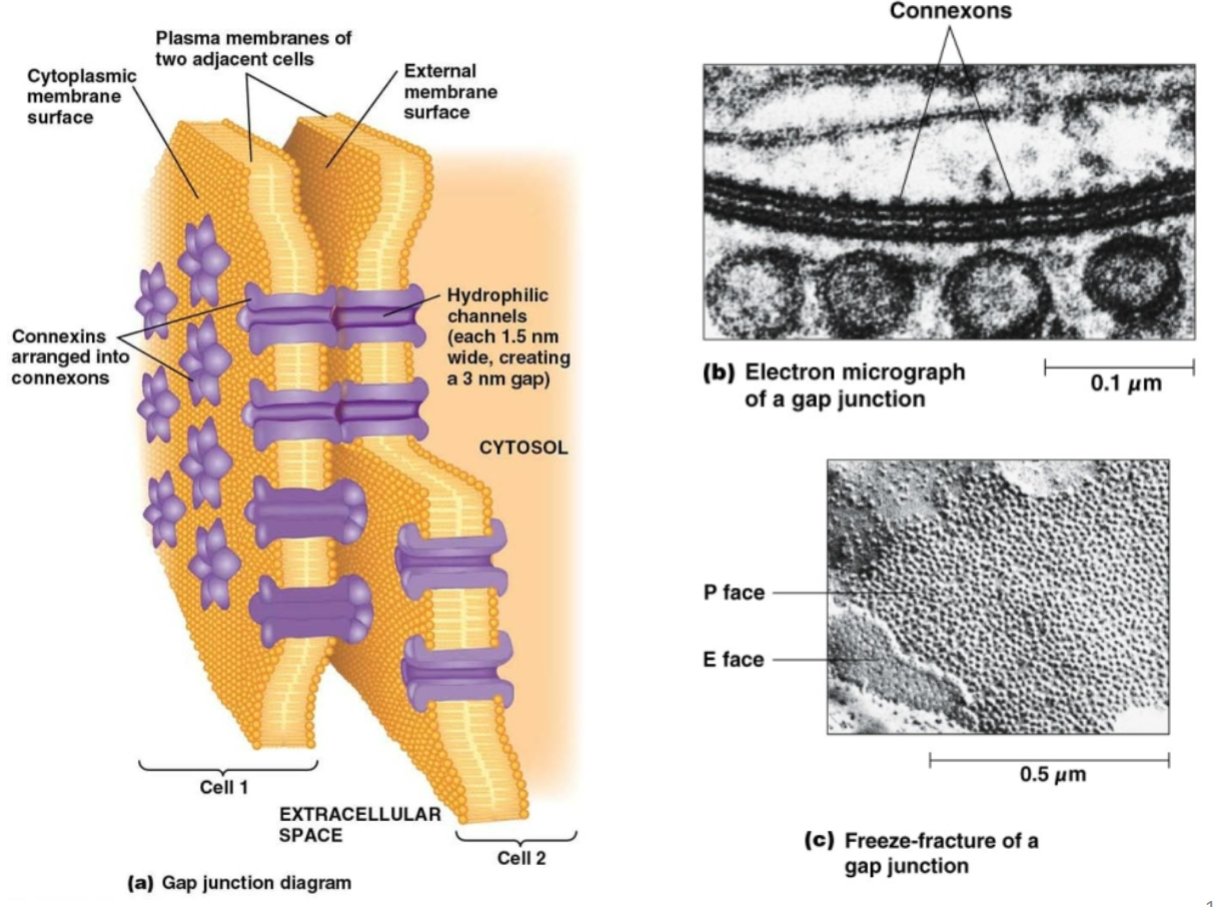
Which of the following do gap junctions allow to pass?
A. RNA.
B. Most enzymes.
C. Plasma membrane proteins.
D. Small water-soluble molecules.
E. Small hydrophobic molecules.
D. Small water-soluble molecules.
How are connexins, connexons, and gap junctions related?
Connexins are the individual proteins that make up a connexon.
There are 6 connexins per connexon.
Each gap junction is made up of 2 connexons in adjacent cells that interact with each other; each connexon is embedded in the plasma membrane.
Adhesive junctions are different from gap junctions in that _____.
A. only adhesive junctions associate with microfilaments or intermediate filaments.
B. only gap junctions associate with microfilaments or intermediate filaments.
C. only adhesive junctions prevent the movement of molecules across cell layers.
D. only gap junctions prevent the movement of molecules across cell layers.
A. only adhesive junctions associate with microfilaments or intermediate filaments.
True or false? All junctional proteins are transmembrane proteins.
True.
Hemidesmosome:
Purpose?
Key proteins involved?
Another type of adhesive junction. Anchors basal side of epithelial cells (or epithelial strata) to basement membranes.
Integrins are transmembrane proteins with intracellular and extracellular domains—intracellular portion binds to intermediate filaments indirectly through linker proteins; extracellular portion binds to laminin proteins making up the basal lamina (basement membrane).
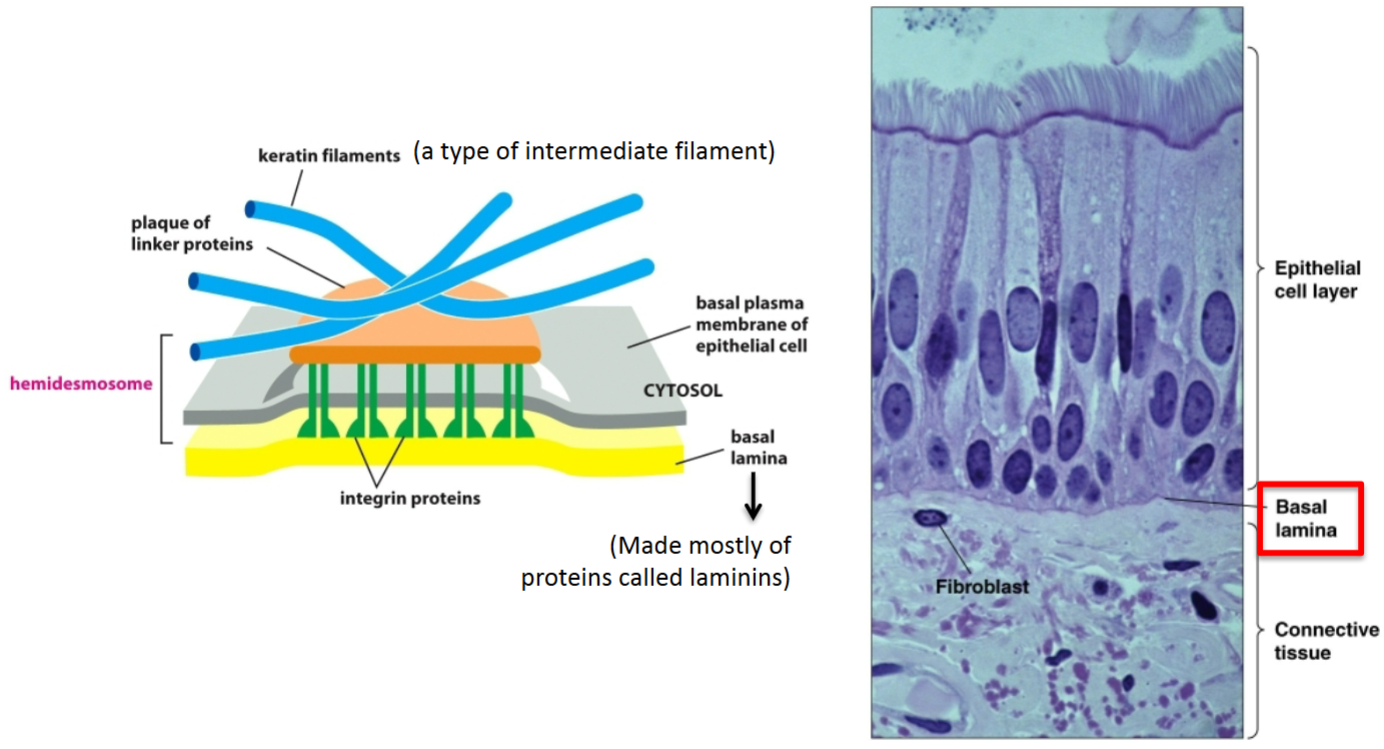
How are epithelial cells connected to the underlying connective tissue?
This interaction happens at discrete locations called hemidesmosomes. The basal lamina, which is a sheet of extracellular matrix composed of several proteins, the most abundant of which is laminin, binds to integrins in the plasma membrane of cells. The other side of integrins is connected to a plaque linker protein on the inside of the cell, which is attached to intermediate filaments inside the cells.
Fibroblast cells
Part of connective tissue.
Not connected to each other.
Connected to the ECM around the entire perimeter of the cell.
Claudin-4 (a specific tight junction protein) normally prohibits movement of Na+ ions across epithelial cells that express it. However, when positively charged amino acids near the tip of the large extracellular loop of claudin-4 are changed to negatively charged amino acids, Na+ can pass through the epithelium. How do you account for this change in permeability?
Normally, the positively charged amino acids near the tip of claudin cause electrostatic repulsion of Na+, which leads to low paracellular permeability through the tight junction. When these amino acids are changed to negatively charged amino acids, this repulsion is abolished, allowing the Na+ to pass more easily through the tight junction via paracellular permeability.
Elastin
Structural fiber (like collagen).
Proteins help to give its characteristics.
Elastic (e.g., skin).
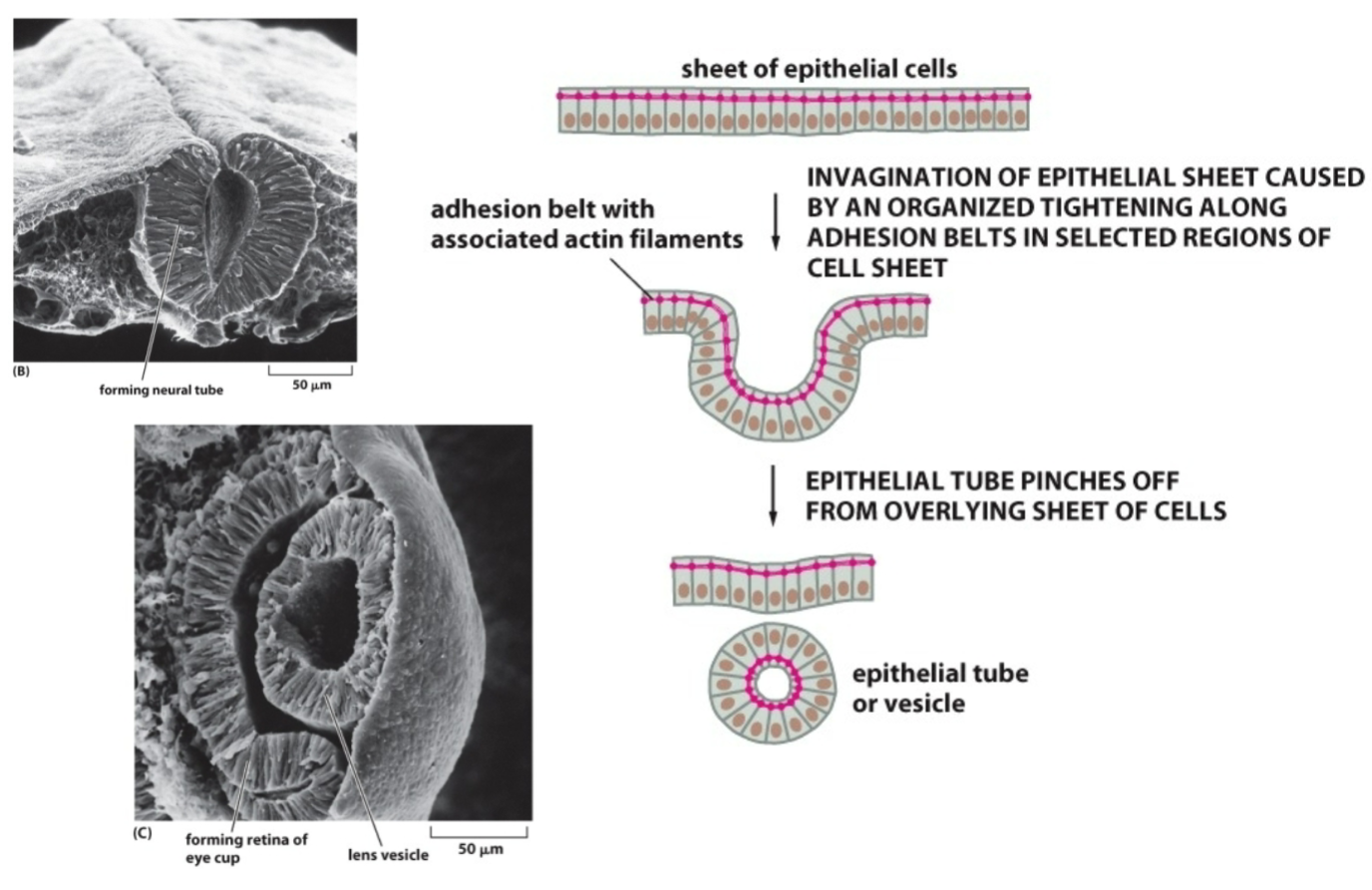
How is a structure like a lens or neural tube formed? What proteins are necessary?
Adherens junctions between cells allow actin (cytoskeletal protein) to span across multiple cells (e.g., epithelial sheet). Actin filaments can contract (polymerize and depolymerize with the help of motor proteins) and cause mass movement of epithelial sheets.
When the actin filaments contract, it causes bending at the apical surface of the cell, causing the sheet to roll up on itself.
What are the 4 ECM related proteins we are concerned with?
Laminin
Fibronectin
Collagen
Proteoglycans
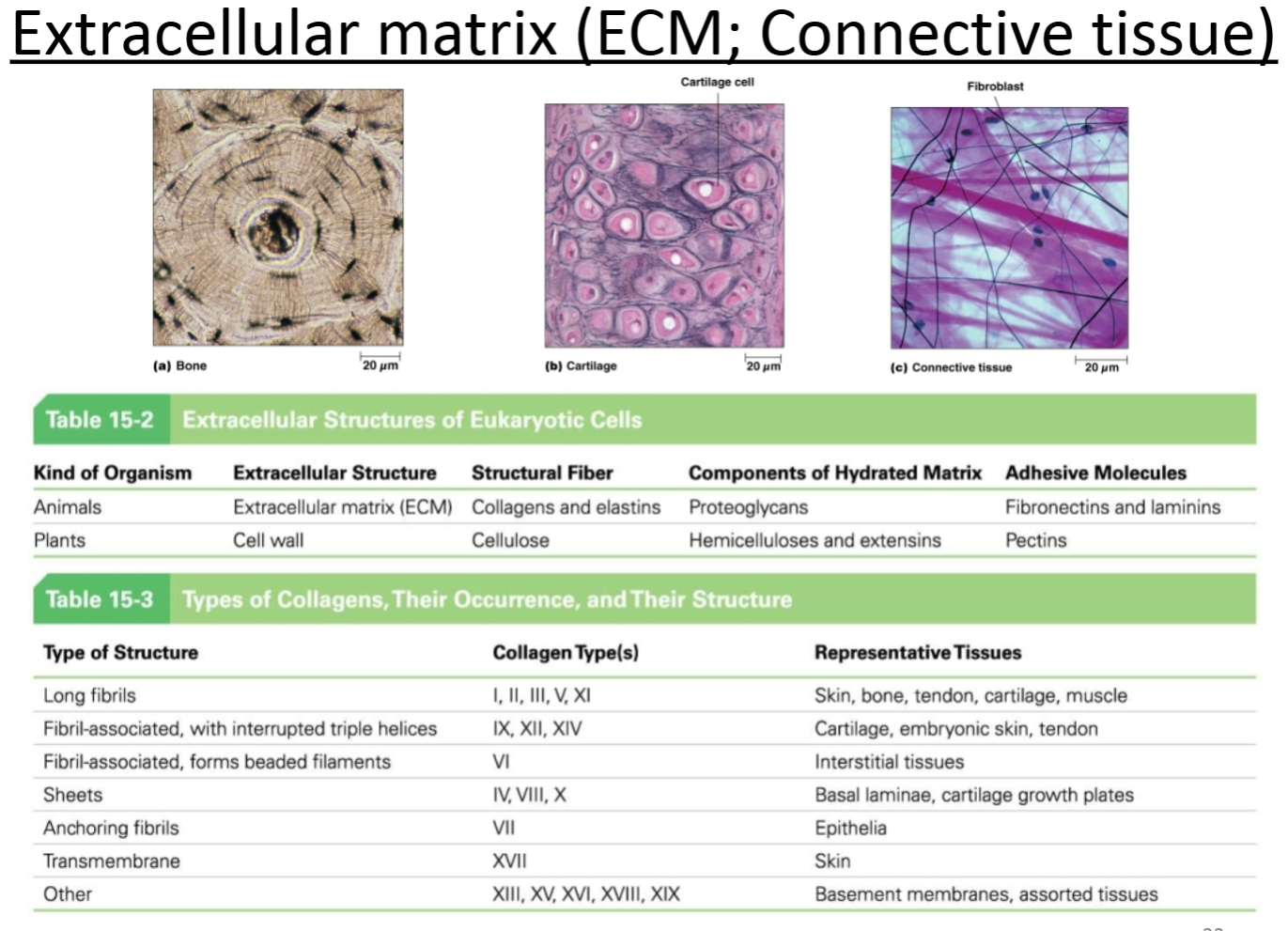
ECM related protein: Laminin:
Purpose?
Location with respect to cells; other molecules with which it interacts?
Heterotrimeric protein that makes a “cross” shape. Laminins create a thin mat of ECM that separates epithelial sheets from underlying connective tissues. Acts as a foundation for epithelial cells. Laminin is part of the basal lamina (basement membrane).
Extracellular protein matrix below epithelial cells. Interacts with integrins as well as other ECM proteins.
