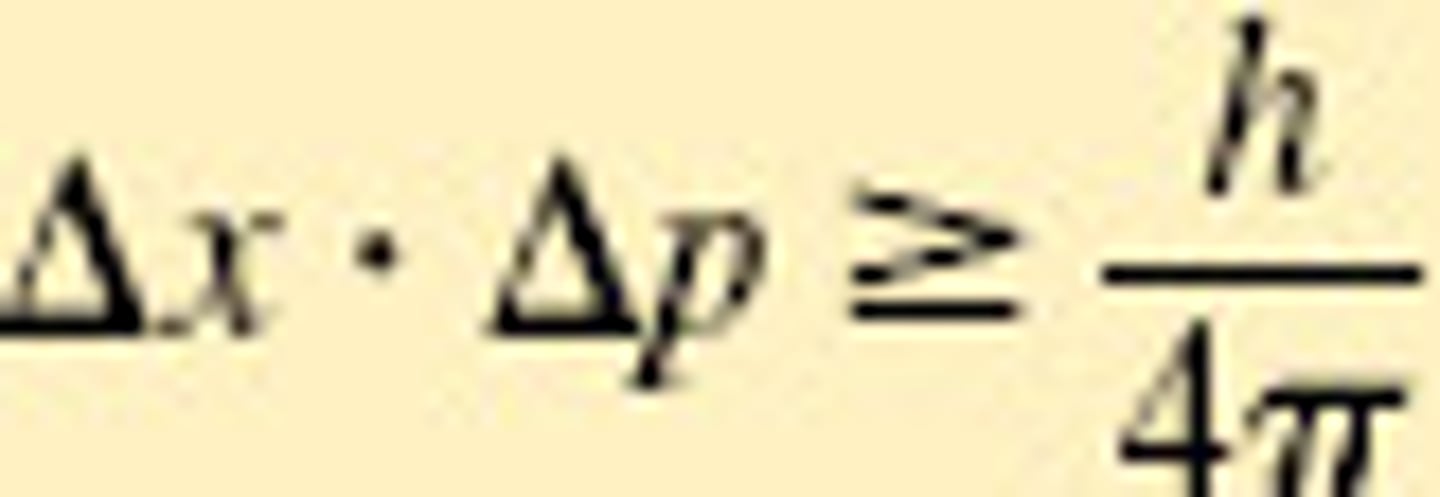Chem 1601 Exam 1 Key Terms
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
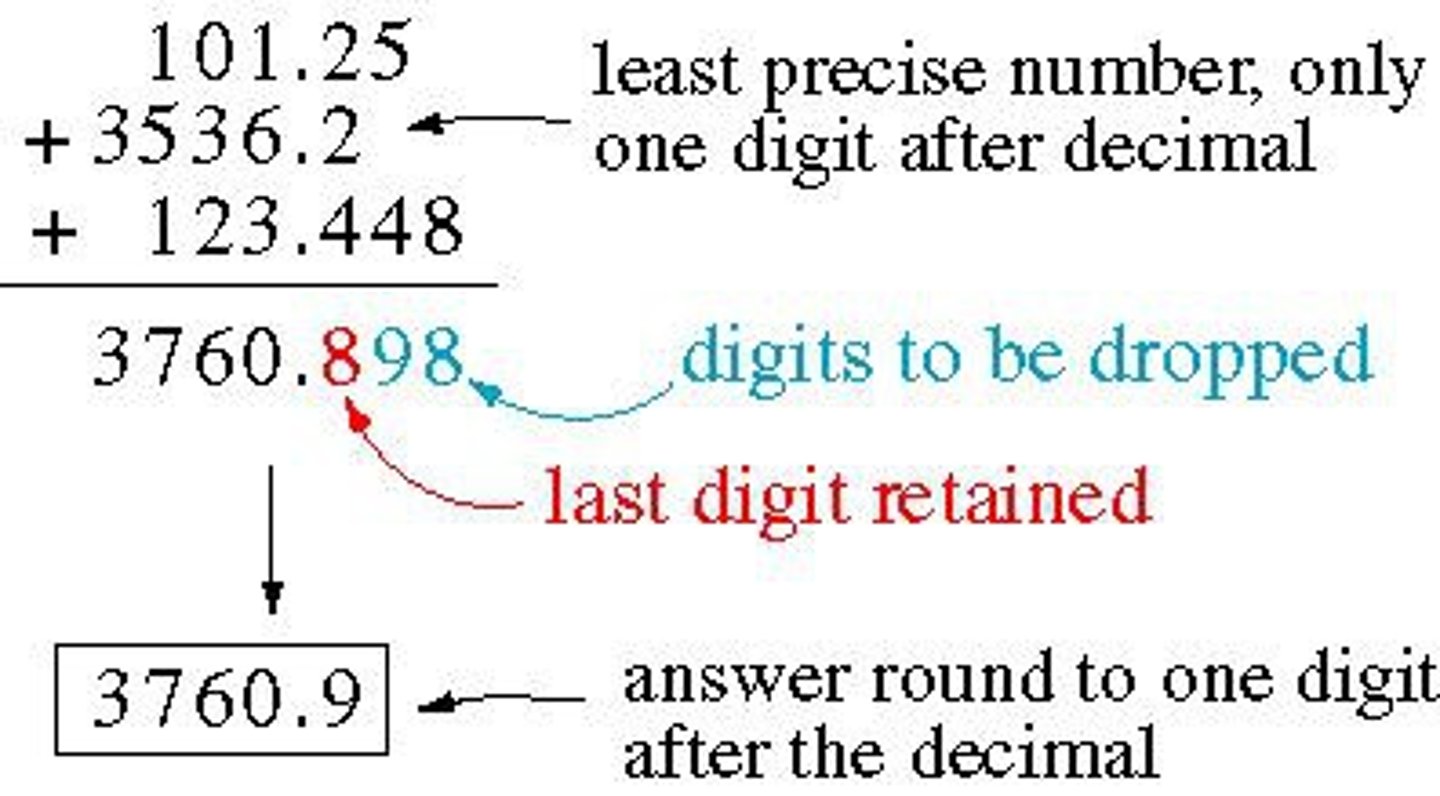
significant figures in addition and subtraction
least number of decimal places
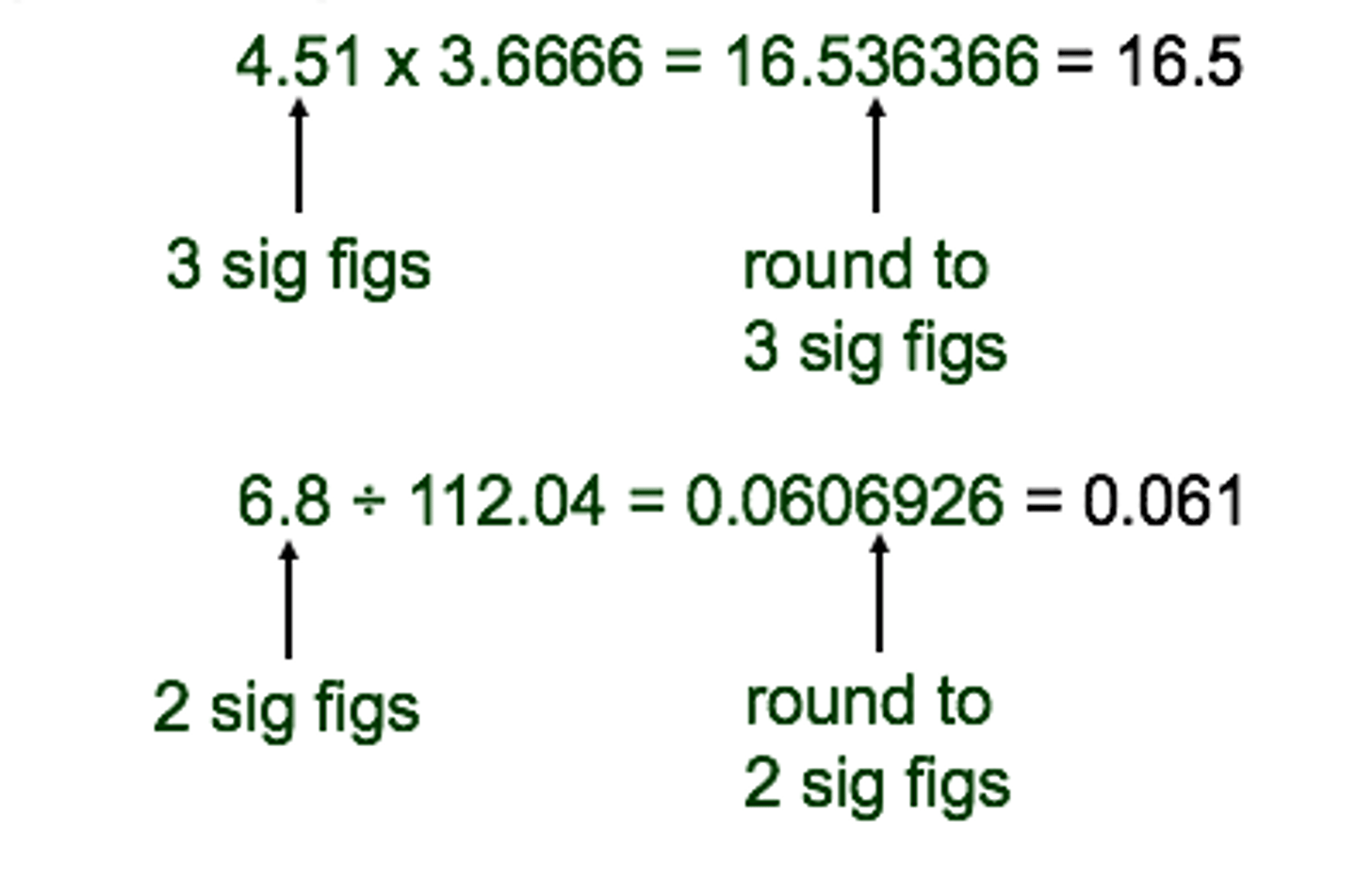
significant figures multiplication and division
least number of significant figures
percent error
the percent that a measured value differs from the accepted value
Avogadro's number
6.02 x 10^23
kinetic energy
energy of motion
-electromagnetic
-magnetic
-electrical
-thermal
electromagnetic energy
The energy of light and other forms of radiation.