Adhesive
Basal lamina below epithelial cells
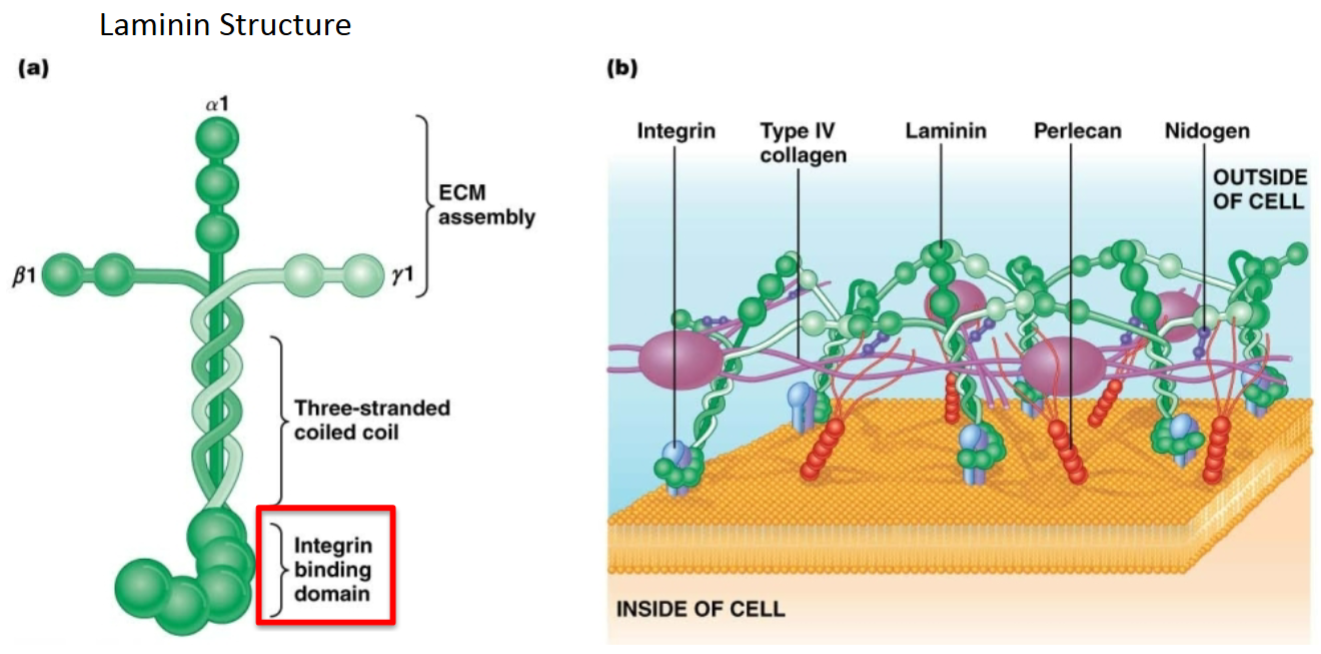
ECM related protein: Fibronectin:
Purpose?
Location with respect to cells; other molecules with which it interacts?
Homodimeric protein that makes a “Y” shape. Involved in cell adhesion—fibronectin has binding sites for many other components of the ECM (e.g., collagen) and for proteins of the cell surface (e.g., integrin).
Outside of cells, in connective tissues; interacts with integrins and links cells to other ECM proteins (e.g., collagen).
Adhesive
Connective tissue
What is the role of fibronectins in the extracellular matrix of animals?
A. To hold water and hydrate tissues.
B. To provide strength to the extracellular matrix.
C. To impart elasticity and flexibility to the extracellular matrix.
D. To serve as a bridge connecting cells to the rest of the extracellular matrix.
D. To serve as a bridge connecting cells to the rest of the extracellular matrix.
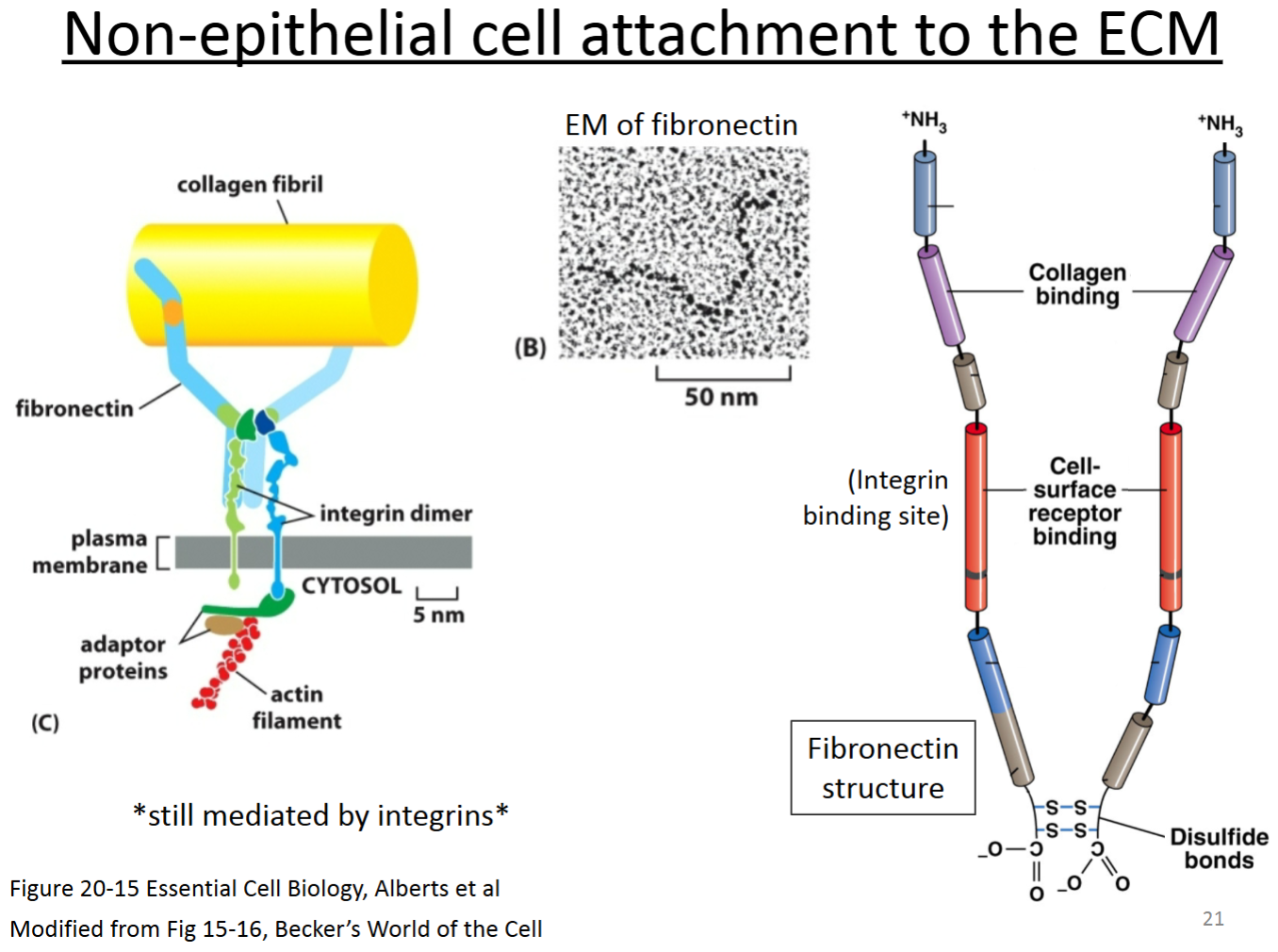
ECM related protein: Collagen:
Purpose?
Location with respect to cells; other molecules with which it interacts?
Rope-like trimeric protein (sometimes heterotrimer, sometimes homotrimer), fibrous protein that is a major component of the ECM in animals. Gives tissues tensile strength.
Outside of cells, in connective tissues; interacts with other ECM proteins (e.g., fibronectin).
Connective tissue
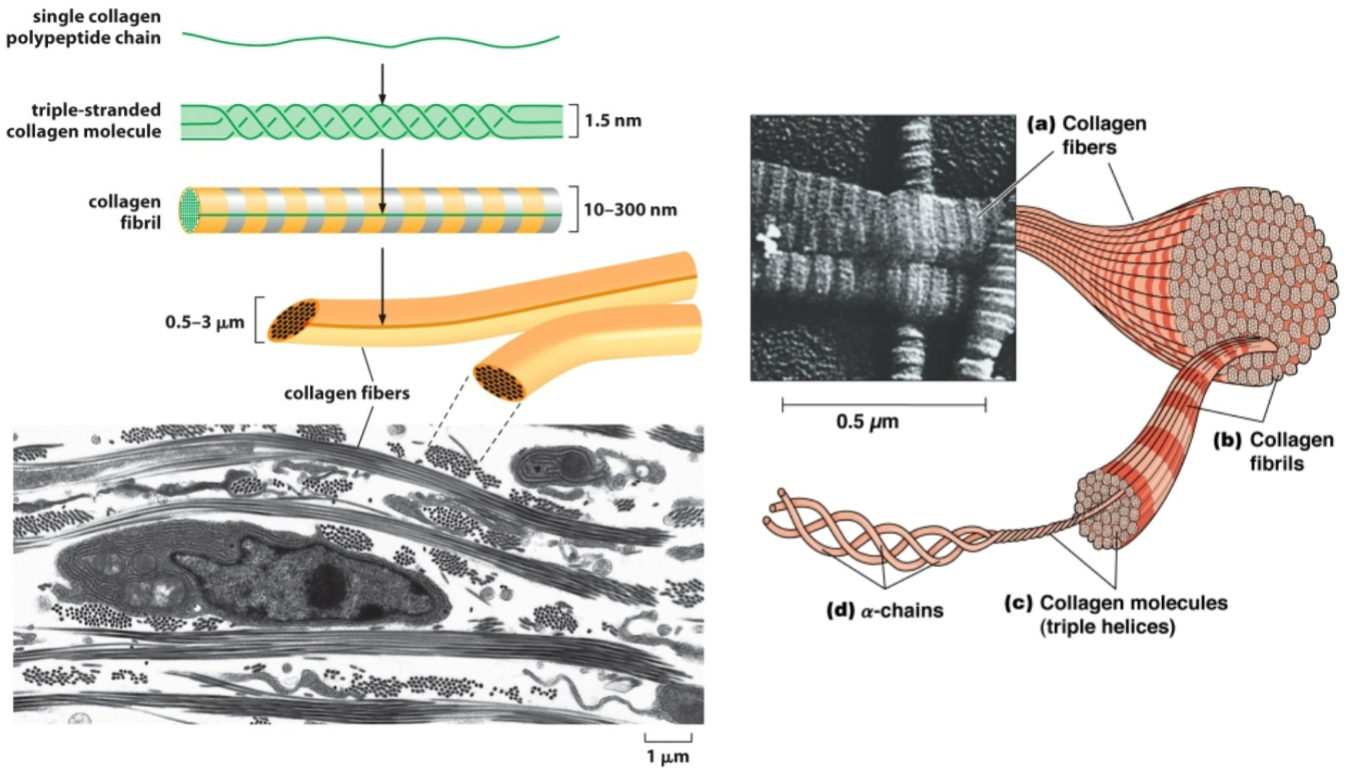
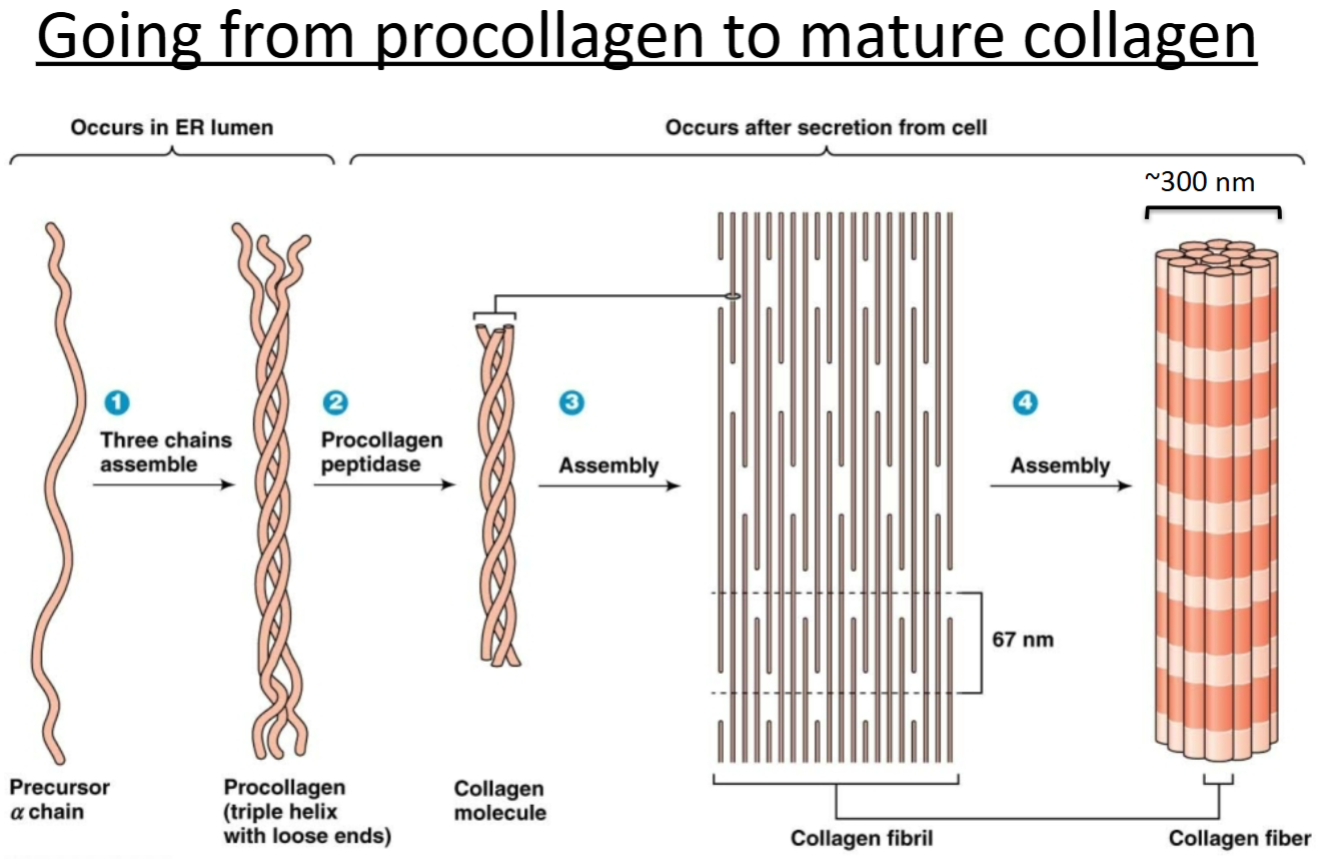
Procollagen
pre molecule initially made but not ready to perform any functions.
Where is collagen’s final destination?
What are the things that happen to collagen from translation to its final destination?
Final destination is outside the cell to hang out as ECM.
Assembles into a triple helix (3 chains wound together) with loose ends (called procollagen) in the ER.
Procollagen gets exported out of the cell.
It gets cleaved by a peptidase. The cleavage products assemble into fibrils, which assemble into fibers.
Collagen must be hydroxylated to be stabilized.
Collagen is the most important ECM protein!
Precursor α chain —(three chains assemble)→ Procollagen (triple helix with loose ends) —(Procollagen peptidase)→ Collagen molecule —(Assembly)→ Collagen fibril —(Assembly)→ Collagen fiber
James Morris
elastic skin man with collagen mutation to have extreme skin elasticity.
ECM related protein: Proteoglycans:
Purpose?
Location with respect to cells; other molecules with which it interacts?
Proteins (core protein) that are highly glycosylated with special sugars called GAGs (glycosaminoglycans—repeating disaccharide units). Connected together through link proteins.
Outside of cells, in connective tissue; interacts with ECM other proteins.
Connective tissue
Glu and Gly
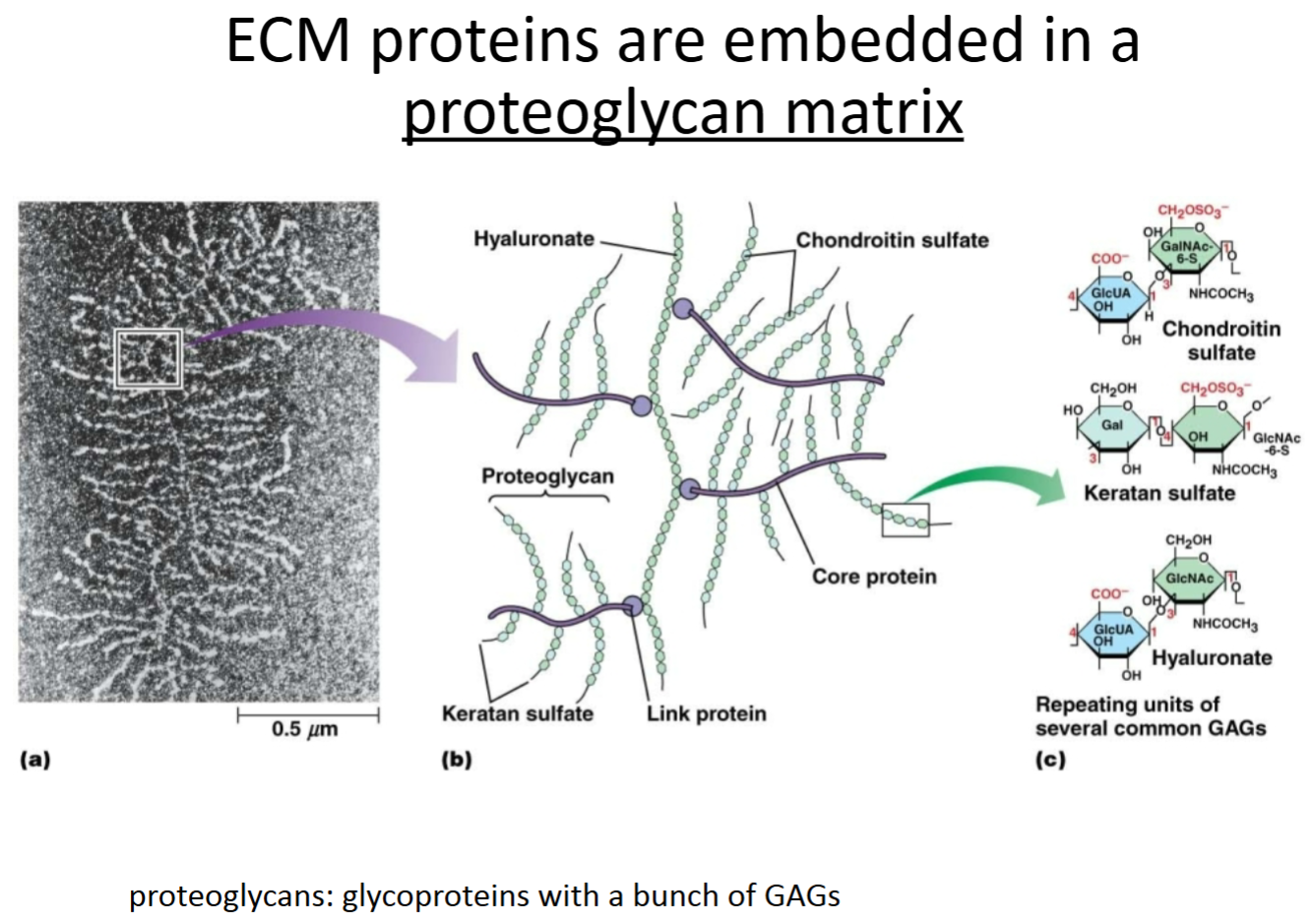
What are proteoglycans?
What is their function/purpose in the ECM?
Are they similar to anything you learned about earlier in the semester?
Proteoglycans are proteins with lots of specialized sugars attached to them, called GAGs (glycosaminoglycans—repeating disaccharide units).
They help to hydrate tissues and have lubrication properties.
Proteoglycans are similar to glycoproteins, but with way more sugar groups, and different sugar groups.
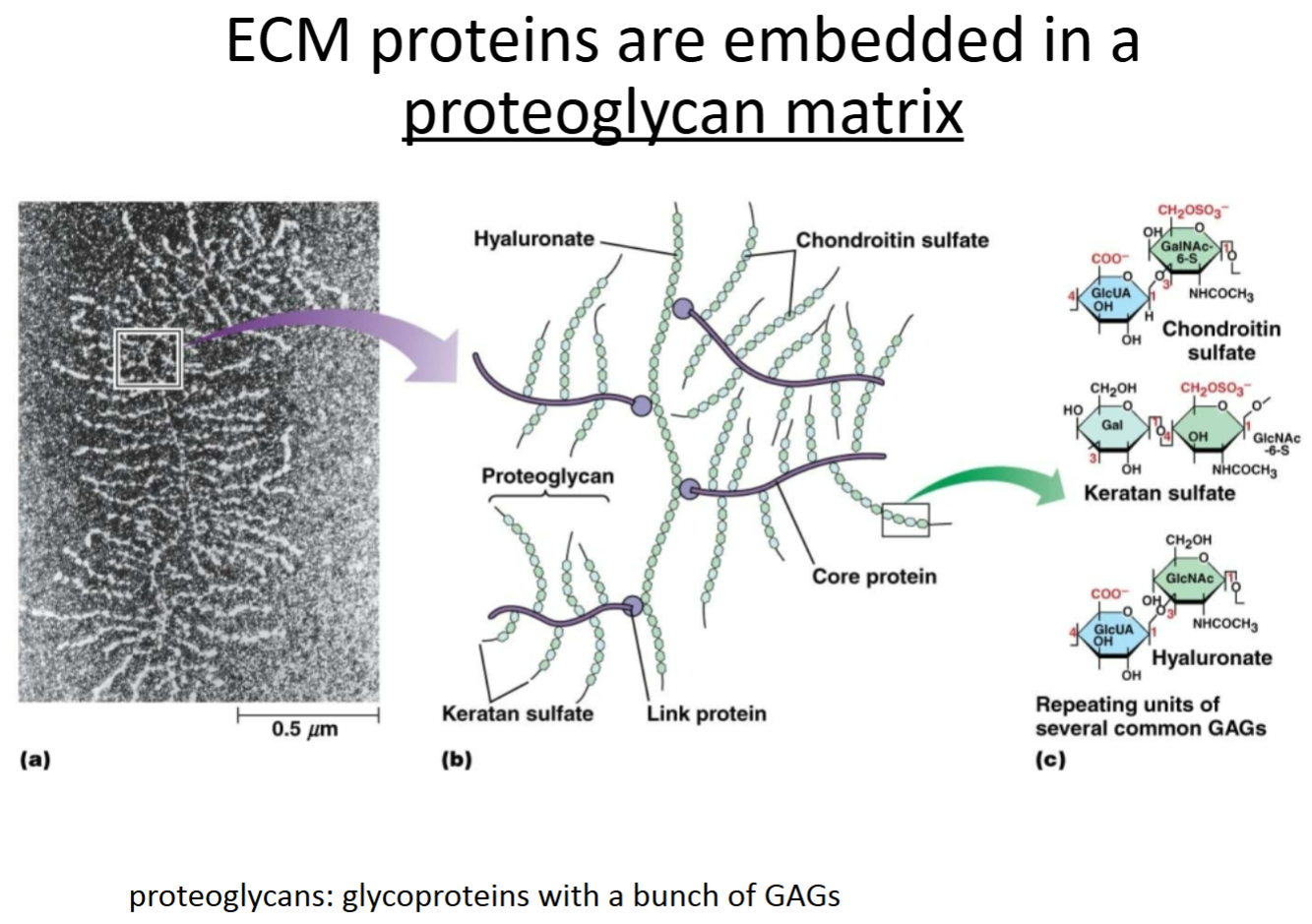
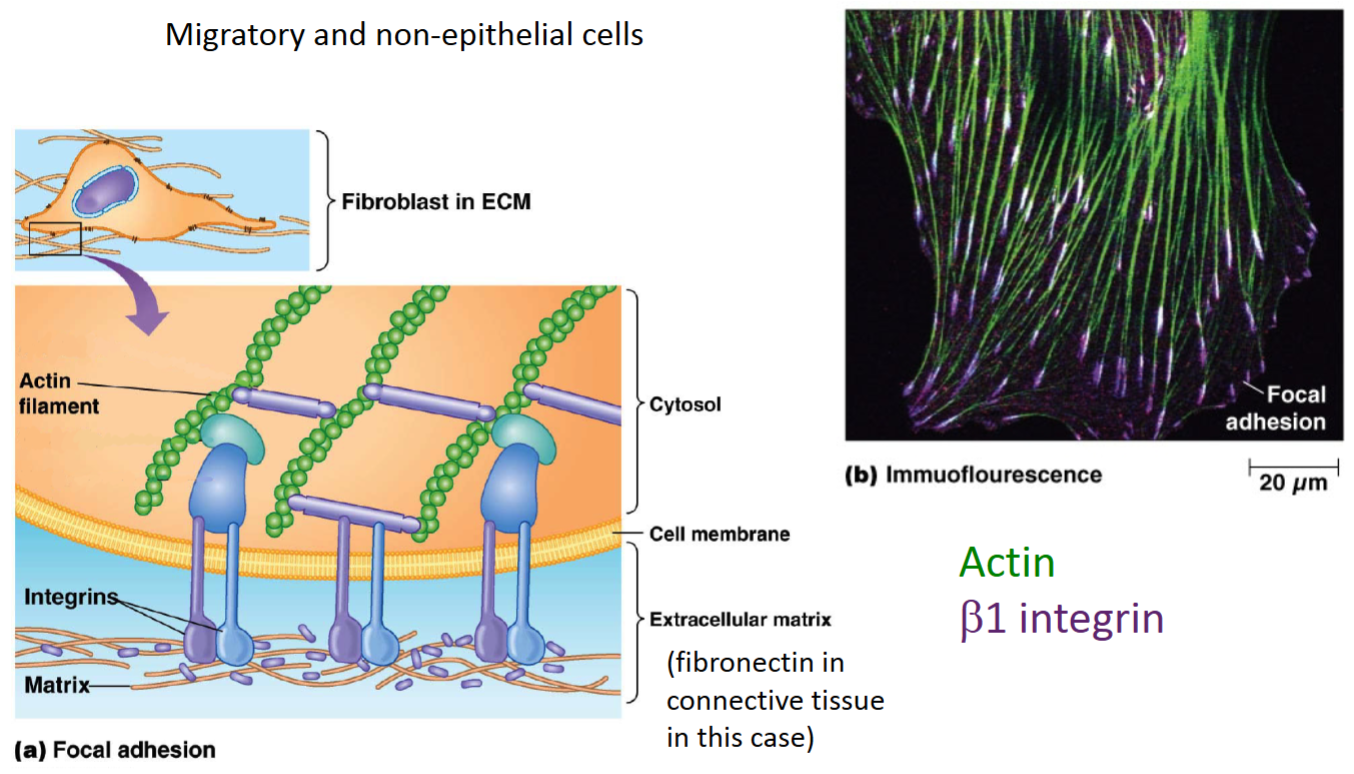
How do integrins anchor epithelial cells to ECM proteins?
Hemidesmosomes.
How do integrins anchor fibroblast cells to ECM proteins?
Focal adhesion/contact.
Which of the following is true of proteoglycans?
A. They connect the inside of cells to the outside.
B. They aid in keeping tissues hydrated.
C. They provide strong structure to tissues.
D. They are found inside cells.
B. They aid in keeping tissues hydrated.
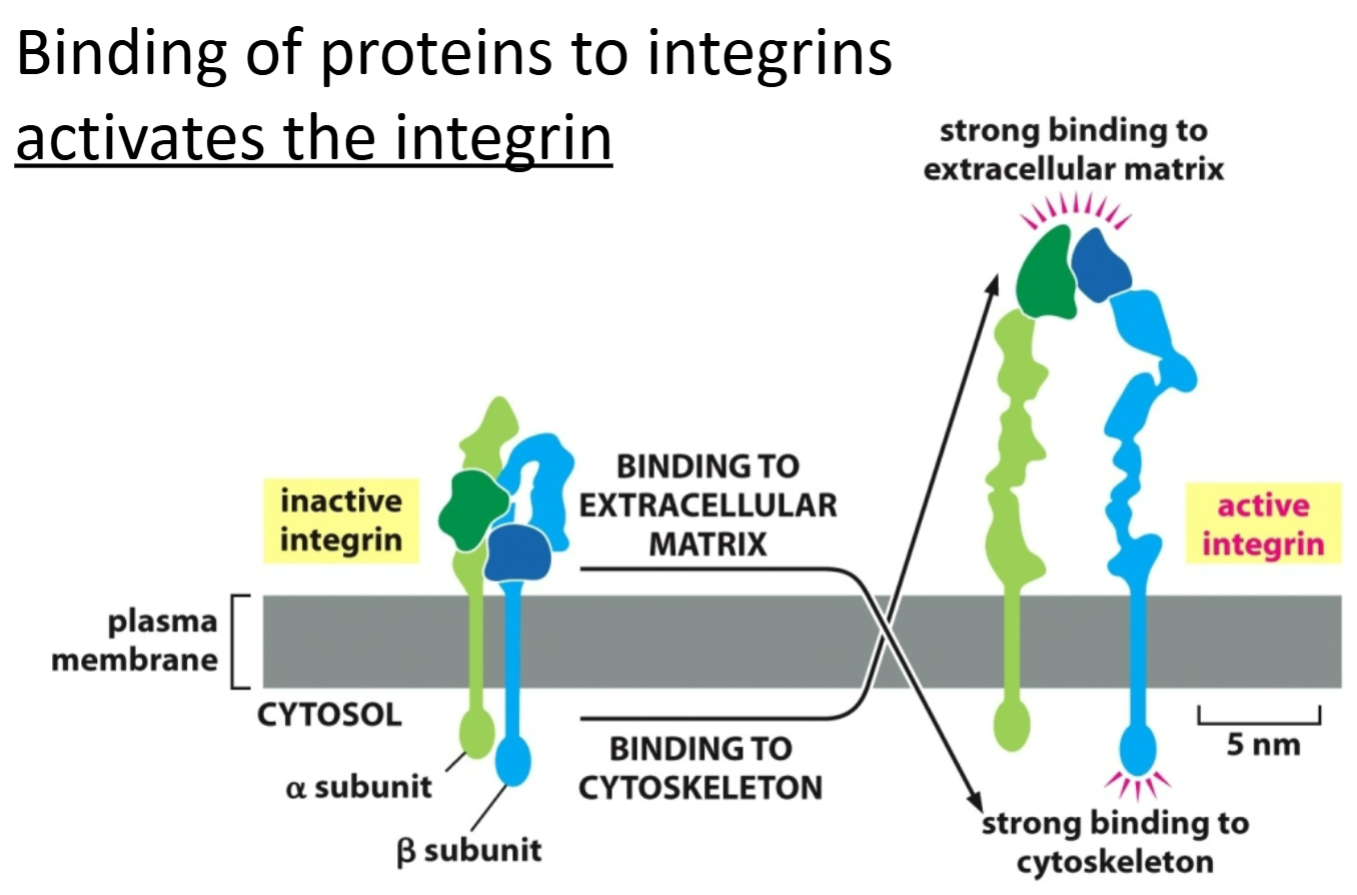
Integrin
Transmembrane protein that links the inside of the cell to the outside.
Binding with proteins activates it.
Connects cell-cell/structural role.
Signaling molecule; both sides can be stimulated.
Relays to inside of cell so the intracellular side can bind to both sides of the protein, working as a receptor to signal the other side.
Which of the following is NOT an extracellular matrix protein?
A. Collagen.
B. Laminin.
C. Integrin.
D. Fibronectin.
C. Integrin.
Cells bind to the ECM via…
A. laminin in the plasma membrane.
B. integrins in the plasma membrane.
C. fibronectin in the plasma membrane.
D. phospholipids in the plasma membrane.
B. integrins in the plasma membrane.
Focal contacts… Choose all that apply.
A. are found in epithelial cells.
B. involve integrins.
C. involve intermediate filaments.
D. are found in fibroblasts.
B. involve integrins.
D. are found in fibroblasts.
How are the connections using integrin that occur between epithelial cells and the ECM different from those that occur between fibroblast cells and the ECM?
For epithelial cells, integrins indirectly bind to intermediate filaments inside the cell and often the basal lamina (laminins; sheet of ECM protein) outside the cell.
For fibroblasts, integrins bind to actin inside the cell and often fibronectin (ECM protein) outside the cell.
Hypothesize why integrins link the ECM to intermediate filaments in epithelial cells whereas the integrins link the ECM to actin filaments in fibroblasts (think about the dynamics of the 2 cytoskeletal components and the way the cells are connected to each other, or not).
Epithelial cells for the most part are stationary and don’t need dynamic actin filaments in order to move. Furthermore, because epithelium often is the lining of tissues and acts as a barrier, it needs to be anchored to a very strong cytoskeletal component, like intermediate filaments.
Fibroblasts are a cell type that moves around quite a bit, and in order to do that, they need to be able to sense their surroundings (through connections with ECM) and need a dynamic protein to help them move (actin).
A basal lamina… Choose all that apply.
A. separates epithelial cells from each other.
B. is impermeable to small organic molecules.
C. is in a thin layer of ECM underlying an epithelium.
D. is linked to the apical surface of an epithelium.
E. is linked to the basolateral surface of an epithelium.
C. is in a thin layer of ECM underlying an epithelium.
E. is linked to the basolateral surface of an epithelium.
Scurvy is a disease that, until the 19th century, was common among sailors and others whose diets were deficient in vitamin C (ascorbic acid). Ascorbic acid serves as a reducing agent responsible for maintaining the activity of prolyl hydroxylase, the enzyme that catalyzes hydroxylation of proline residues within the collagen triple helix. Based on this information, postulate a role for hydroxyproline in collagen triple helices, and explain the sequence of events leading from a dietary vitamin C deficiency to symptoms such as bruising and breakdown of supporting tissues.
Hydroxyproline residues, but not unhydroxylated proline residues, stabilize the collagen triple helix. If vitamin C is deficient in the diet, tissue levels of ascorbic acid will be low, and the enzyme prolyl hydroxylase is not maintained in the reduced form and therefore inactive. Proline is not hydroxylated, resulting in the inadequate stabilization of the triple helix. Collagen therefore breaks down, leading to defects in tissues that depend on collagen to maintain the adhesive strength of the ECM. As a consequence, such tissues are subject to breakdown.
What are the 4 different types of cell signaling?
Endocrine
Paracrine
Synaptic
Contact-dependent
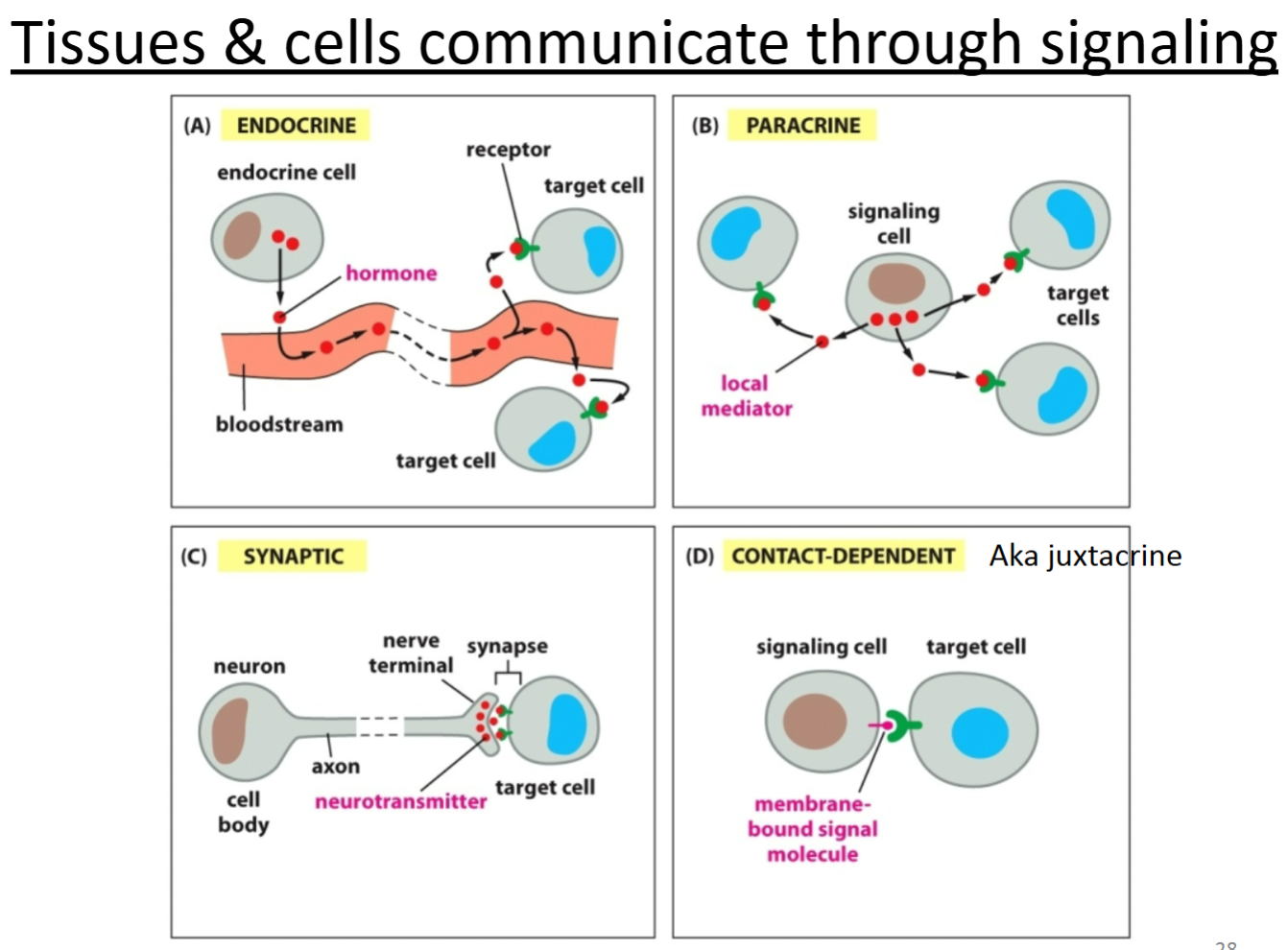
Endocrine signaling
signal cell releases signal to the bloodstream.
How do cells that are separated by long distances communicate?
What types of molecules are involved?
Endocrine signaling is used to communicate between long distances.
Hormones (or other small molecules) are secreted and travel through the bloodstream to cause a signaling cascade within a cell that is far away. Receptor proteins are also required on the cell receiving the signal.
Paracrine signaling
Relies on diffusion through surrounding tissue.
Cell releases a signaling molecule—local migration to same organ/tissue.
Contact-dependent signaling
Relies on cells being in direct contact.
For cells fixed in place—signaling molecule anchored in place so that the target cell can receive a signal if it is in direct contact with the signaling cell.
How do neighboring cells communicate with one another?
Which proteins might be involved?
Contact-dependent (juxtacrine) and paracrine signaling.