mechanical energy
Kinetic or potential energy associated with the motion or position of an object
electrical energy
Energy caused by the movement of electrons.
thermal energy
Heat energy
potential energy
stored energy
-gravitational
-electrostatic
-chemical
-nuclear
gravitational potential energy
Potential energy that depends on the height of an object

electrostatic potential energy
arises from the interactions between charged particles
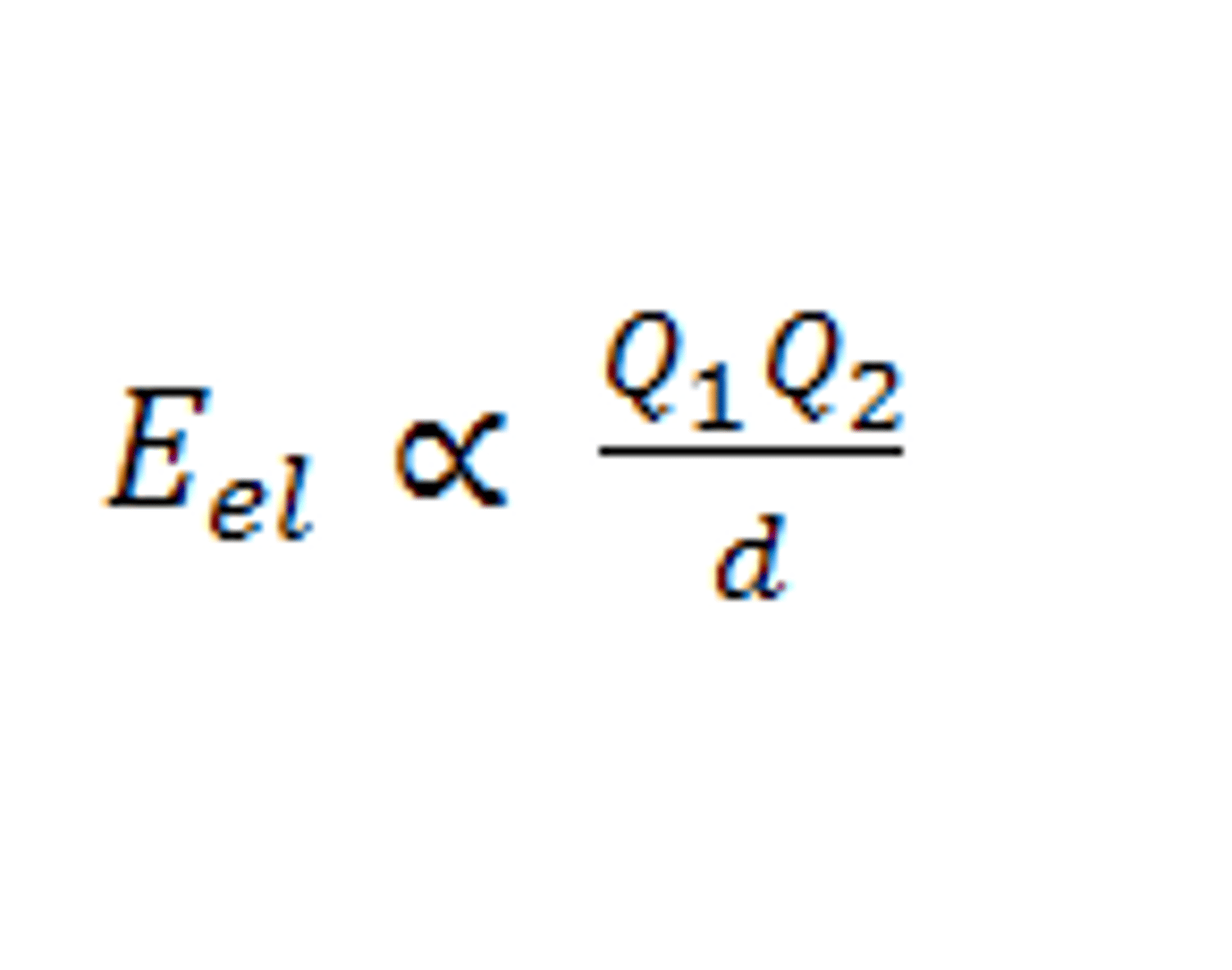
chemical potential energy
Energy stored in chemical bonds
nuclear potential energy
Energy stored in the nucleus of an atom
what happens when bonds are made
energy is released in form of heat
when bonds are destroyed
energy is absorbed
Accuracy
refers to how close a measured value is to an accepted value
Precision
a measure of how close a series of measurements are to one another
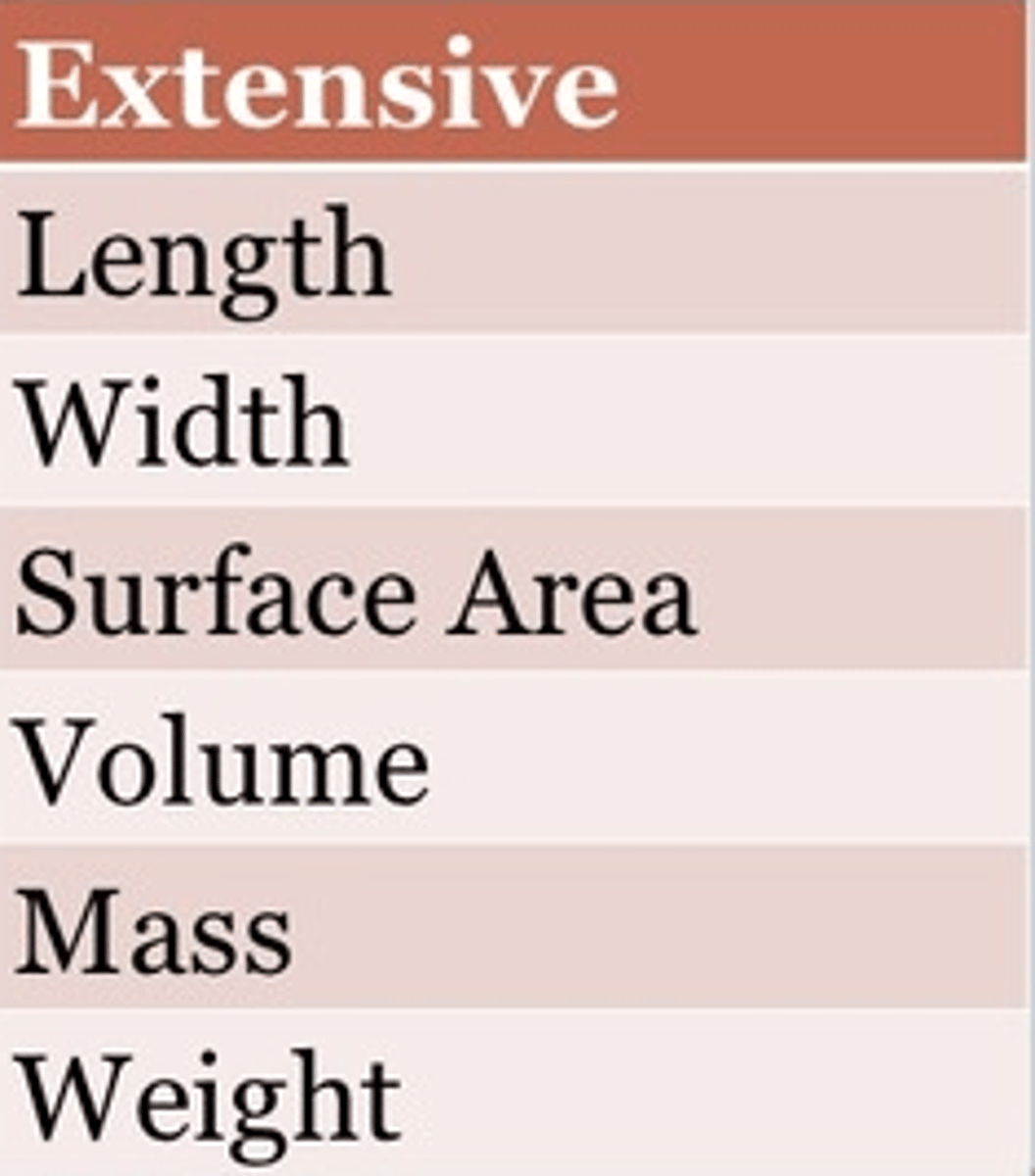
extensive property
depends on the amount of matter in a sample
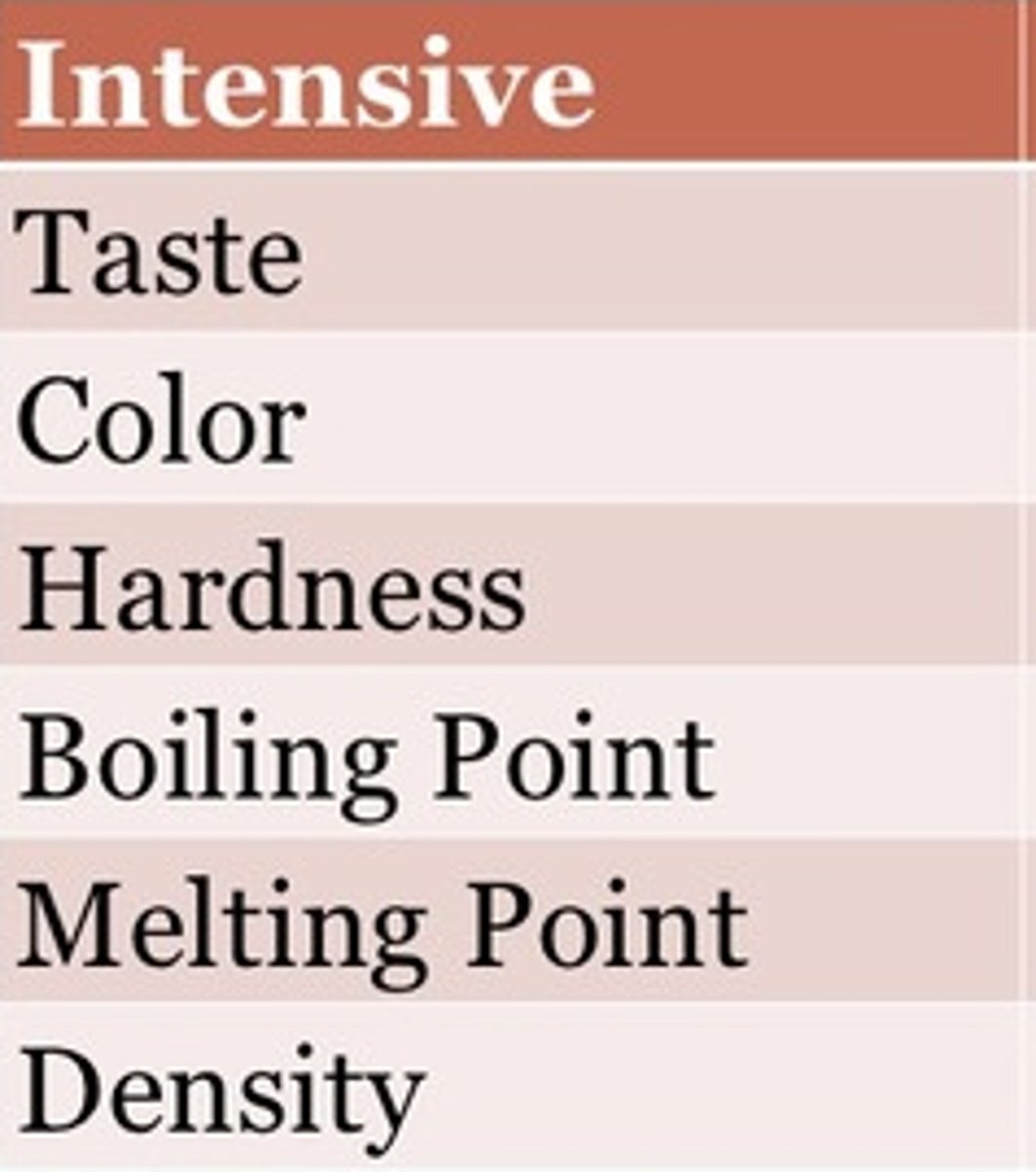
intensive property
a physical property that remains the same no matter how much of a substance is present
Cation
A positively charged ion
-lose electron
Anion
A negatively charged ion
-gain electron
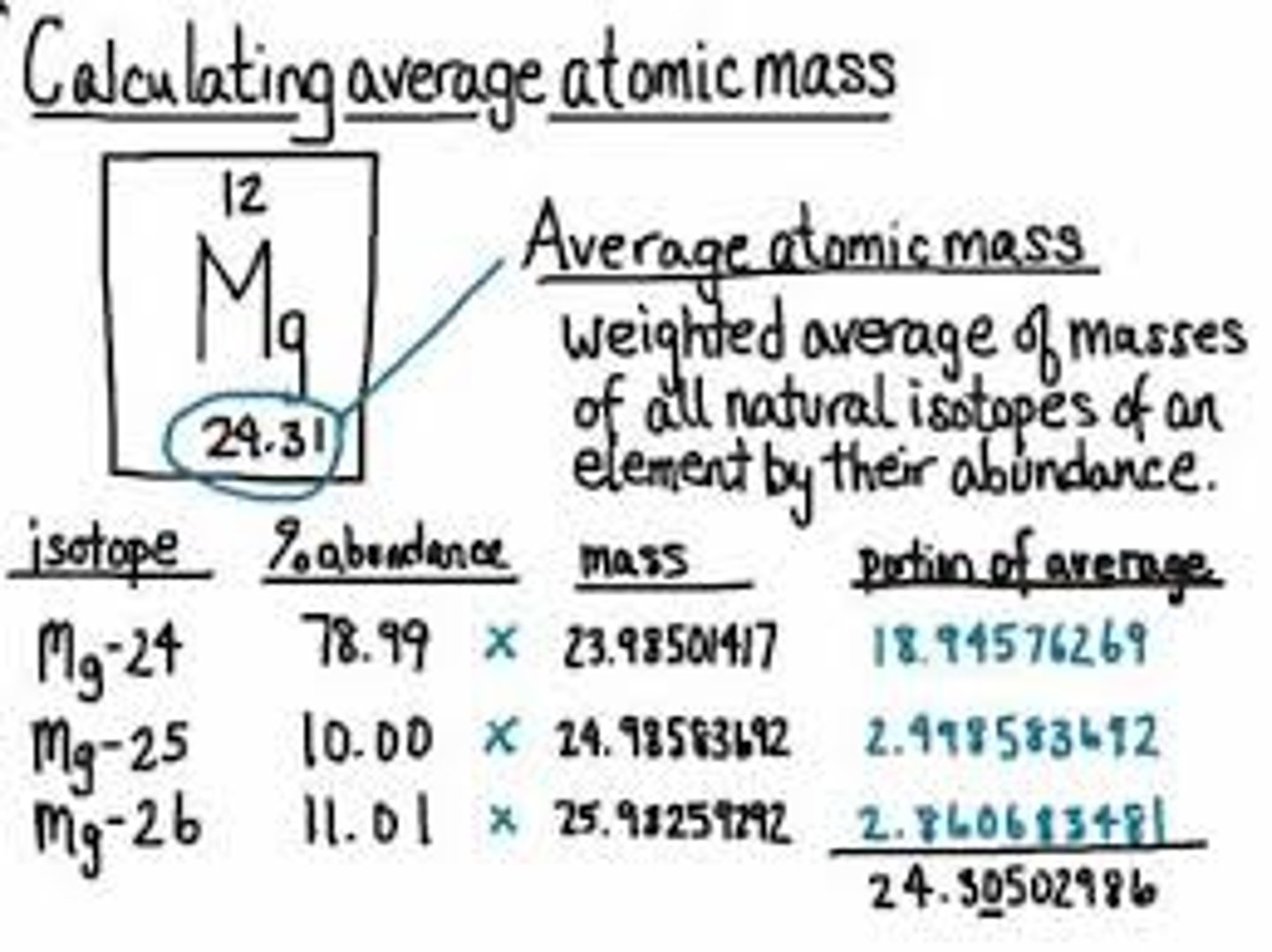
percent relative abundance
the percentage of a particular isotope that occurs in nature
natural abundance