For contact-dependent signaling in epithelial cells, it is sometimes through the use of gap junctions. Other times, a membrane-bound signaling molecule interacts with a receptor on an adjacent cell.
For paracrine, hormones (or other local mediators like neurotransmitters, growth factors, etc.) are secreted, diffuse through the ECM and act on nearby cells. Neurons are an example that use paracrine signaling. A receptor protein is required on the receiving cell.
How is paracrine signaling different than endocrine signaling? How are they similar?
Different:
Paracrine signaling occurs over short distances and the signaling molecules don’t travel through the bloodstream.
For endocrine signaling, the signaling molecules get to their target cell by entering the bloodstream.
Similar:
Both use hormones as the signaling molecule (ligand of the receptor).
Both use receptors on the target cell.
Totipotent cells
can give rise to all cell types of the body, as well as extraembryonic cells (e.g., the placental cells).
An example of these are embryonic cells within the first couple of cell divisions after fertilization.
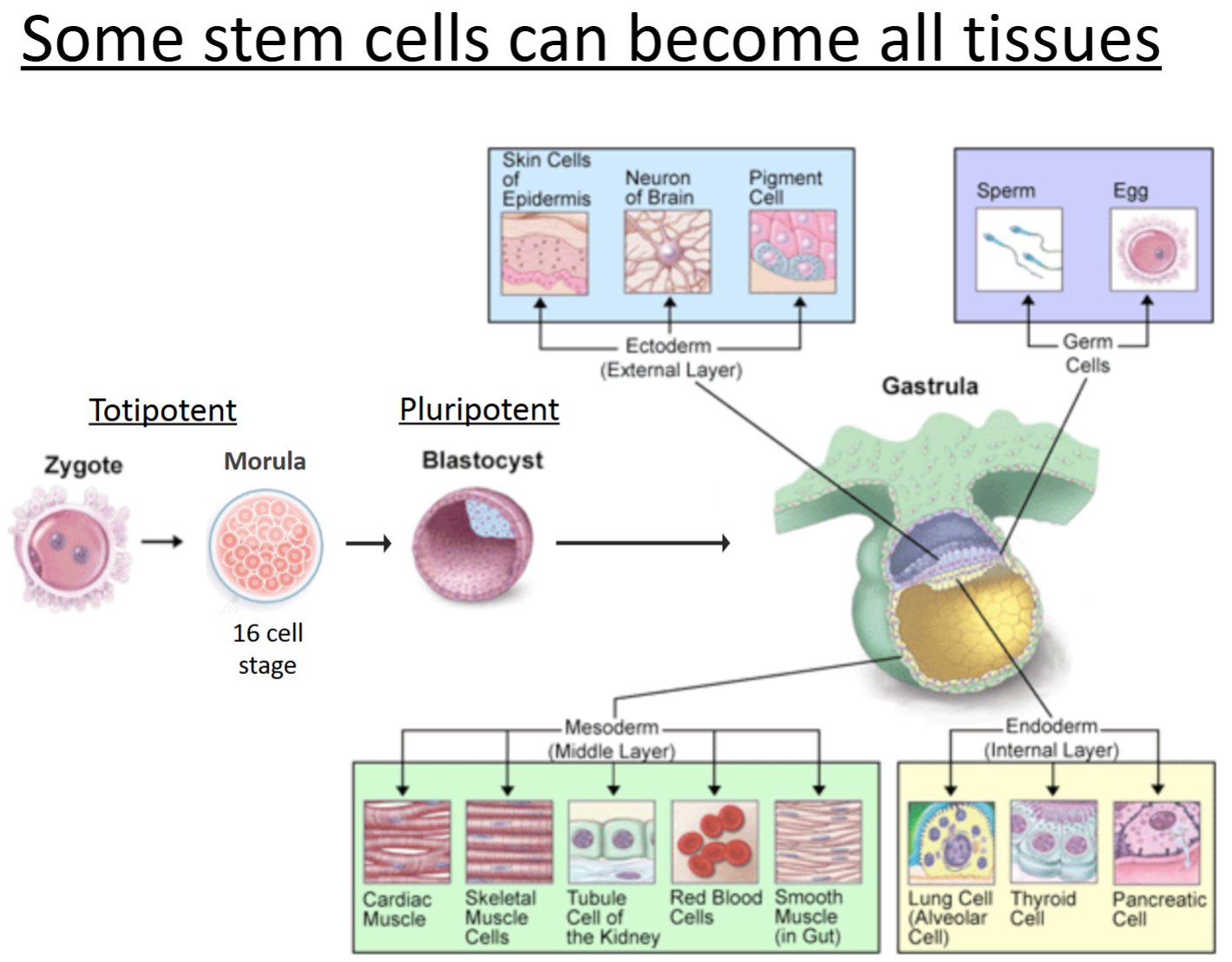
Pluripotent cells
can give rise to all the tissues and cell types in the body, but NOT extraembryonic.
An example of these are embryonic stem cells.
Blastocyst: inner cell mass where these cells are as a ball of undifferentiated cells.
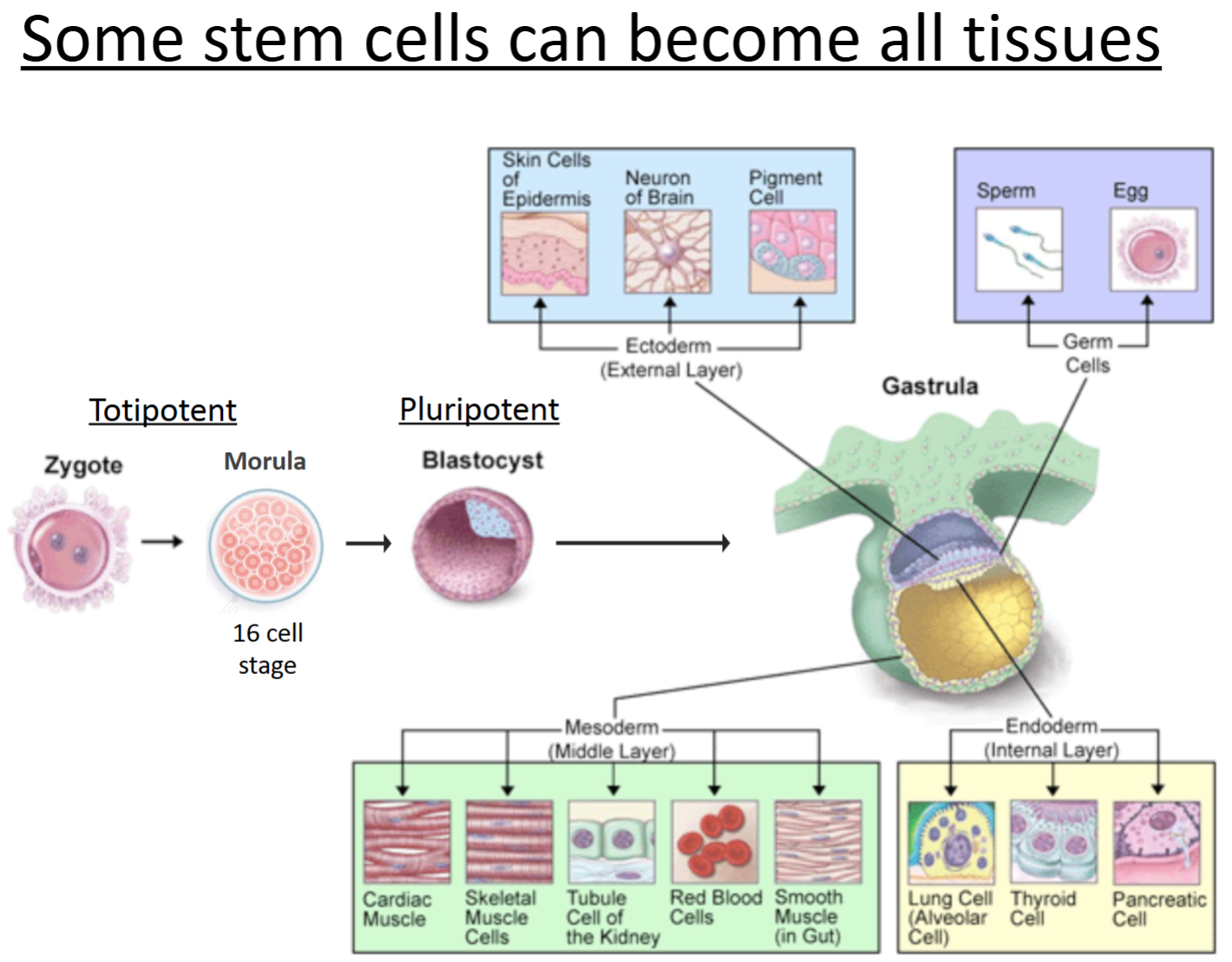
Multipotent cells
stem cells of an adult tissue.
They can become multiple different cell types but are much more limited.
CANNOT give rise to all tissues of the body!
For example, blood stem cells wouldn’t be able to differentiate into skin cells.
Asymmetric cell division
makes functionally different cells by way of transcription regulators.
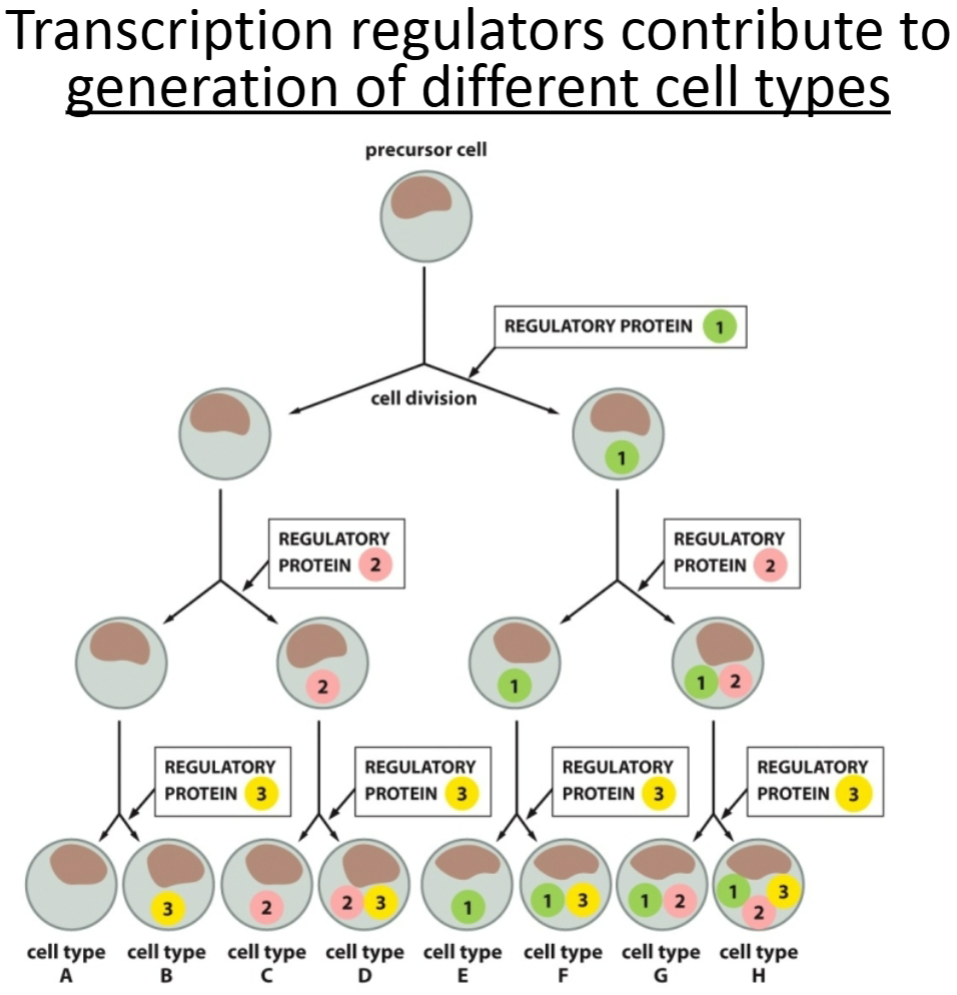
How are cells able to differentiate into so many different cell types in an organism and within a single tissue?
Through expression of different genes by activation of different transcription factors. Which transcription factors are functioning, and which genes are expressed in each cell is determined by several factors, one of which is where the cell is physically located as development is happening.
One important pathway that can control cell fate is Delta-Notch signaling. The Delta protein on one cell binds to the Notch protein on another cell. This causes part of the Notch protein to be cleaved, which then enters the nucleus. This activates a transcription factor to cause expression of genes and other regulatory proteins that determine cell fate.
Often times, the cell expressing Delta causes the other cell to become something different than itself.
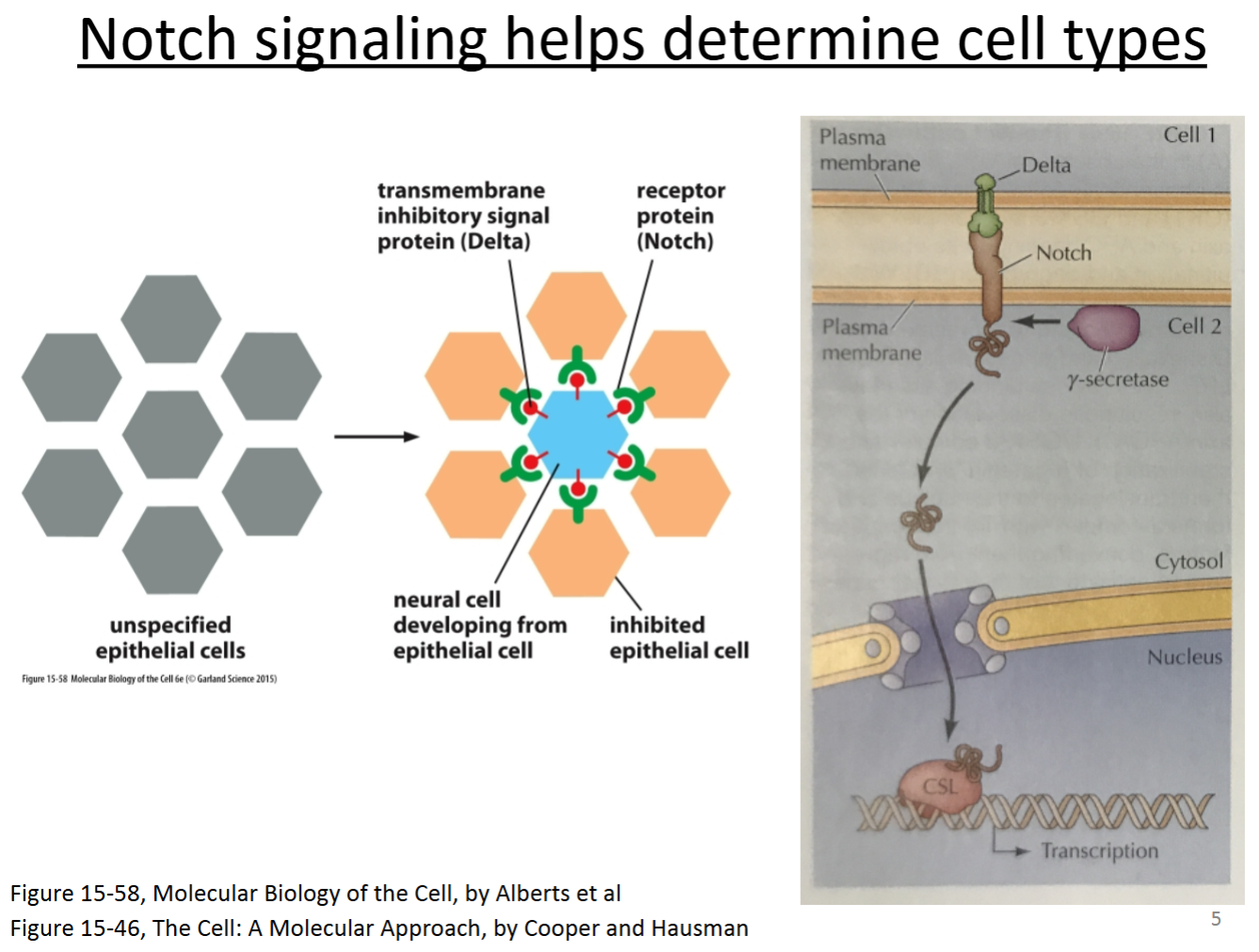
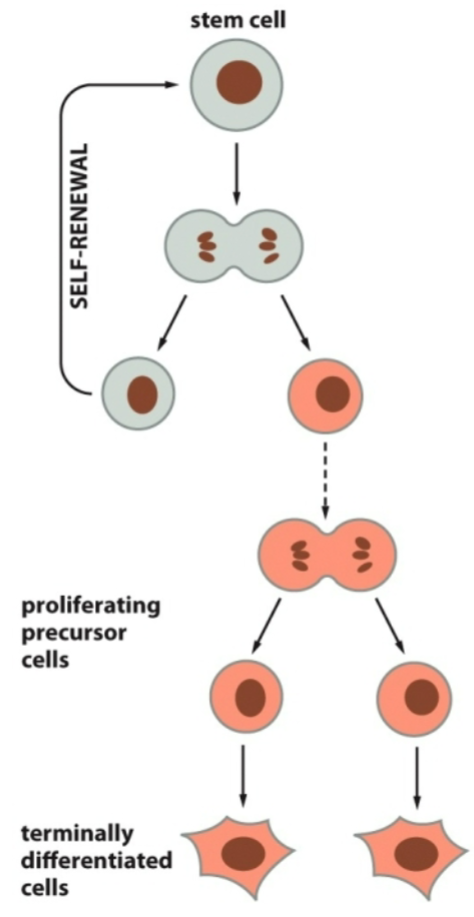
Which cell type maintains tissues?
Multipotent.
Adult tissue homeostasis depends on multipotent stem cells (e.g., skin, cornea, blood, intestine, bone, etc.).
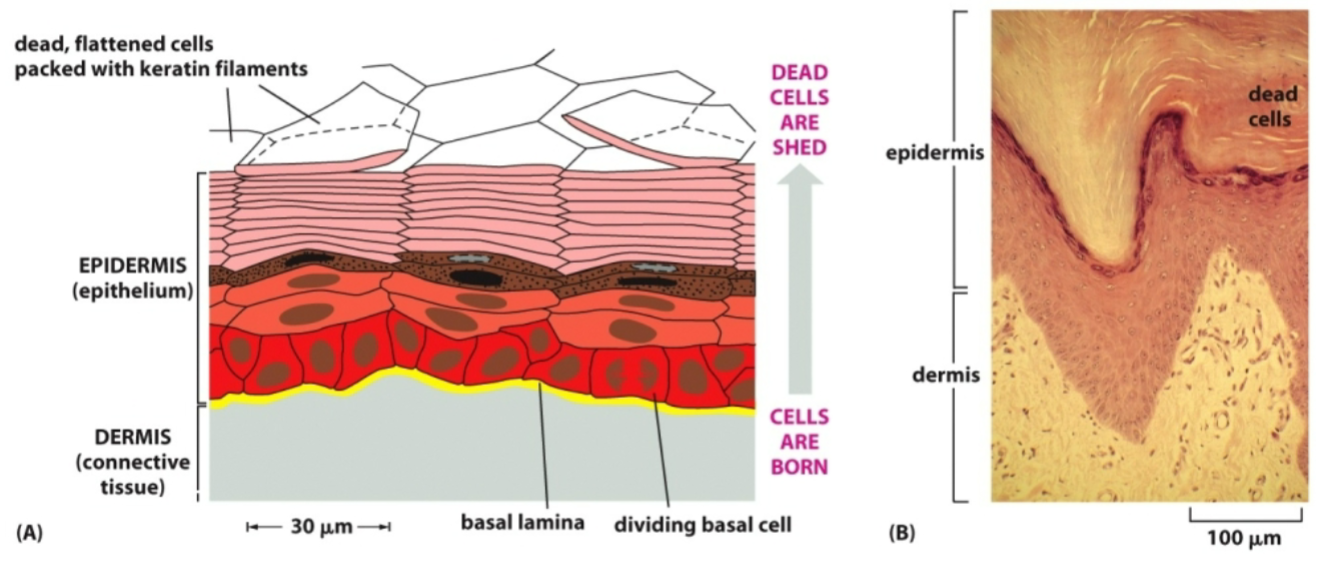
How does tissue renewal of the skin work?