the relative percentage of a particular isotope in a naturally occurring sample with respect to other isotopes of the same element
periodic table groups
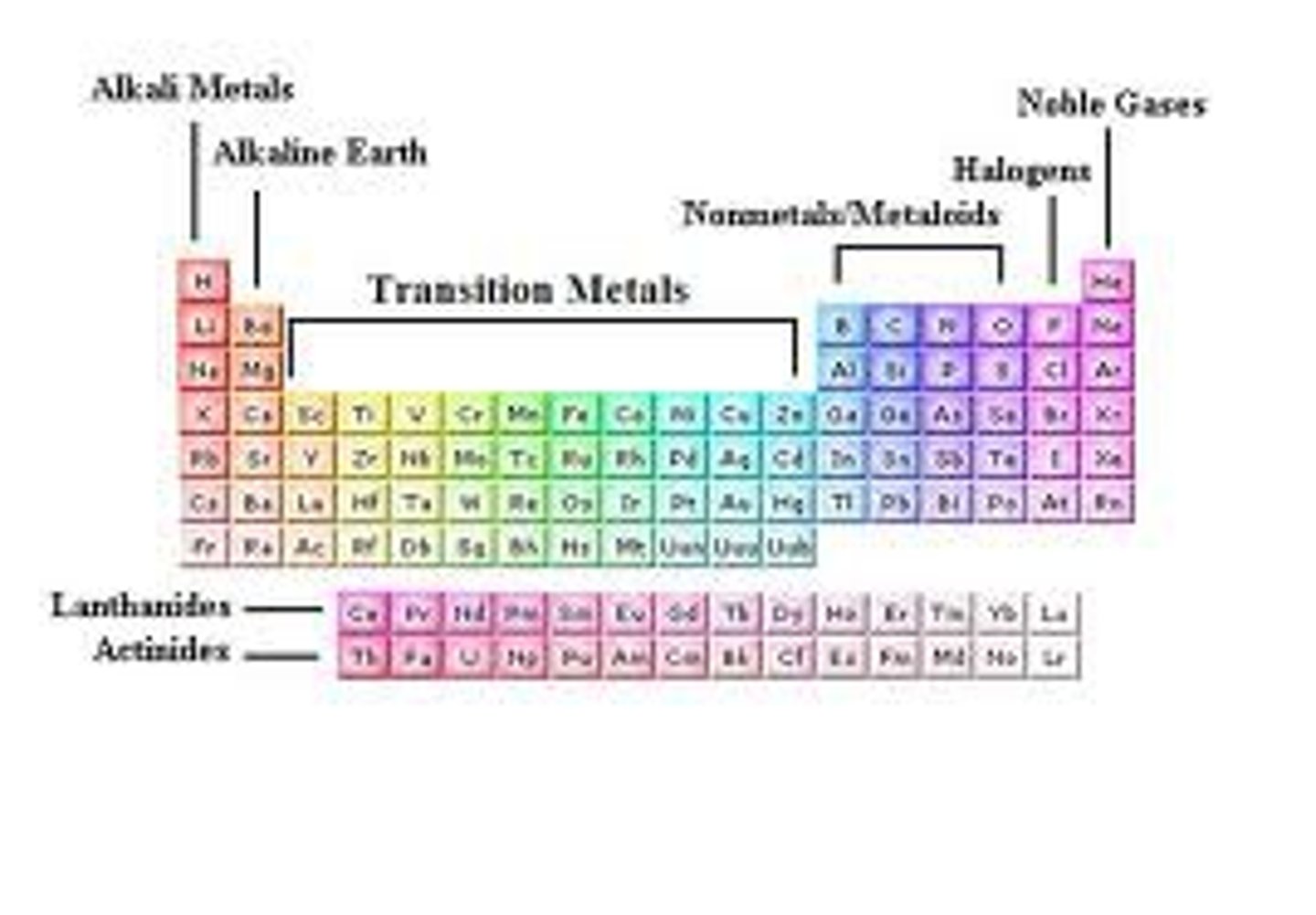
elements in same group
have the same number of valence electrons and similar chemical properties
elements in same period
have the same number of electron shells
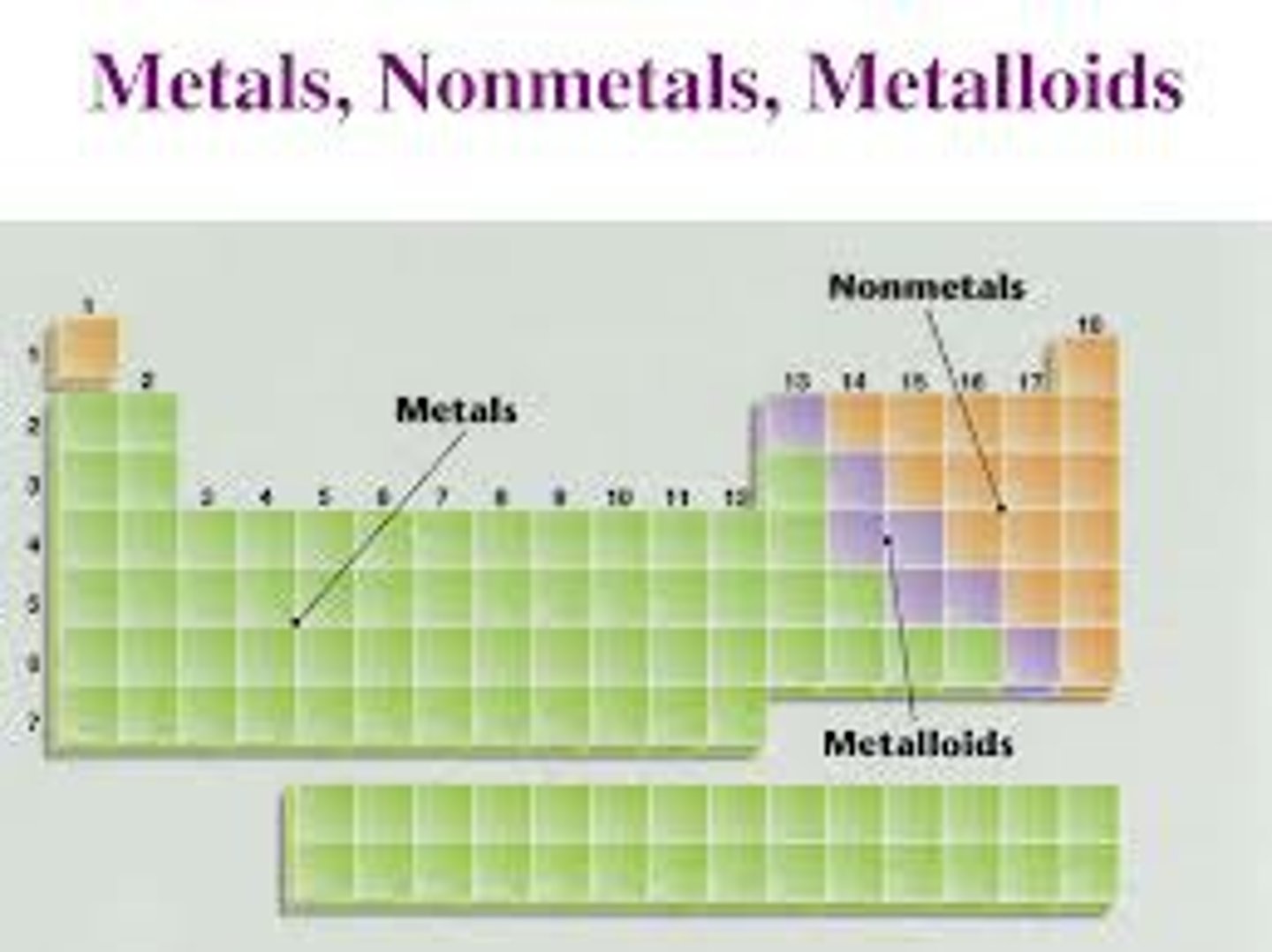
characteristics of metals
-Malleable: can be formed into thin sheets
-Ductile: can be pulled into thin wires
-Lustrous: have a shiny appearance
-Heat and electricity conductors
-Relatively large densities
-Relatively high melting and boiling points
-All are solids at room temperature, except mercury (Hg)
-Tend to form cations