The basal layer cells divide and a daughter cell is pushed up. Dead cells at the surface are shed.
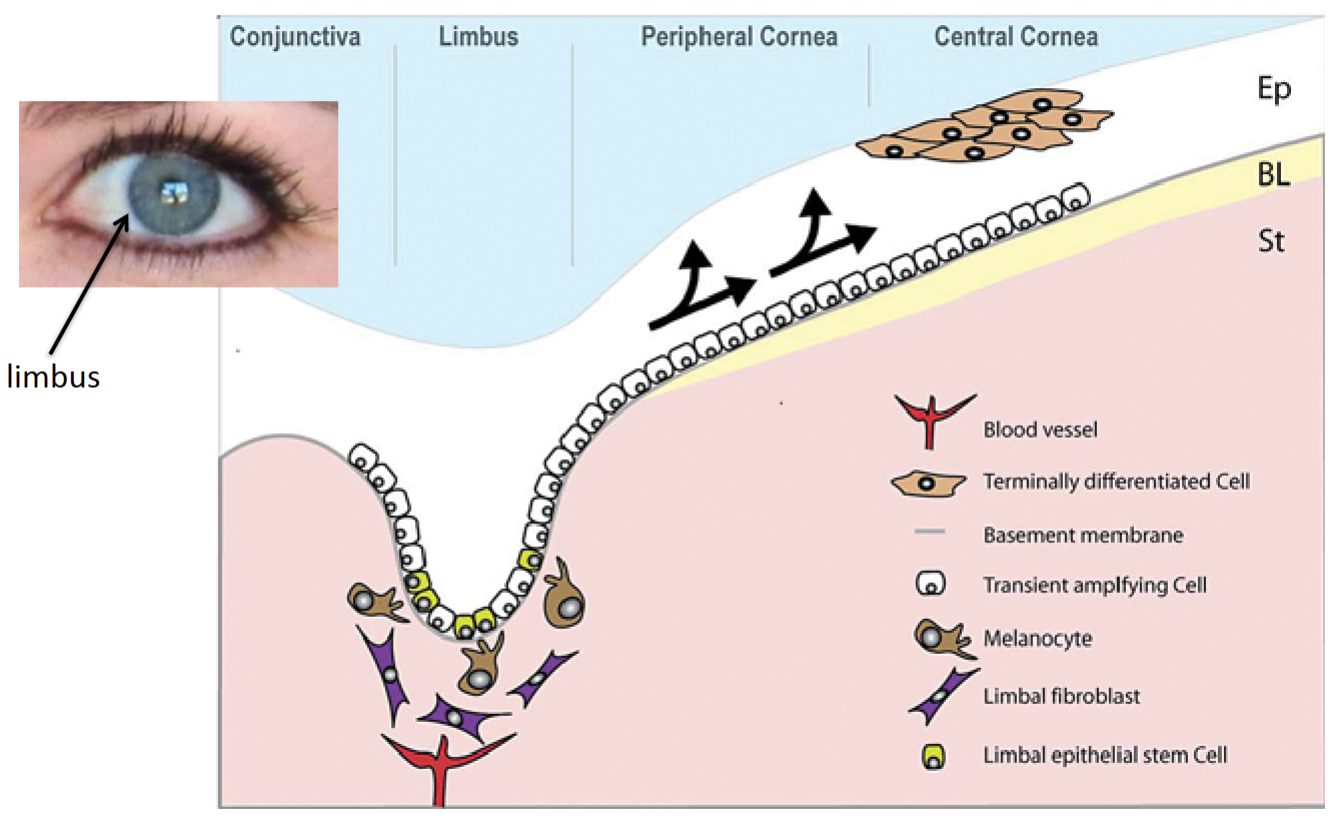
How does tissue renewal of the cornea work?
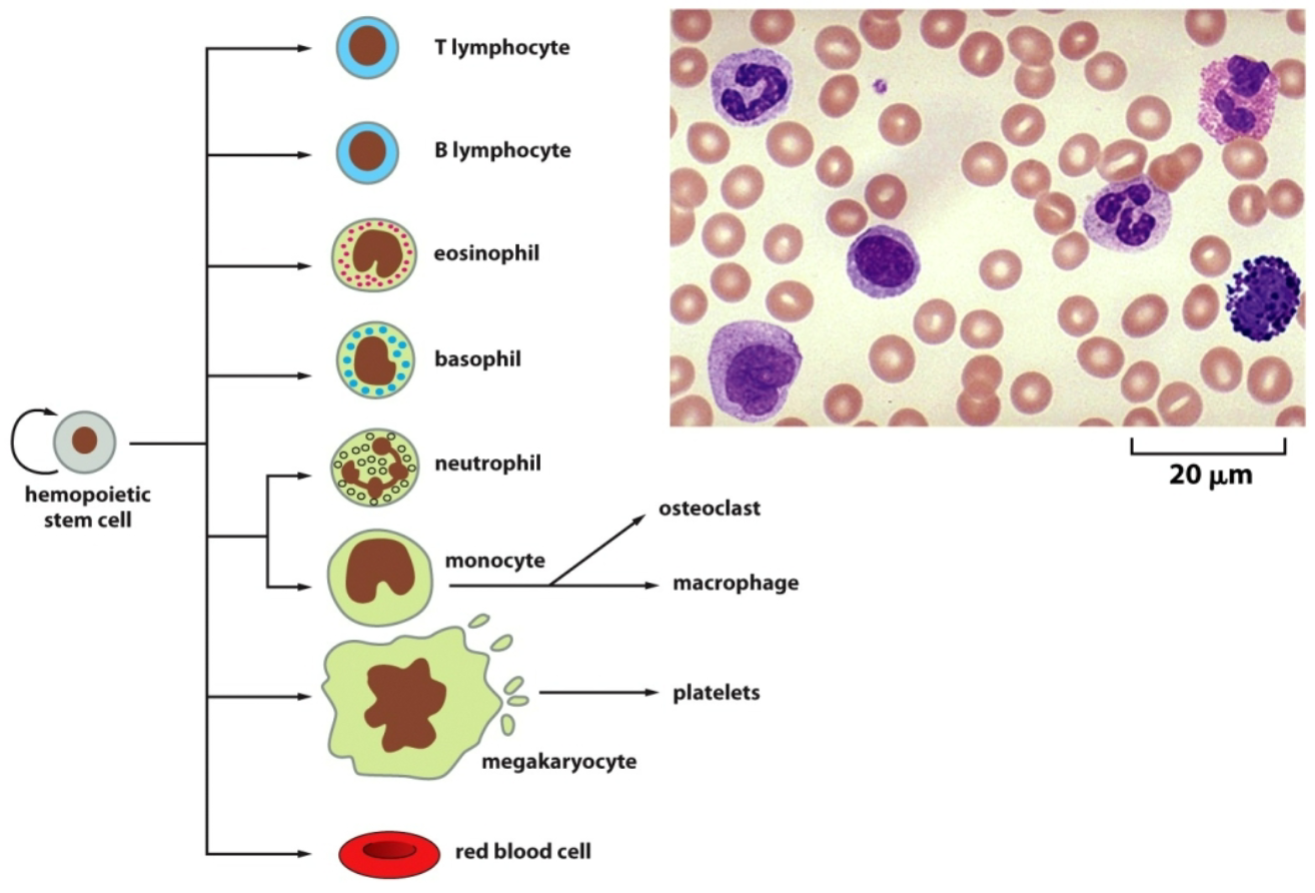
How does tissue renewal of the blood work?
T lymphocytes, B lymphocytes, eosinophils, basophils, neutrophils, and monocytes are white blood cells.
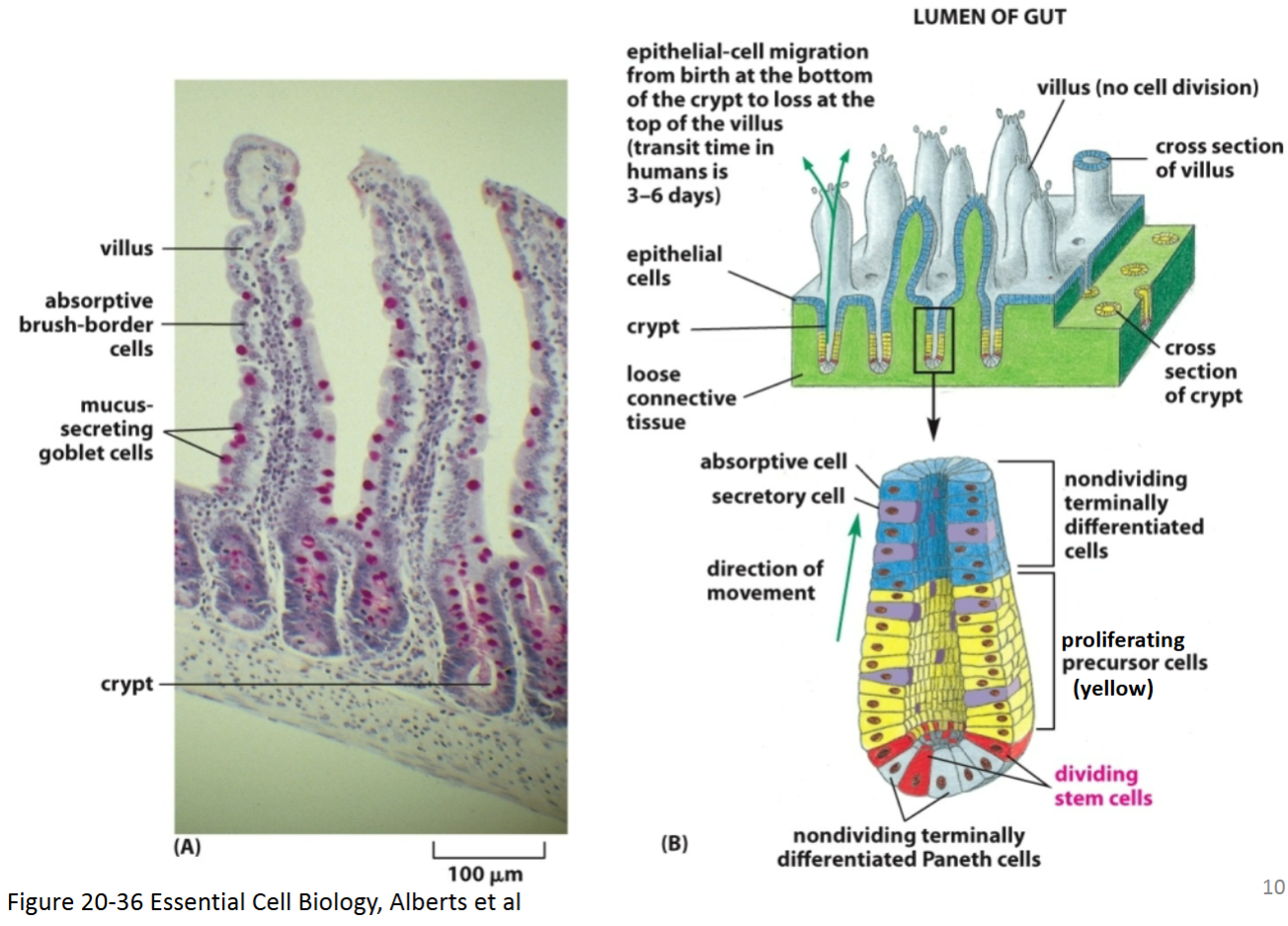
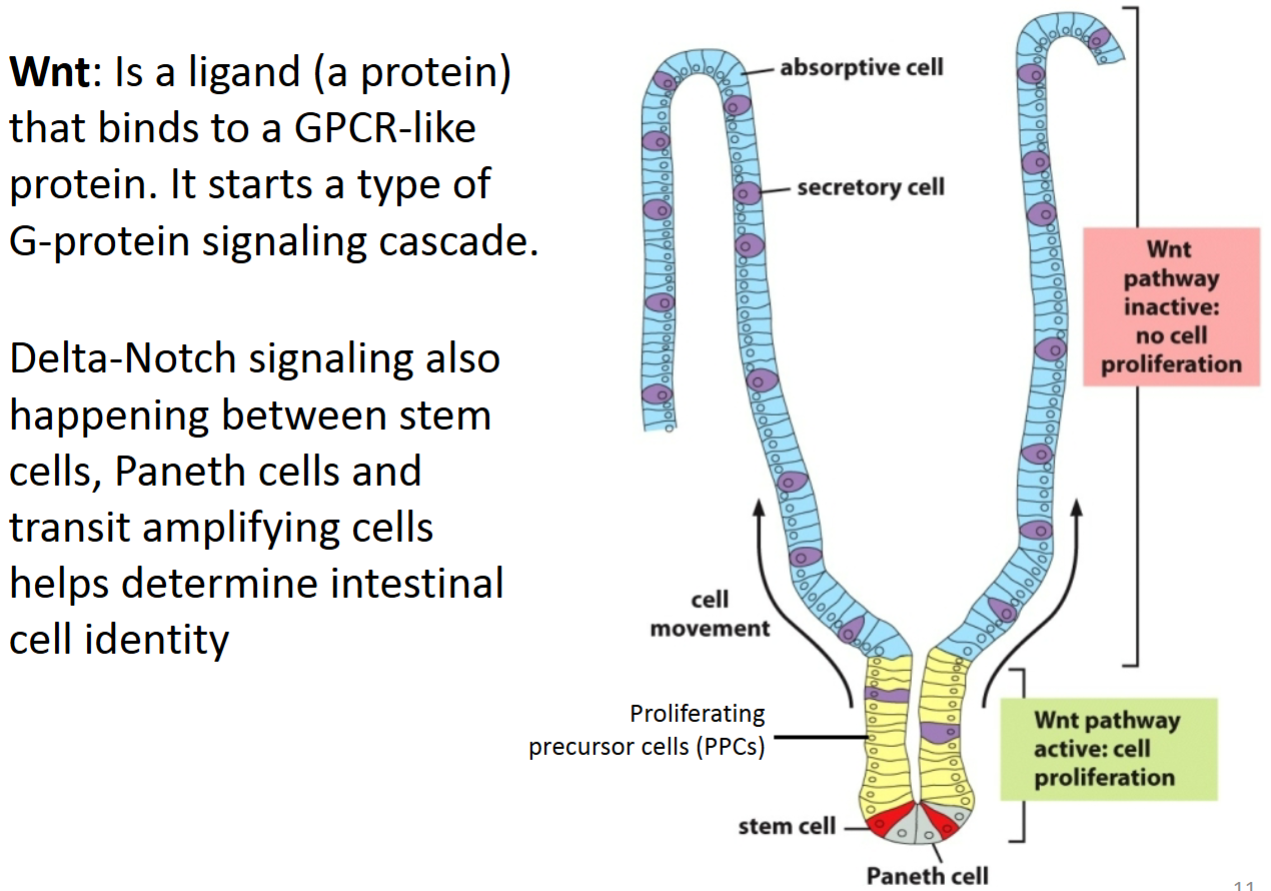
How does Wnt signaling control cell proliferation and contribute to differentiation in intestinal cells?
Paneth cells (differentiated cells in the bottom of the crypt that are derived from the intestinal stem cells) secrete the diffusible Wnt ligand, which binds to the GPCR receptor (called Frizzled) on stem cells and proliferation precursor cells—PPCs (also called transient amplifying cells) via paracrine signaling.
This causes a signaling cascade that allows β-catenin to enter the nucleus and promote transcription of genes required for proliferation.
As these cells divide, the stem cells remain in the bottom of the crypt while PPCs are pushed upward.
Once these cells move far enough out of the crypt, they are no longer close enough to receive the Wnt ligand.
Because of this, the Wnt pathway is turned off, β-catenin is degraded, cell division stops, and differentiation occurs.
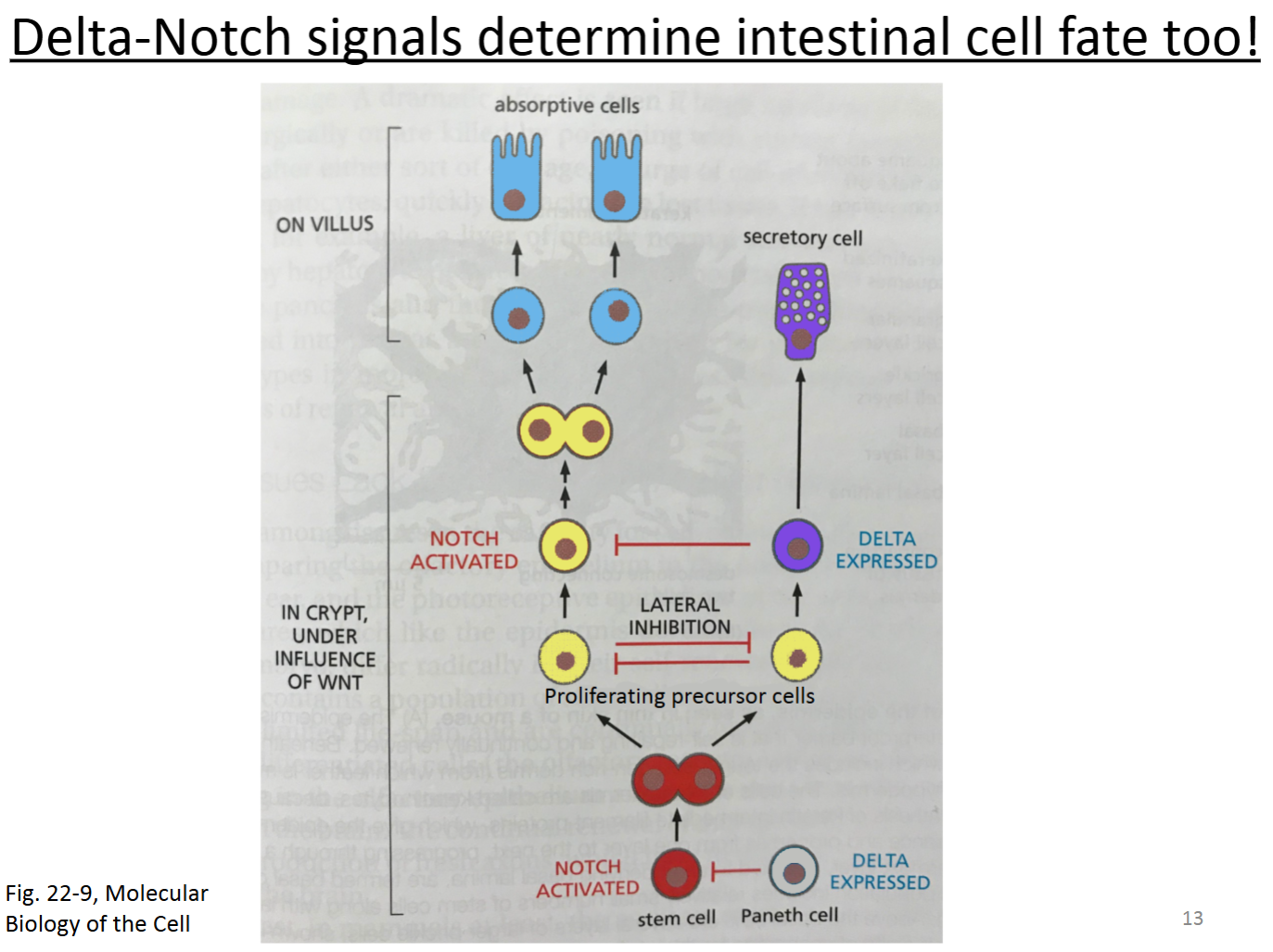
How is Delta-Notch signaling related to Wnt signaling in homeostasis of intestinal tissue?