-Very few colors: grey, silver, gold/copper
characteristics of nonmetals
-Brittle: deform easily, break upon impact, not malleable or ductile
-Dull appearance
-Insulators: poor conductors of heat and electricity
-Relatively lower densities, melting and boiling points than metals
-Various physical states at room temperature
-Tend to form anions
-Variety of colors
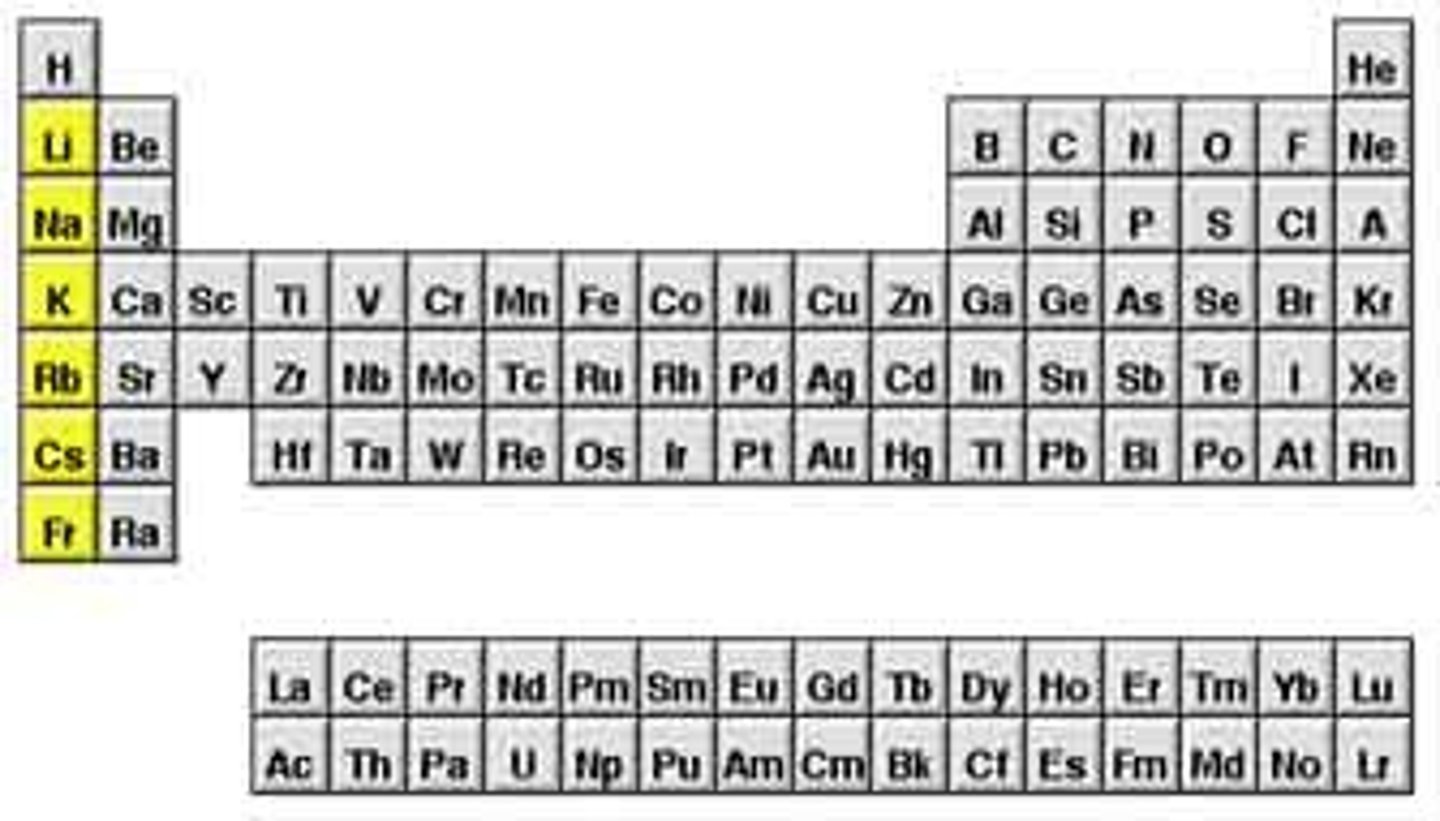
alkali metals
Group 1: 1 electron in outer level, very reactive, soft, silver, shiny, low density; Lithium, Sodium, Potassium, Rubidium, Cesium, Francium
-form +1 ions
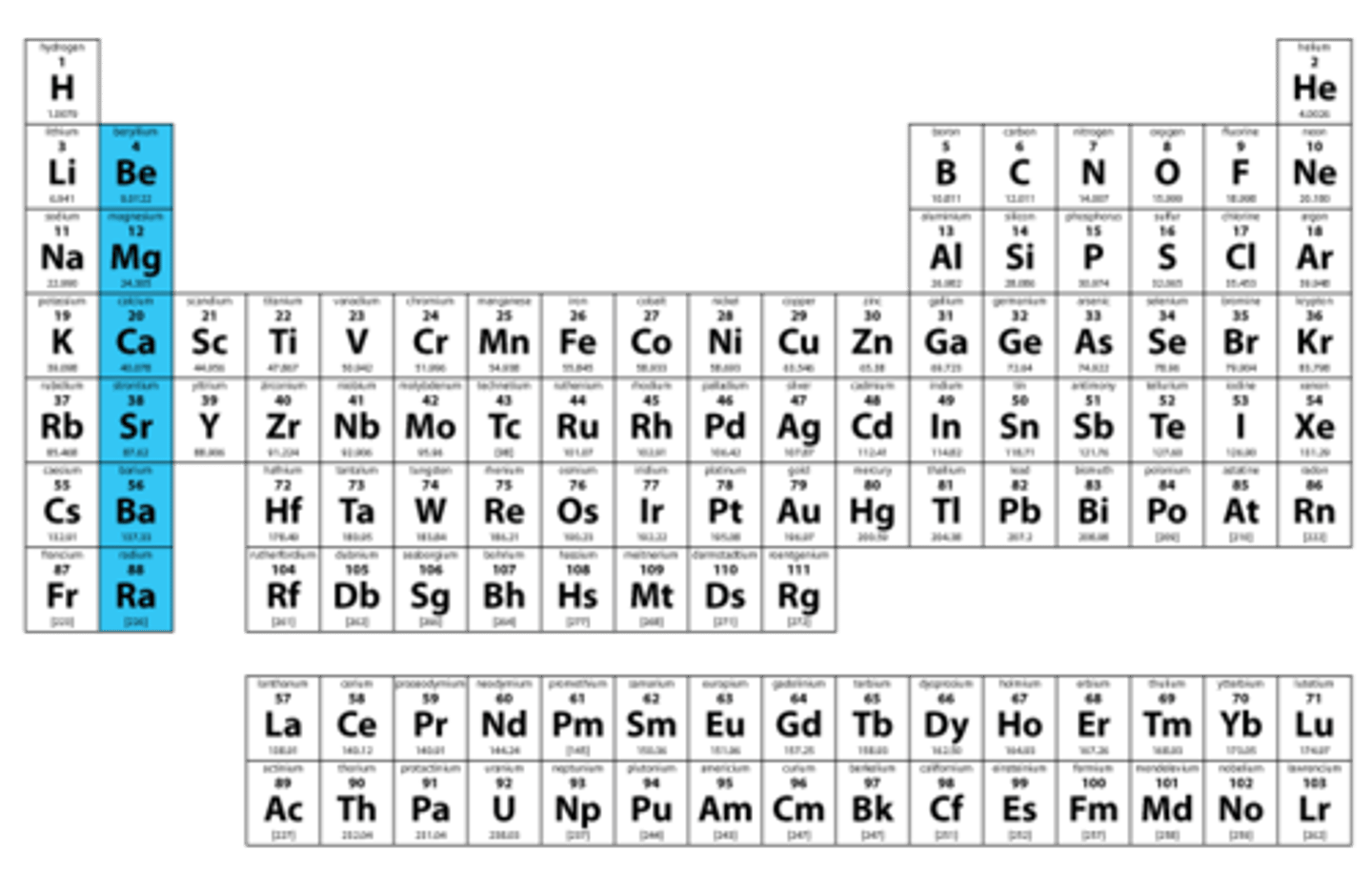
alkaline earth metals
Group 2 on PT. Two valence electrons, form +2 ions. reactive
Halogens
Contains nonmetals, 7 valence electrons in it's outermost energy level. Very reactive
noble gases
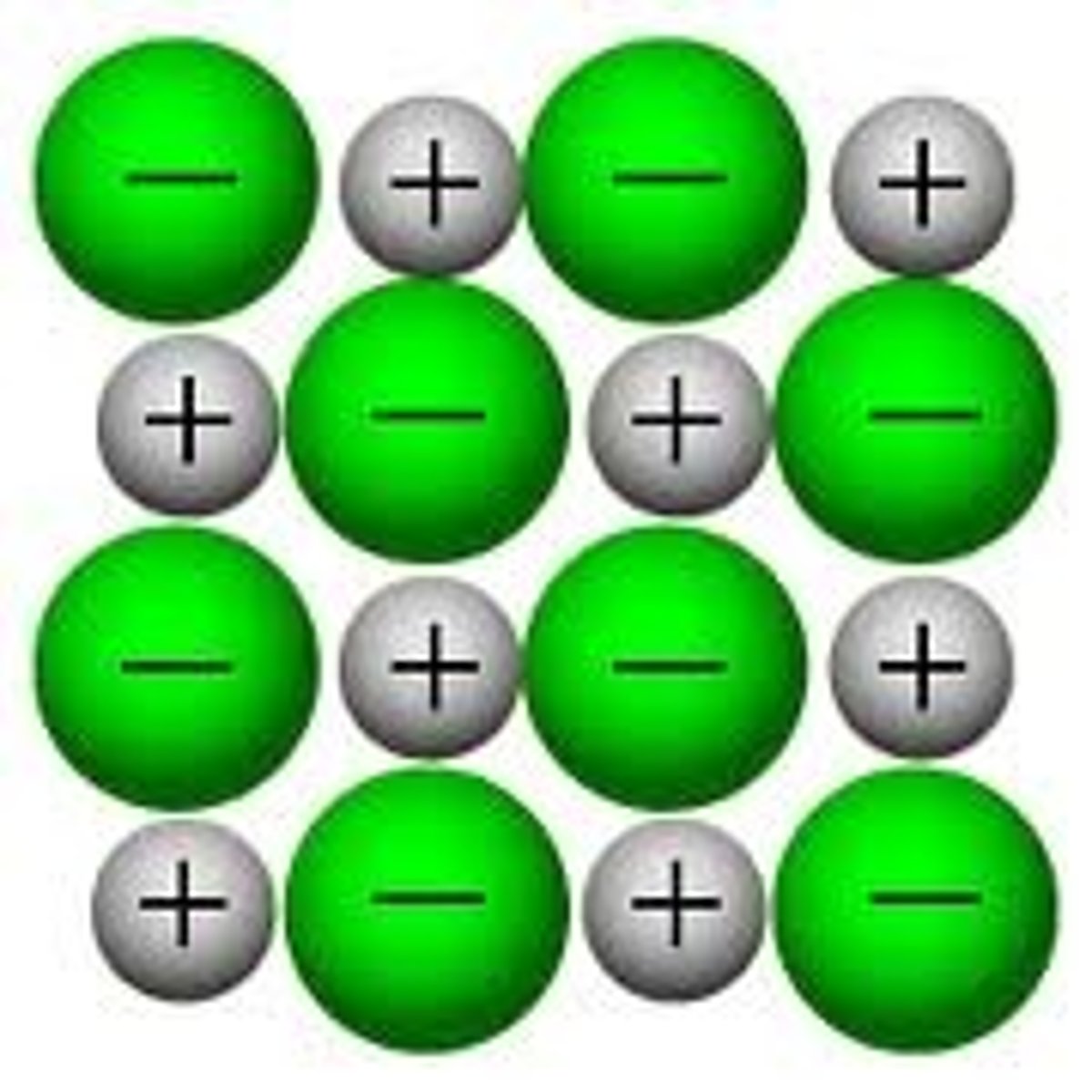
ionic compounds
compounds composed of cations and anions
-held together by electrostatic (or ionic) forces
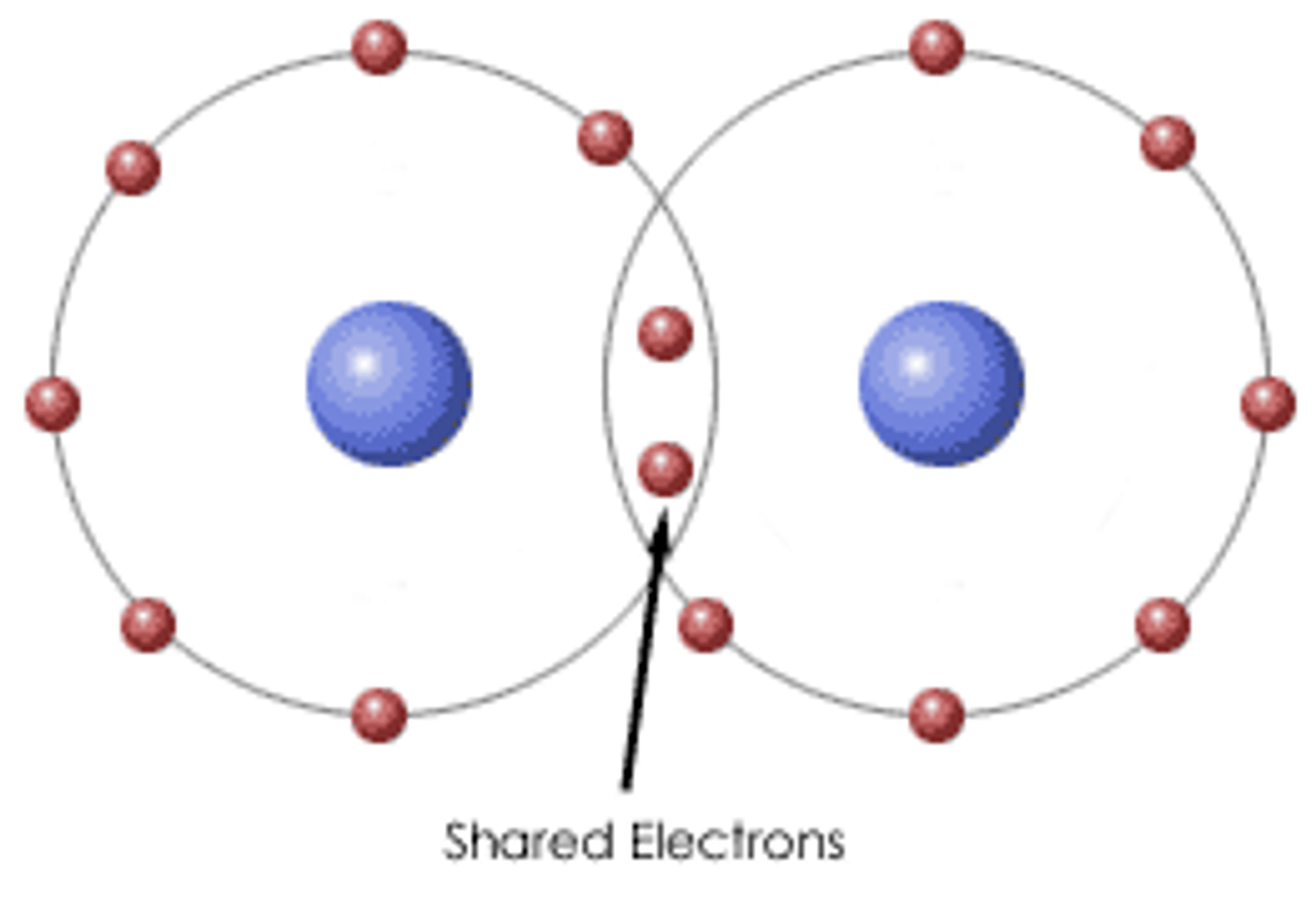
covalent compounds
typically made of two or more nonmetals and characterized by the atoms sharing their electrons in covalent bonds
Rutherfold's Gold Foil Experiment
positively charged alpha-particles were directed at gold foil, but very few were deflected, leading to the discovery of the nucleus and disproving the Plum Pudding Model
-discovered that atom mostly empty space-since some particles went through.