Two of the transcriptional targets of Wnt signaling in the crypts of the intestine are genes that encode the Delta and Notch proteins.
In the base of the crypt, Paneth cells express Delta and are in a terminally differentiated state.
The multipotent stem cells express Notch.
The Delta-Notch interaction between the Paneth and stem cells causes the stem cells to remain in an undifferentiated stem state through lateral inhibition.
When these stem cells divide, they will give rise to more stem cells, but some cells will be pushed up further from the base.
These cells (proliferation precursor cells—PPCs; also known as transit amplifying cells—TACs) will laterally inhibit each other and also divide.
Proliferating precursor cells receive Wnt and divide. They stay in precursor form by expressing just enough Delta and Notch simultaneously to remain in a proliferated precursor state and inhibit nearby cells.
As the PPCs get pushed up further, more Delta-Notch signaling occurs, with cells expressing Delta becoming terminally differentiated into secretory cells.
The PPCs (further up the crypt) that express Notch will keep dividing until they are too far to receive the Wnt signal and will become terminally differentiated into absorptive cells.
Why do you suppose skin cells and intestinal cells are renewed frequently, whereas most neurons last for a lifetime of the organism?
Skin cells are constantly being sloughed off and are constantly in contact with the environment (water, clothes, etc.). These cells also form a first line of defense against potentially hazardous compounds and mutagens that are ubiquitous in our environment. The rapid turnover protects the organism from harmful consequences, as wounded and sick cells are discarded.
A neuron, on the other hand, lives in a very protected environment, insulated from the outside world. Its function depends on a complex system of connections with other neurons—a system that is created during development and is not easy to reconstruct if the neuron subsequently dies.
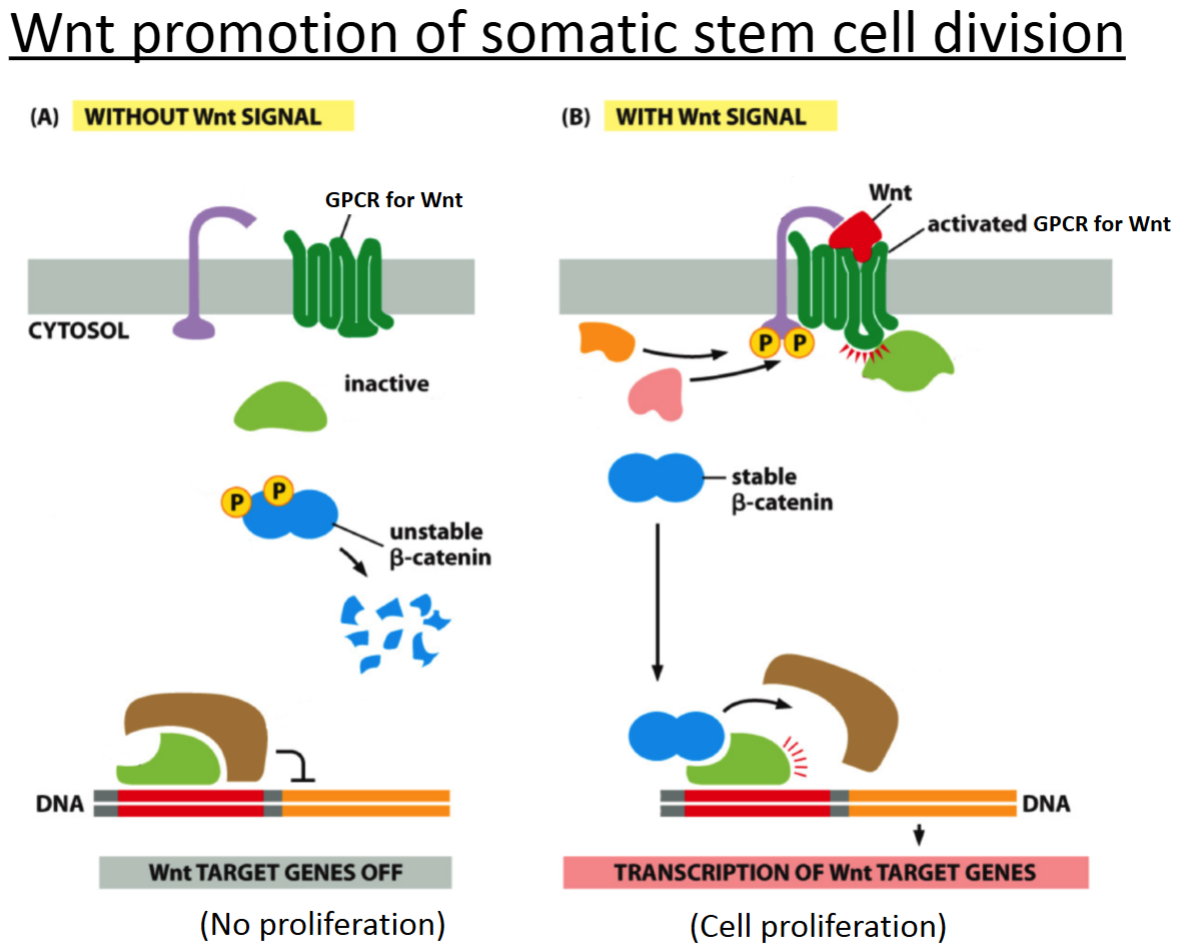
How does Wnt signaling work?
Wnt binds and activates GCPR.
There is a G protein cascade and a kinase cascade.
If the β-catenin is stable, then the cell can divide.
The stable β-catenin binds to the transcription factor and transcription of genes occurs for cell division.
What are the 3 main events of a signal transduction pathway? Provide examples that might be specific to each event.
Activation of a receptor by a signal (e.g., small molecule binding to a receptor, light or sound stimulating a receptor).
Relay system of the signal to inside the cell (e.g., second messengers like calcium used).
Cellular response (e.g., changes in gene expression, cell division, apoptosis, secretion of a protein, etc.).
The type of signaling for delta-notch signaling is…
A. synaptic.
B. paracrine.
C. endocrine.
D. contact-dependent.
D. contact-dependent.
With Delta-Notch signaling, determine the:
Signal?
Receptor?
Transduction mechanism?
Cellular response?
Delta.
Notch.
Cleavage of the intracellular domain and movement of it into the nucleus.
Transcription of genes that determine cell type.
When a stem cell divides, it often does so “asymmetrically". What does this mean?
A. That the 2 daughter cells both become stem cells.
B. That the 2 daughter cells both become non-stem cells.
C. That the 2 daughter cells are no longer genetically identical.
D. That the 2 daughter cells become different types of cells from each other.
D. That the 2 daughter cells become different types of cells from each other.
Cells that are terminally differentiated _____. Choose all that apply.
A. will undergo apoptosis within a few days
B. no longer produce RNAs or proteins
C. no longer undergo cell division
D. are unable to move
E. will sit in G0
C. no longer undergo cell division
E. will sit in G0
What is true of proliferating precursor cells (PPCs)? Choose all that apply.
A. They are a type of stem cell.
B. They can divide to produce stem cells.
C. They can divide to produce more PPCs.
D. They can be daughter cells from PPC division.
E. They can be daughter cells from stem cell division.
F. They can divide to produce terminally differentiated cells.
C. They can divide to produce more PPCs.
D. They can be daughter cells from PPC division.
E. They can be daughter cells from stem cell division.
F. They can divide to produce terminally differentiated cells.
An adult hemopoietic stem cell found in the bone marrow _____.
A. is pluripotent
B. can produce only red blood cells
C. will express all the same genes as those found in an unfertilized egg
D. can undergo self-renewing divisions for the lifetime of a healthy animal
D. can undergo self-renewing divisions for the lifetime of a healthy animal
What are the problems and alternatives of stem cells in research and therapies?
Problems:
Ethics
Immune response
Alternative:
Induced pluripotent stem cells
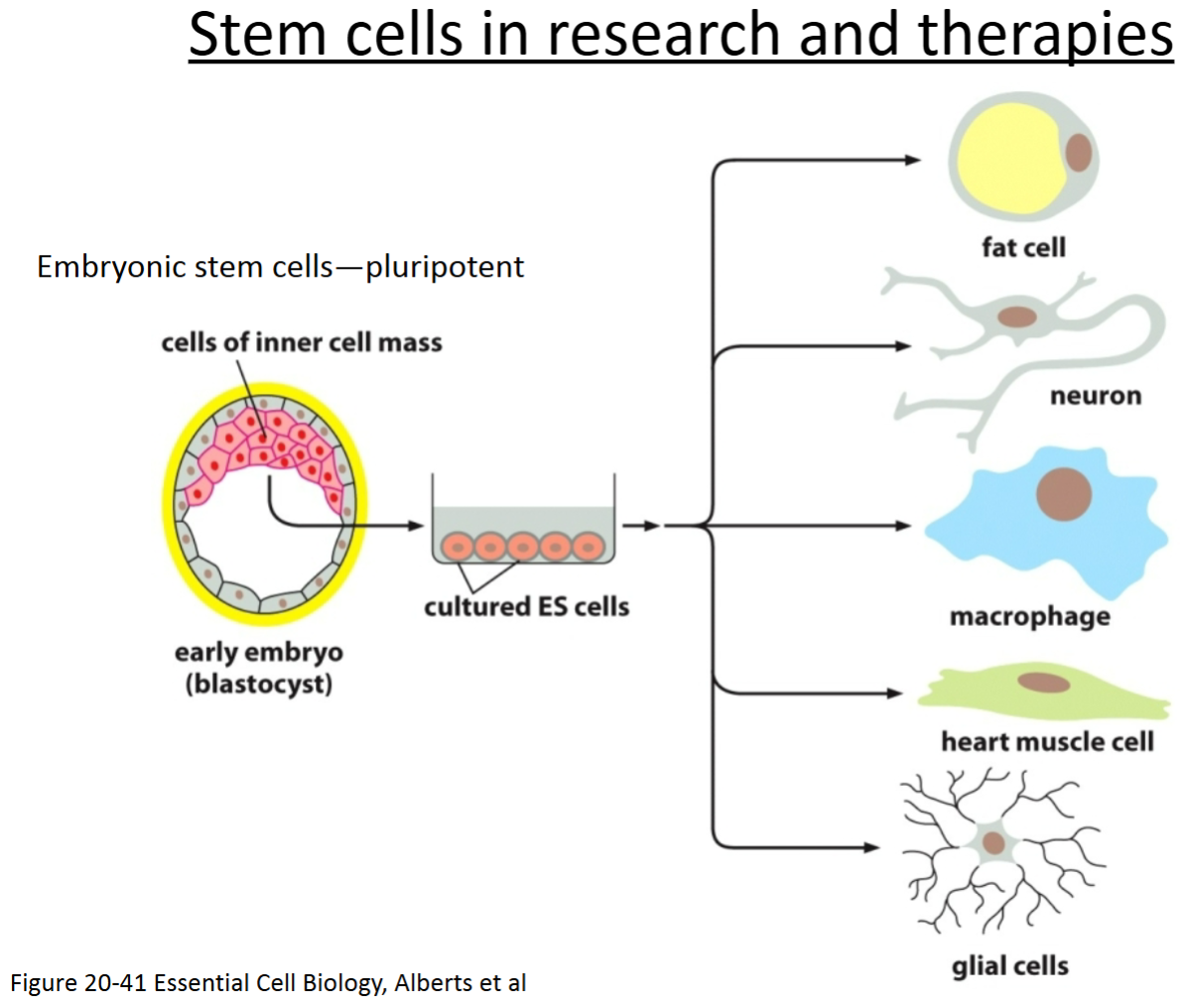
How are skin cells induced to become pluripotent?
Embryonic stem cells are pluripotent—from inner cell mass of blastocyst.
Yamanaka factors: transcriptional factors active early in development causing cells to revert to a pluripotent state—can get them to turn into whatever cell type from the body is needed.
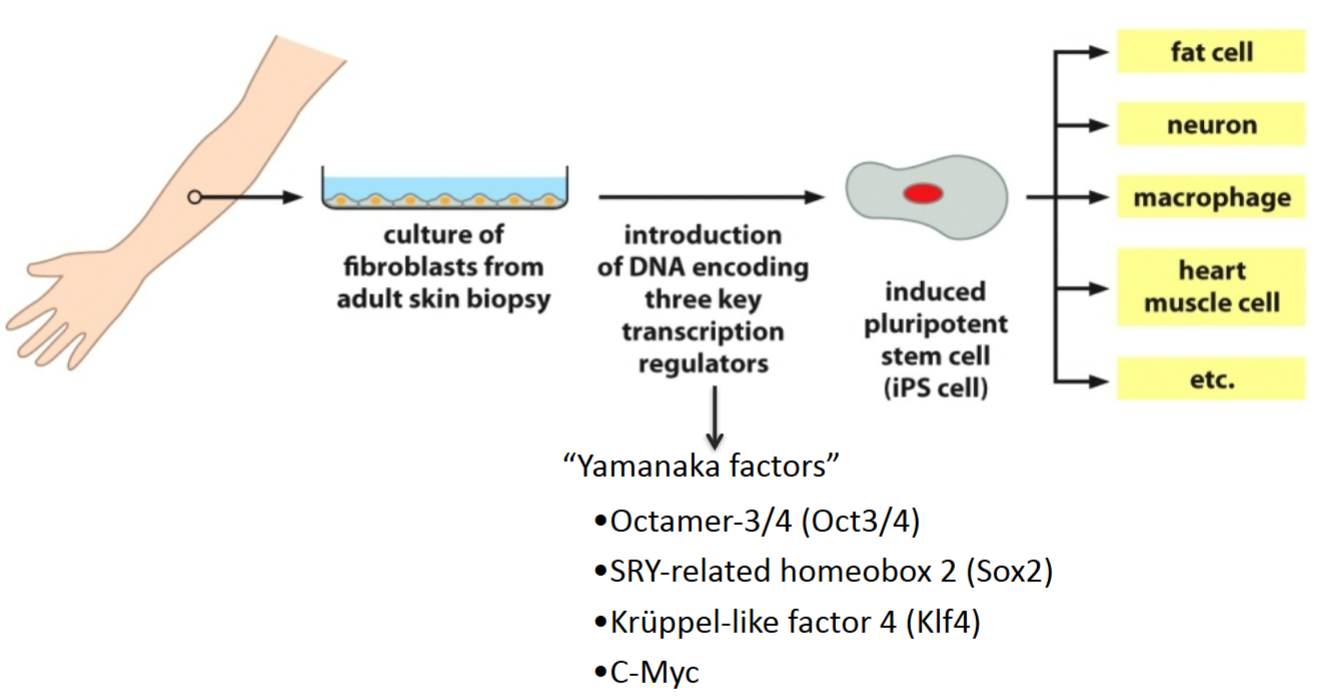
How can adult cells be converted into cells with properties of ES cells (e.g., induced pluripotent stem cells)?
By transfecting them with Yamanaka factors, which are transcription factors. They reverse differentiate cells back into a pluripotent state. All of these transcription factors are involved in growth and development, and 3 of them are involved in maintaining the pluripotency of stem cells.
From where are mouse embryonic stem (ES) cells derived?
They come from the inner cell mass of early embryos (blastocyst stage).
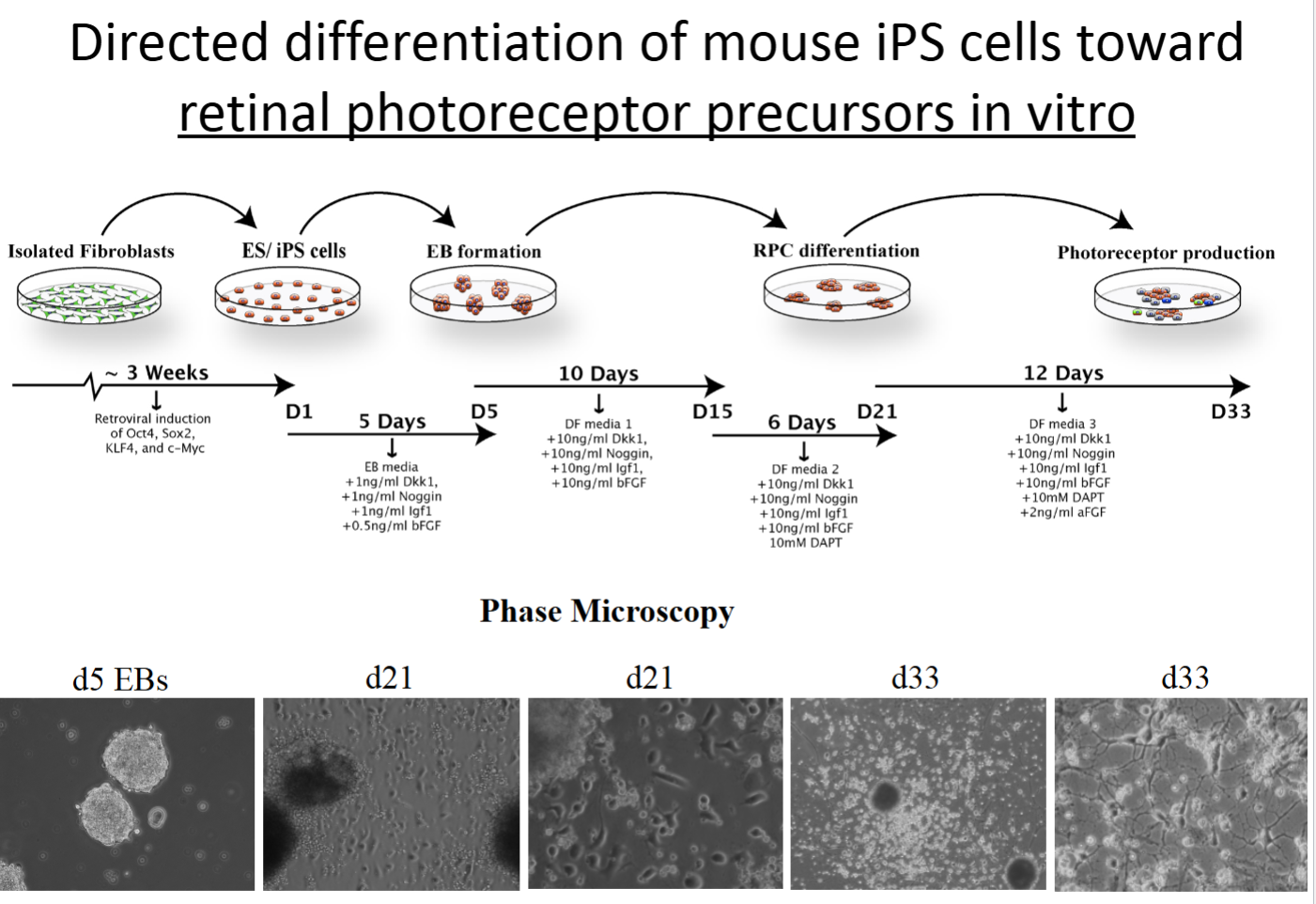
iPS cells
Derived from adult somatic cells (e.g., fibroblasts).