-some density in middle, since some particles did not get through
-and a + charge since some + alpha particles were deflected awat
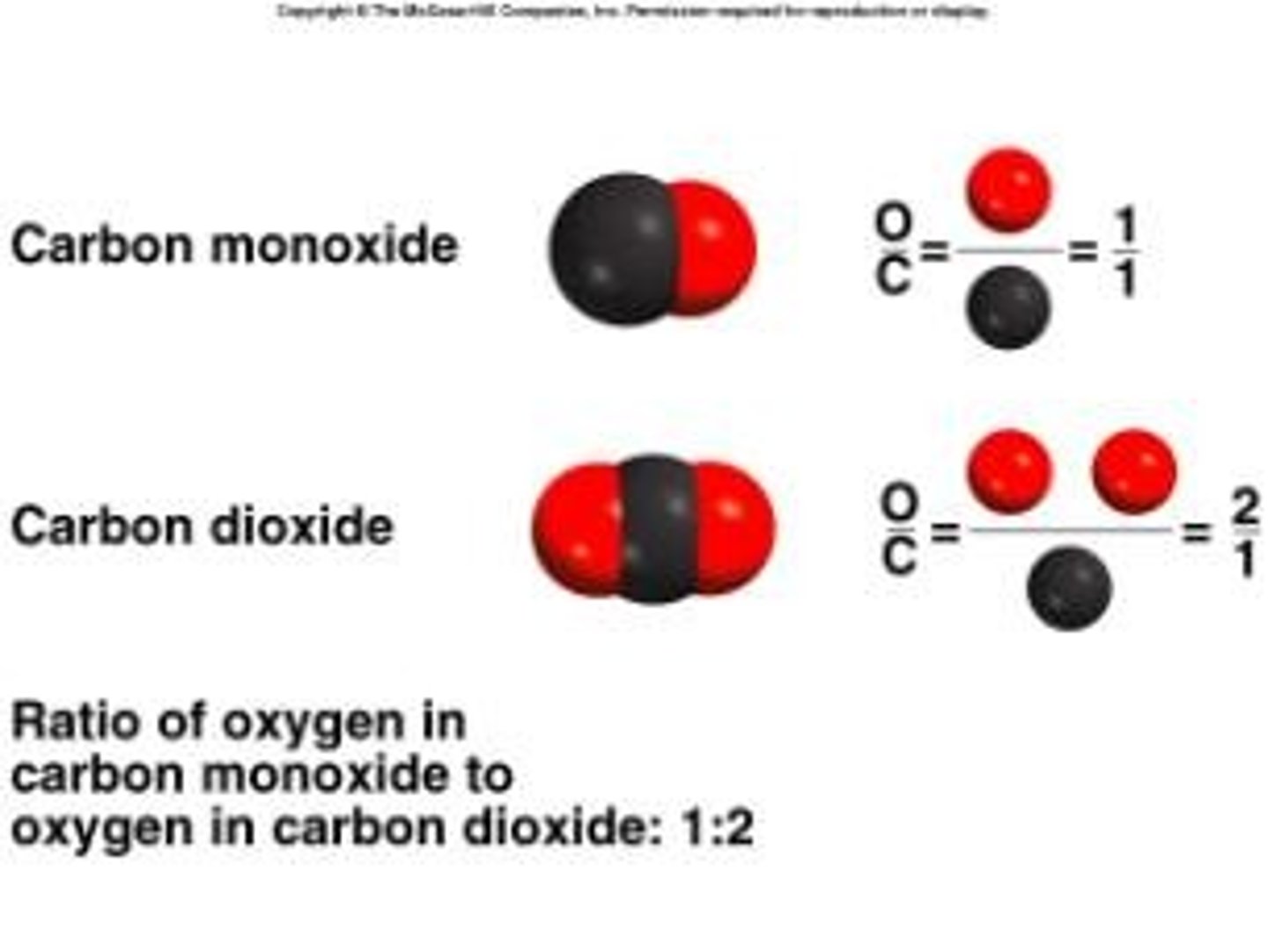
law of definite proportions
a given compound always contains exactly the same proportion of elements by mass

Law of Multiple Proportions
if two or more different compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers
Metals, nonmetals, metalloids on periodic table
Lanthanides
Actinides
types of bonds in molecular compounds
covalent
basic unit of molecular compounds
molecule
formula of molecular compounds
molecular formula
bonds in ionic compounds
ionic bonds in lattice structure
Basic unit of ionic compounds
The formula unit
formula of ionic compounds
empirical formula
binary acid
an acid composed of only two elements, one of which is hydrogen
oxacids
Acids that are formed by the combination of polyatomic ions with hydrogen ions
alkenes
Hydrocarbons with one or more carbon-carbon double bonds
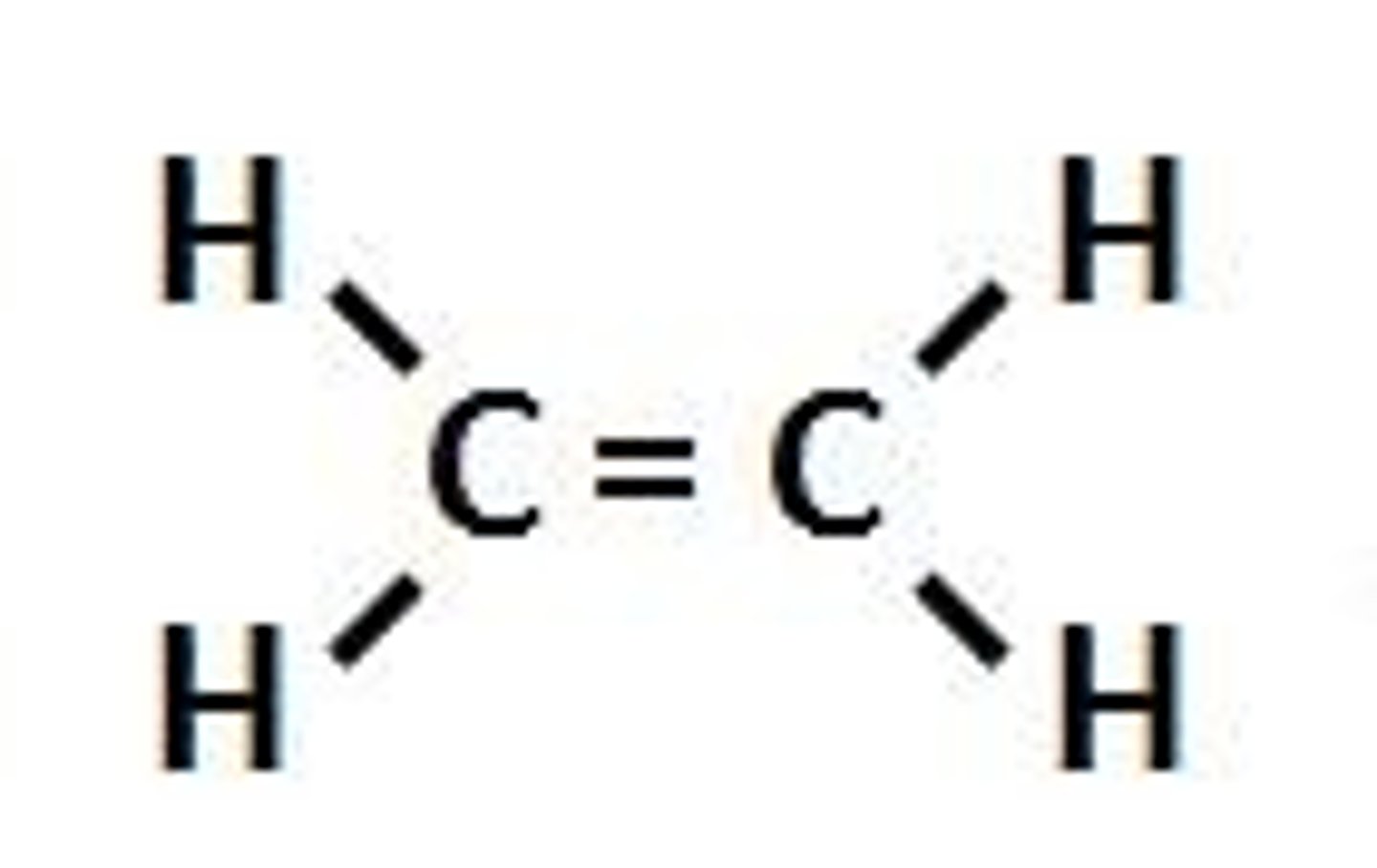
Alkynes
a carbon compound with a carbon-carbon triple bond.