De-differentiated via specific transcription factors (Yamanaka factors).
Re-differentiated with appropriate hormones, growth factors, transcription factors, etc.
Can be used for cell therapies!
How does gene expression compare in iPS cells versus ES cells?
They have similar levels of gene expression, so iPS should be a viable alternative for ES.
What does a microarray examine?
What are the pros and cons of this type of experiment?
Have we done anything similar to a microarray in lab?
Microarrays determine which genes are being expressed by examining which RNA molecules (transcripts) are present in a population of cells or tissue. They can also compare the amount of these transcripts (RNAs) in two or more populations of cells or tissues. In the case of iPSCs with the example in the PowerPoint, it was comparing the degree to which certain genes are expressed in embryonic stem cells versus induced pluripotent stem cells.
Pros: RNA is relatively cheap and easy to work with. It’s a quantitative assay.
Cons: Not examining the actual proteins made—only their transcripts so it’s hard to know if all those transcripts were actually translated. Could also end up with false positive data.
We have NOT done anything similar to a microarray in lab.
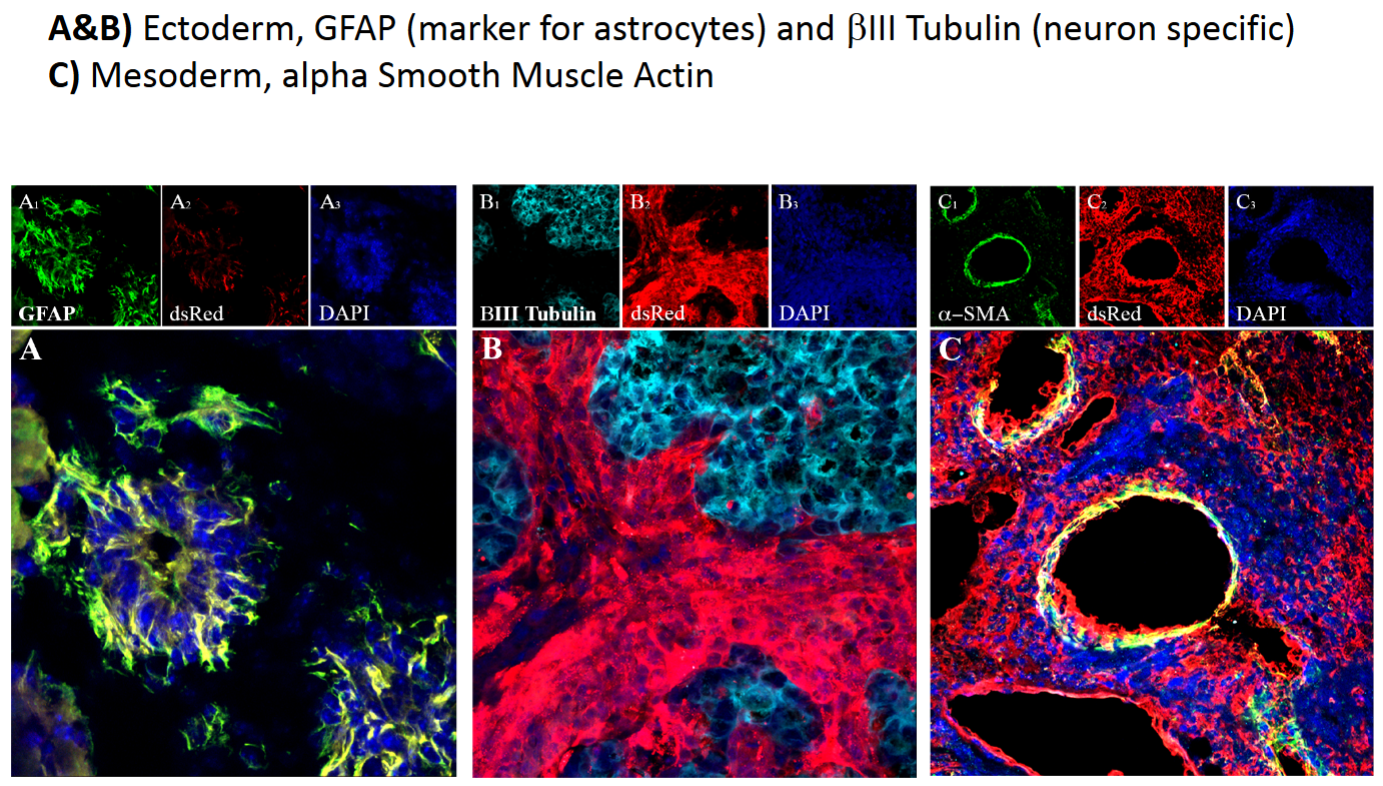
What is immunochemistry (IHC) and what does it examine?
What are the pros and cons of this type of experiment?
Have we done anything similar to IHC in lab?
IHC examines specific proteins that are expressed in tissues with the use of antibodies that are fluorescently labeled. It allows one to see specifically where in a tissue that a protein is expressed but is not very quantitative for determining relative amounts of proteins. In the PowerPoint example, it was used to show that IPSCs were capable of being all of the germ layers of development by expressing the correct proteins.
Pros: Examining actual proteins and their localization.
Cons: This is not a quantitative type of experiment. Also, antibodies are expensive and can be unreliable. This technique is prone to experimental artifacts, again usually due to crappy antibodies.
We have done immunocytochemistry (ICC) in lab, which is the same as IHC, except done on cells instead of tissue sections.
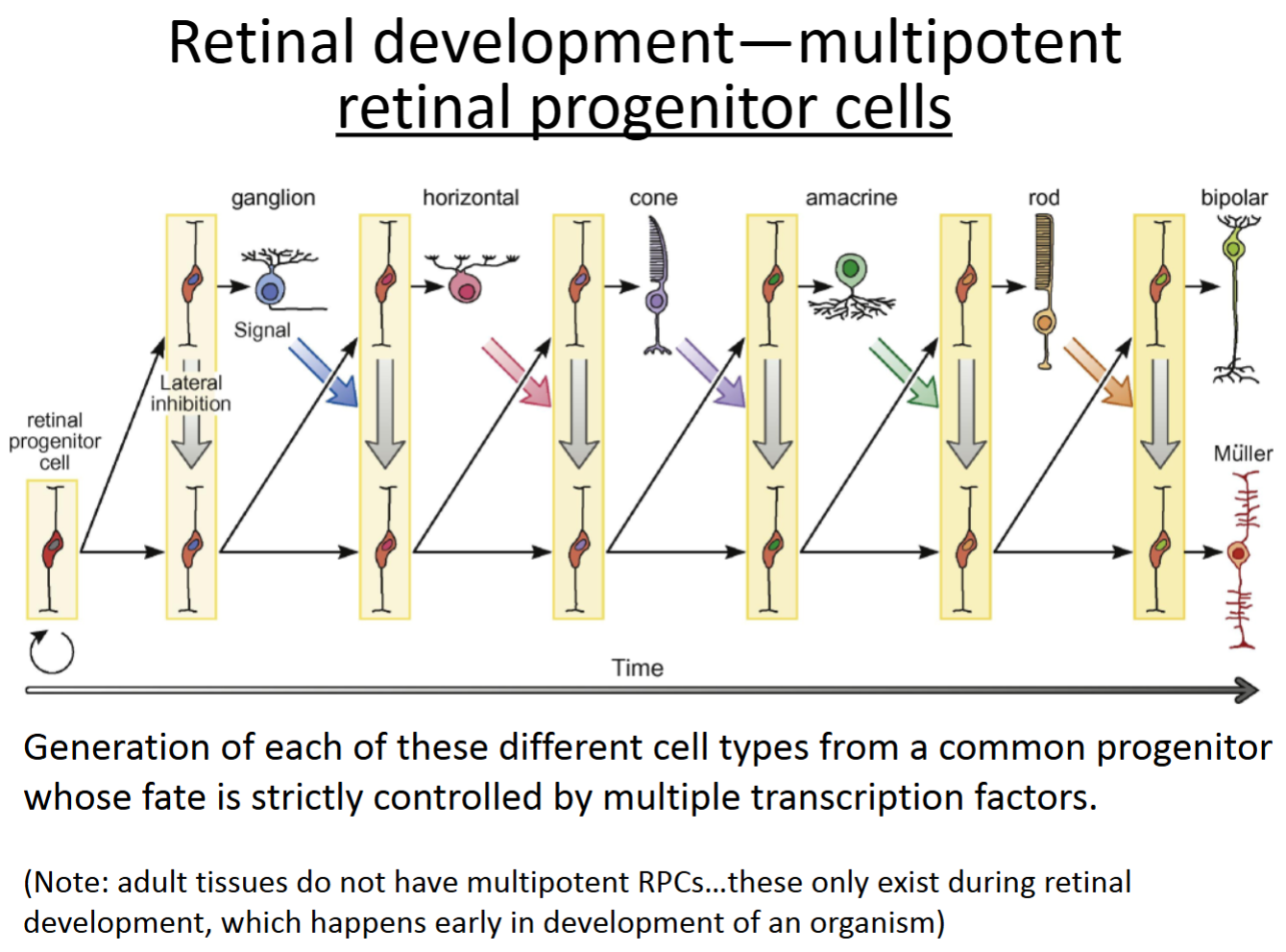
Multipotent retinal progenitor cells
multipotent stem cells only present when the eye and retina are developing—they do asymmetric division to become any cell type of the adult retina.
What are some limitations of iPSCs?
Introduction of genetic mutations during the reprogramming process, incomplete/imperfect reversion to pluripotent state, if any of the induced pluripotent cells don’t re-differentiate properly (can lead to tumor growth if they remain in a pluripotent state).
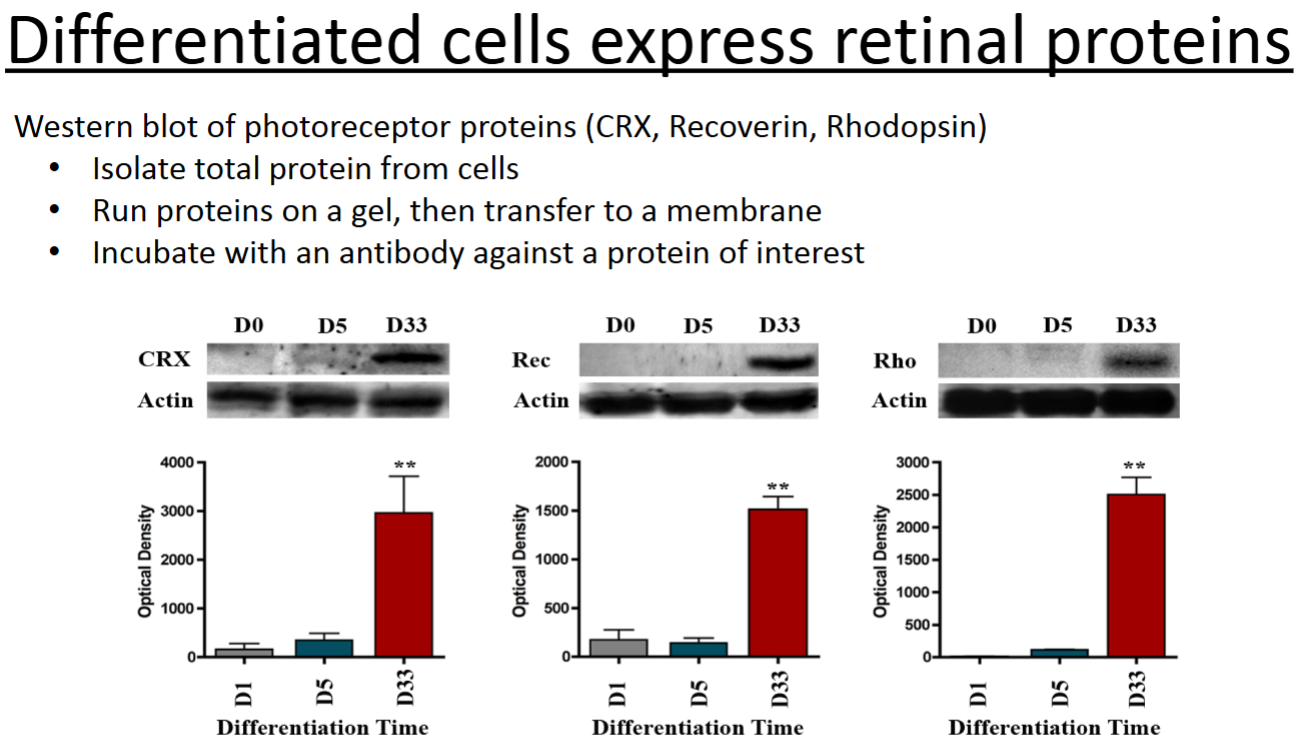
What is a western blot and what does it examine?
A western blot can quantitatively determine the amount of protein present in a population of cells or within tissues. In the example in the PowerPoint, it was used to determine the amount of specific retinal proteins that were expressed at various time points throughout differentiation of iPSCs into photoreceptor cells.
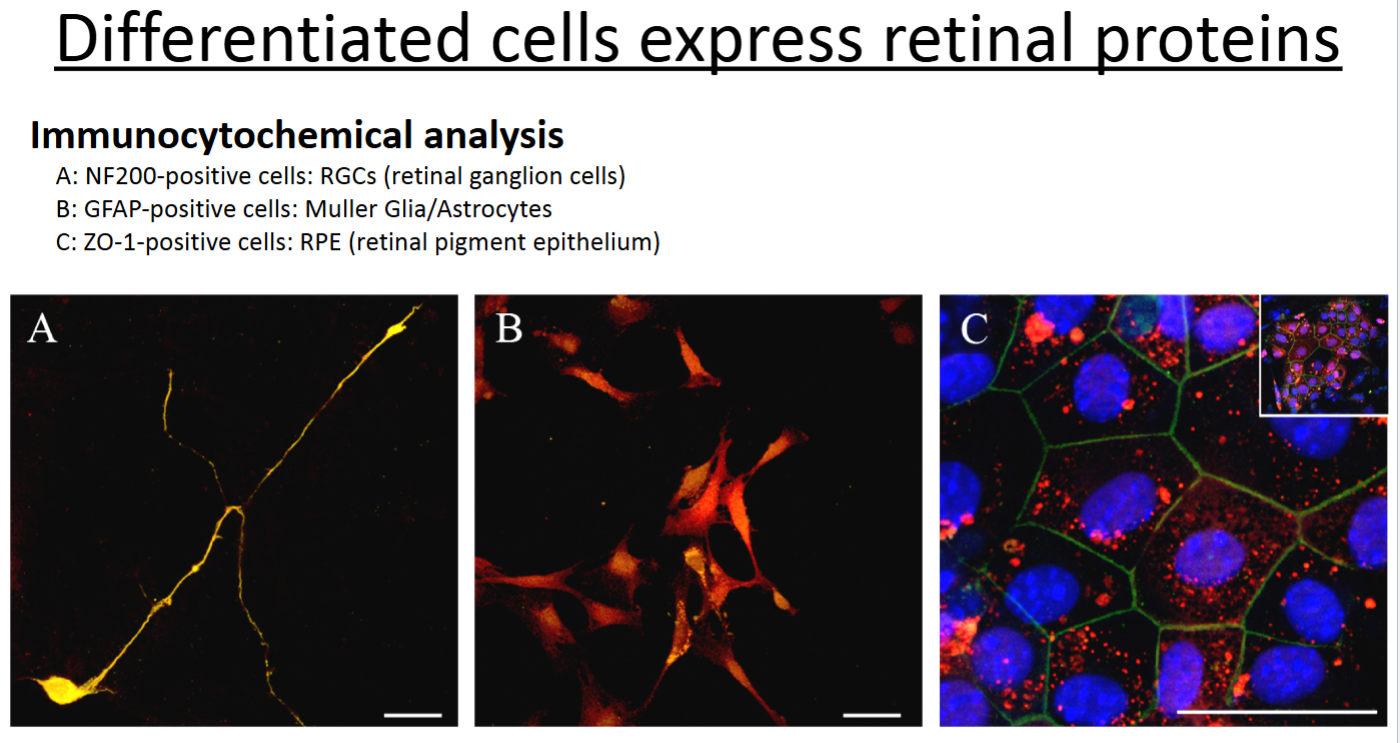
What is immunocytochemistry (ICC) and what does it examine?
ICC is the same thing as IHC, except it is performed on cells in culture rather than on tissue/organ sections.
Why were the molecular/cellular tools of microarray, western blots, and immunocytochemistry used on the iPS cells and the cells differentiated into retinal cells?
For the iPS cells, it was to make sure the genes and proteins that are known to be expressed in normal ES cells are being expressed. It gives more confidence/evidence that the cells truly are pluripotent.
For the retinal cells, it was done to be sure the correct proteins are in fact being expressed in the in vitro experimental cells. If they are, you can be confident that you are studying the cell type you are trying to study. Of course, there needs to be prior knowledge of what proteins the cells express in vivo.
When ES cells or iPS cells are injected back into the donor, they often form teratomas (benign tumor with tissue or organ components resembling normal derivatives of more than one germ layer). Speculate why this might be.
A teratoma is a (usually benign) tumor with tissue or organ components resembling normal derivatives of more than one germ layer. All tumors of this class are the result of abnormal development of pluripotent cells: germ cells and embryonal cells. Since these stem cells are being put back into an already developed organism/tissue, these cells sometimes don’t receive the correct cues and signals and develop into undesired cells/tissues. Groups are doing experiments to figure out a way to drastically reduce the occurrence of teratomas.
What cytoskeletal component do crawling cells use?
What is the basic structure of this protein?
Actin.
It’s composed of G-actin monomers that twist together into a rope-like structure called F-actin; the monomers have tertiary structure whereas F-actin has quaternary structure, but all the subunits are identical.
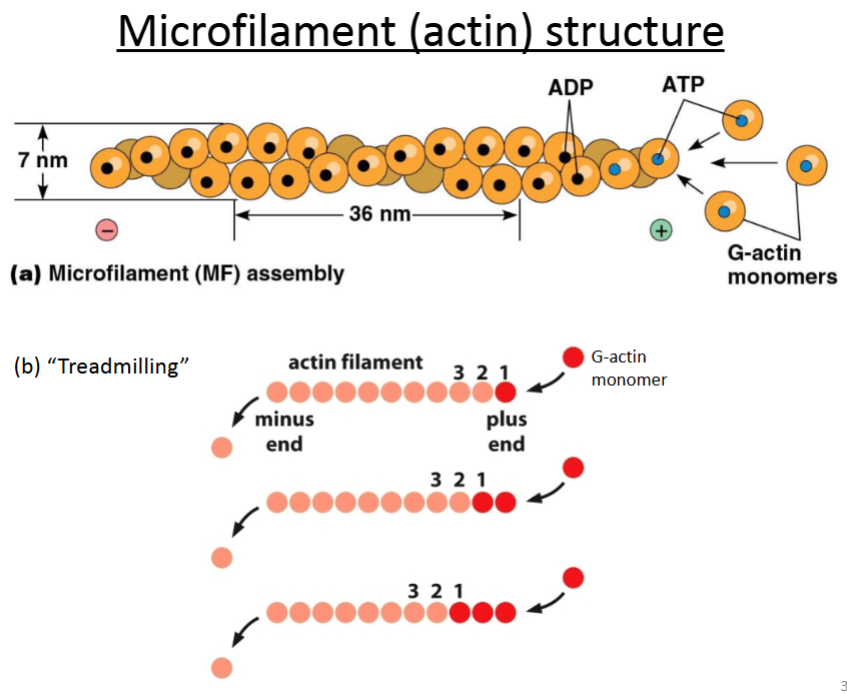
Are microfilaments and actin monomers or polymers?
Microfilaments are actin in polymer form.
Actin can be monomeric or polymeric.
Globular actin (G-actin): monomer.
Filamentous actin (F-actin): polymer.
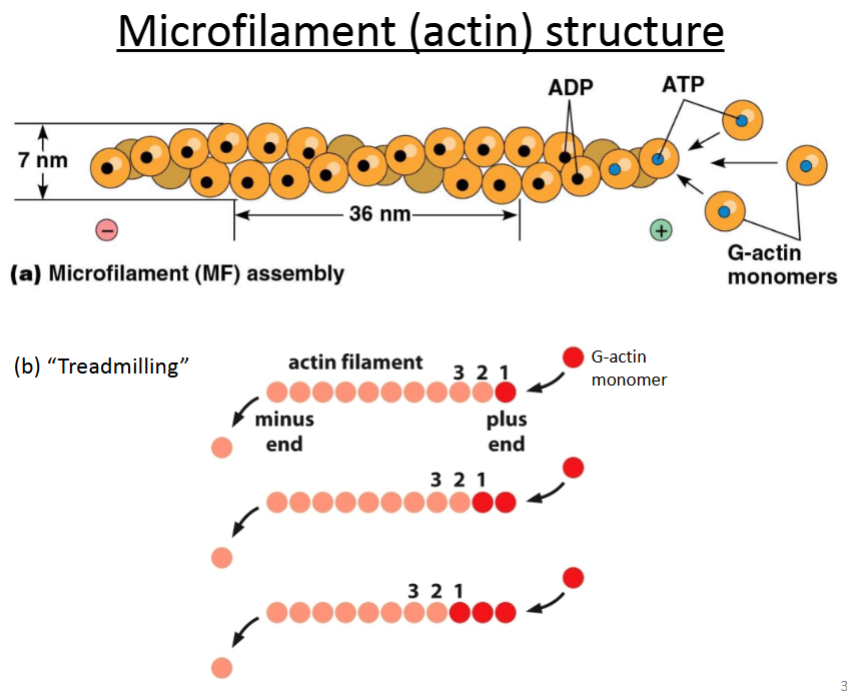
What are the microfilament (actin) functions?
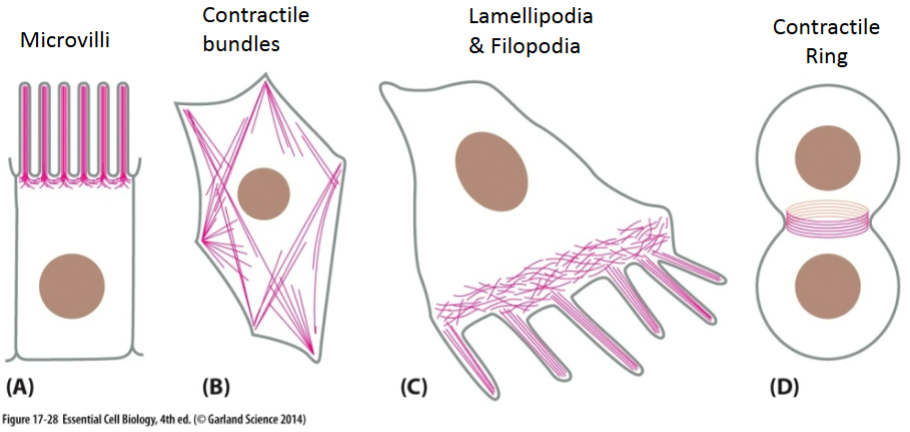
Cell cortex—strengthen the plasma membrane.
Bending of epithelial sheets at adherens junctions to form tissue structures.
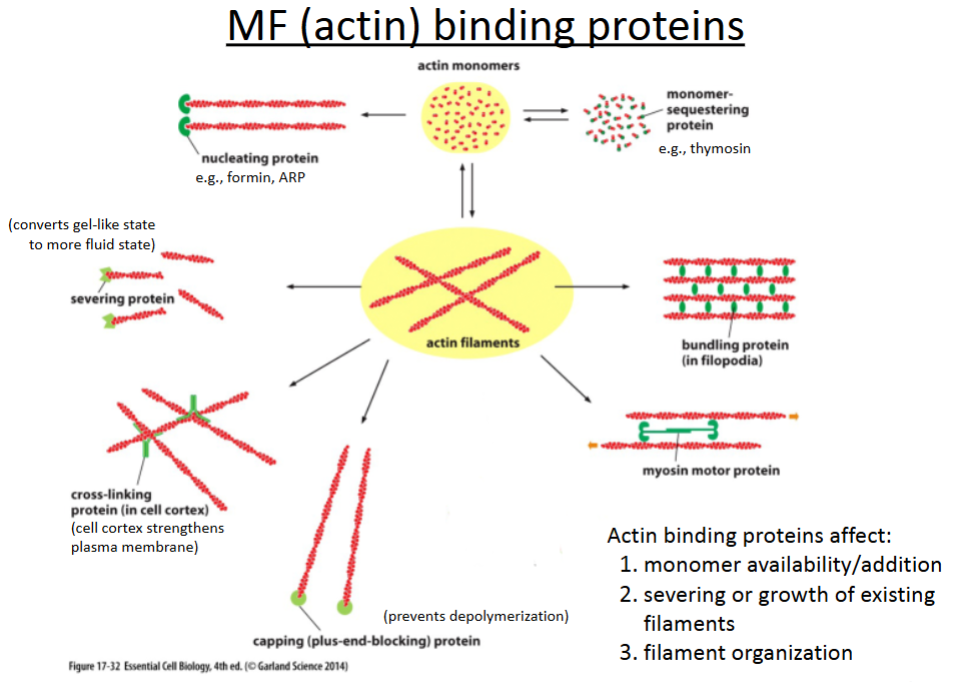
What are the 6 actin binding proteins?
Nucleating proteins
Severing proteins
Crosslinking proteins
Capping proteins
Bundling proteins
Branching proteins
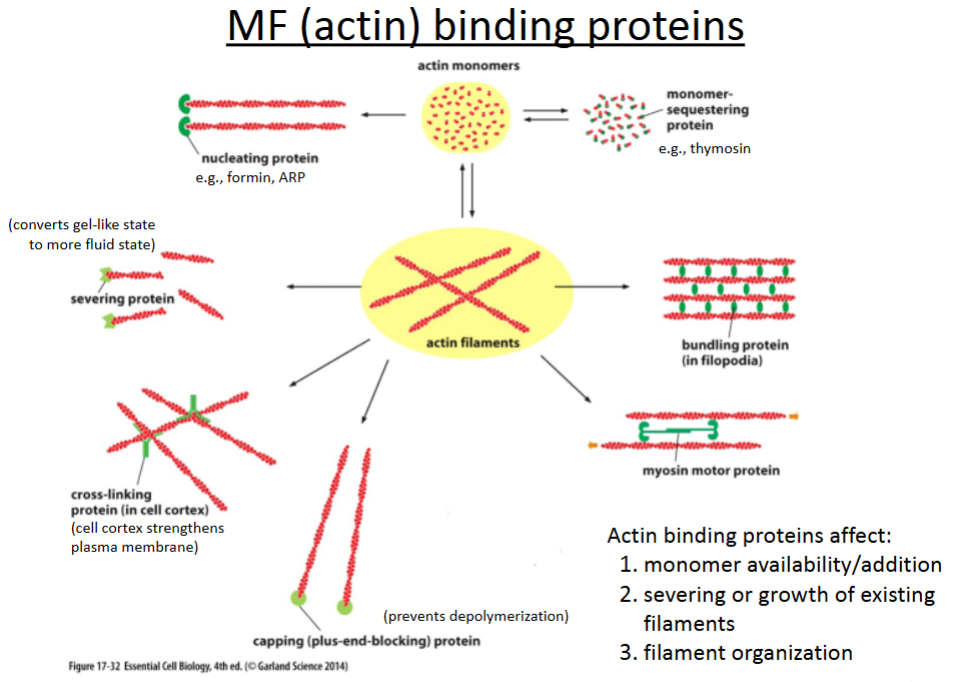
Nucleating proteins
promotes polymerization of actin filaments; involved in cell movement (crawling).
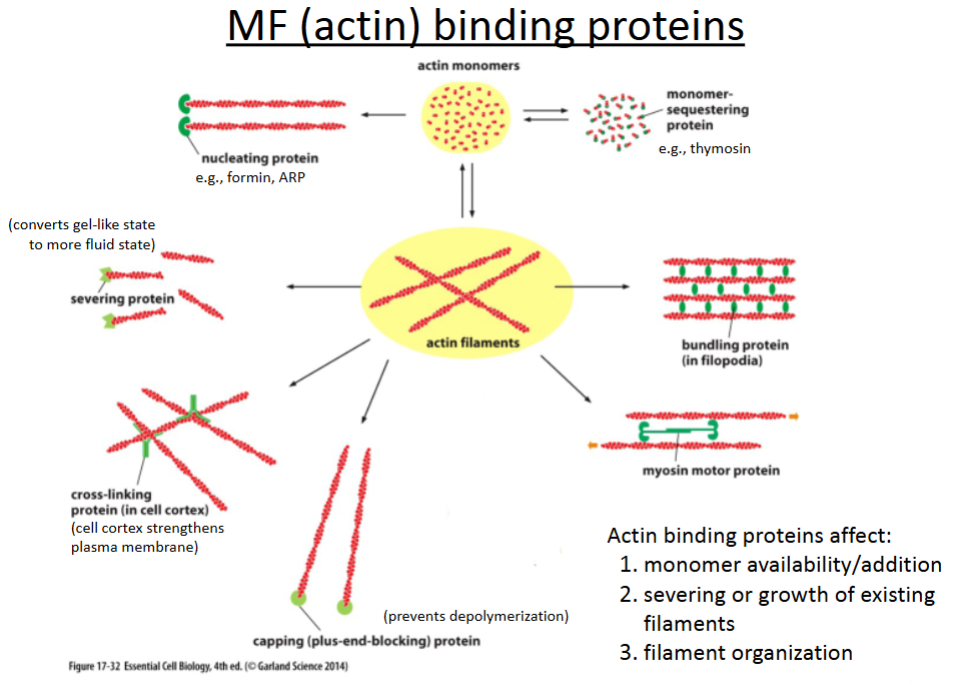
Severing proteins
cuts actin filaments to shorter lengths and can convert actin gel into a more fluid state.
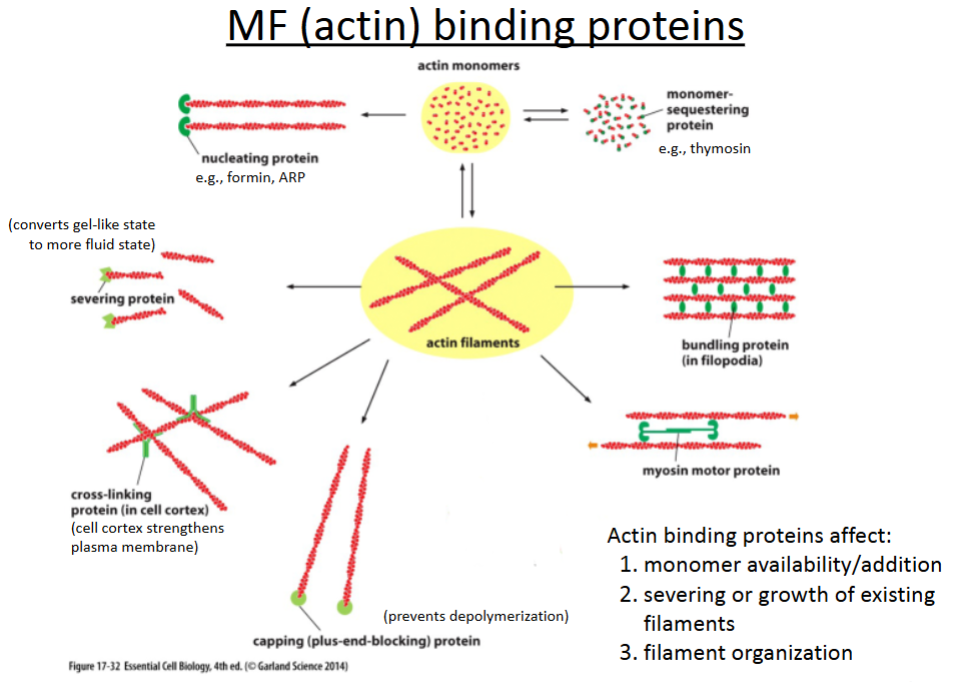
Crosslinking proteins
connect different actin filaments together to form a meshwork (e.g., the cell cortex, contractile bundles); strengthens the cell and also supports the plasma membrane.
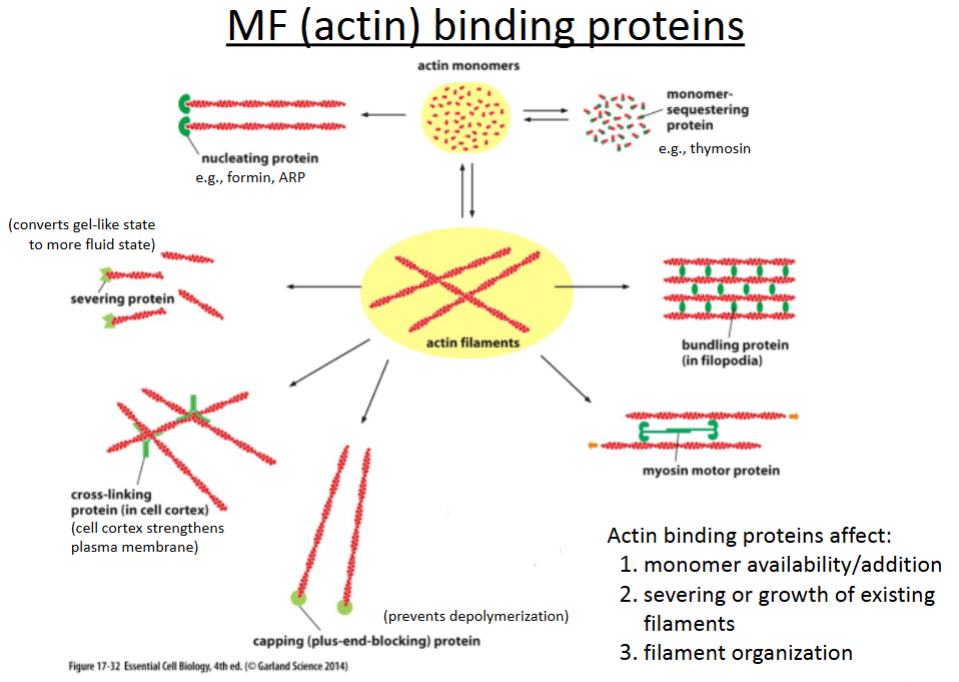
Capping proteins
prevents further polymerization but also prevents depolymerization; involved in cell crawling.
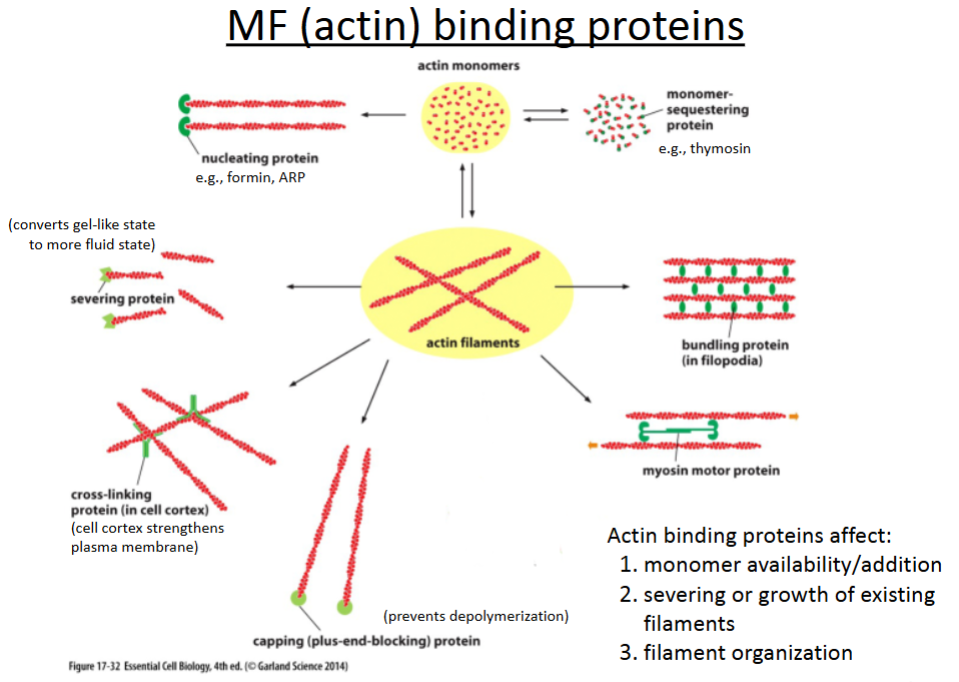
Bundling proteins
hold growing actin filaments in filopodia together; involved in cell crawling.