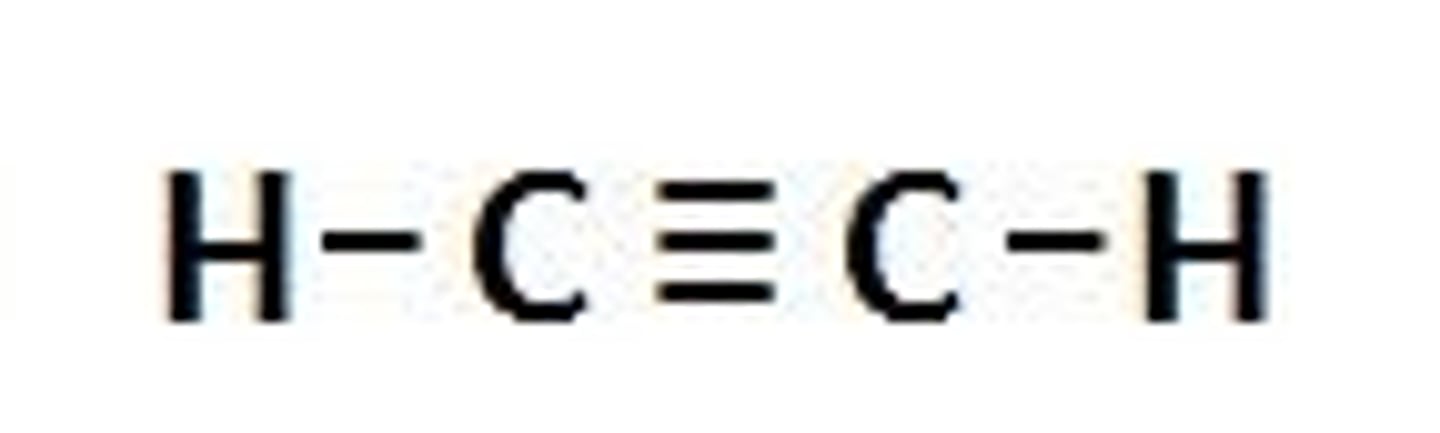
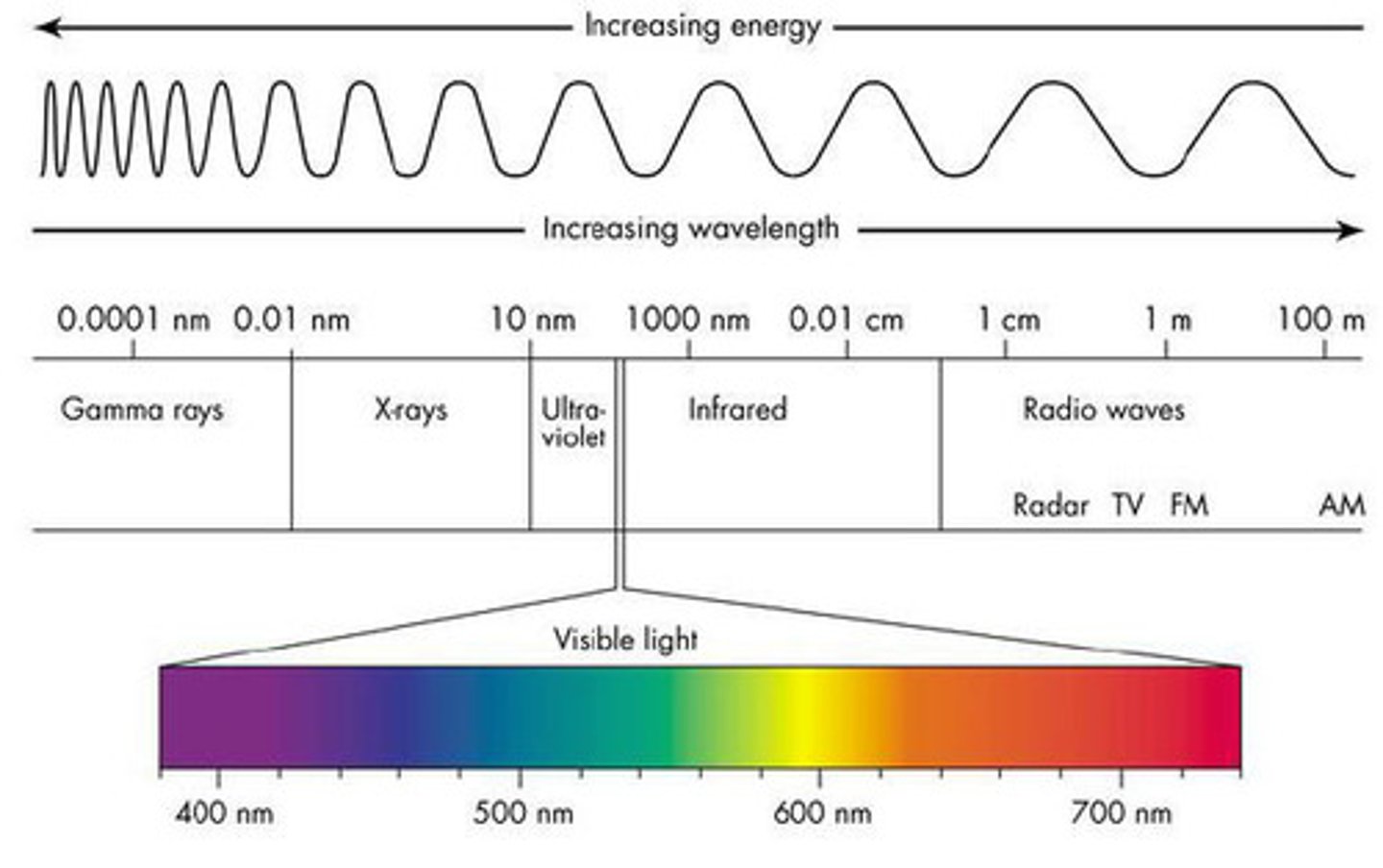
frequency vs. wavelength vs. energy
-long wavelength=low frequency and low energy
-short wavelength=high frequency and high energy
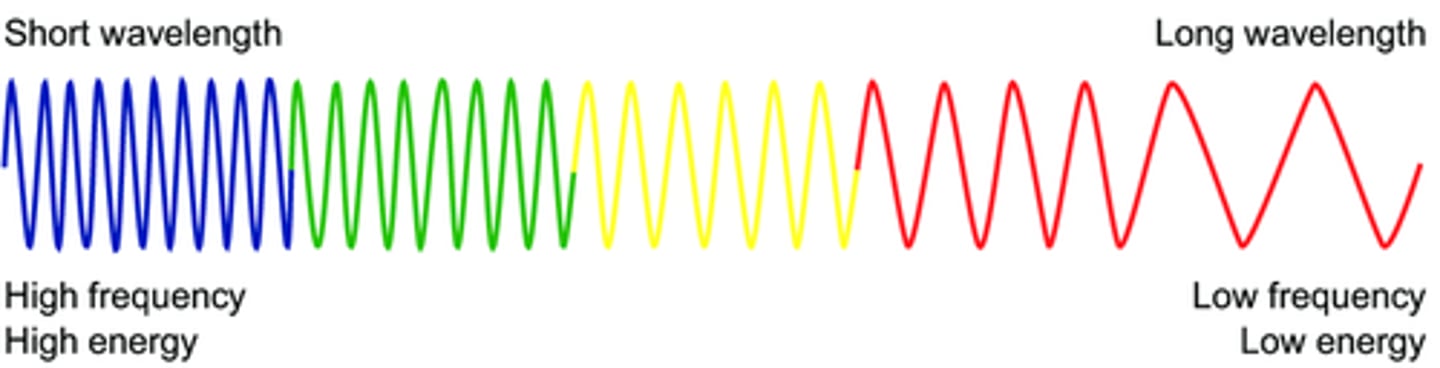
wavelength
The distance between two corresponding parts of a wave

frequency
the number of complete wavelengths that pass a point in a given time
speed of light equation
c=λv
the order of light on the electromagnetic spectrum
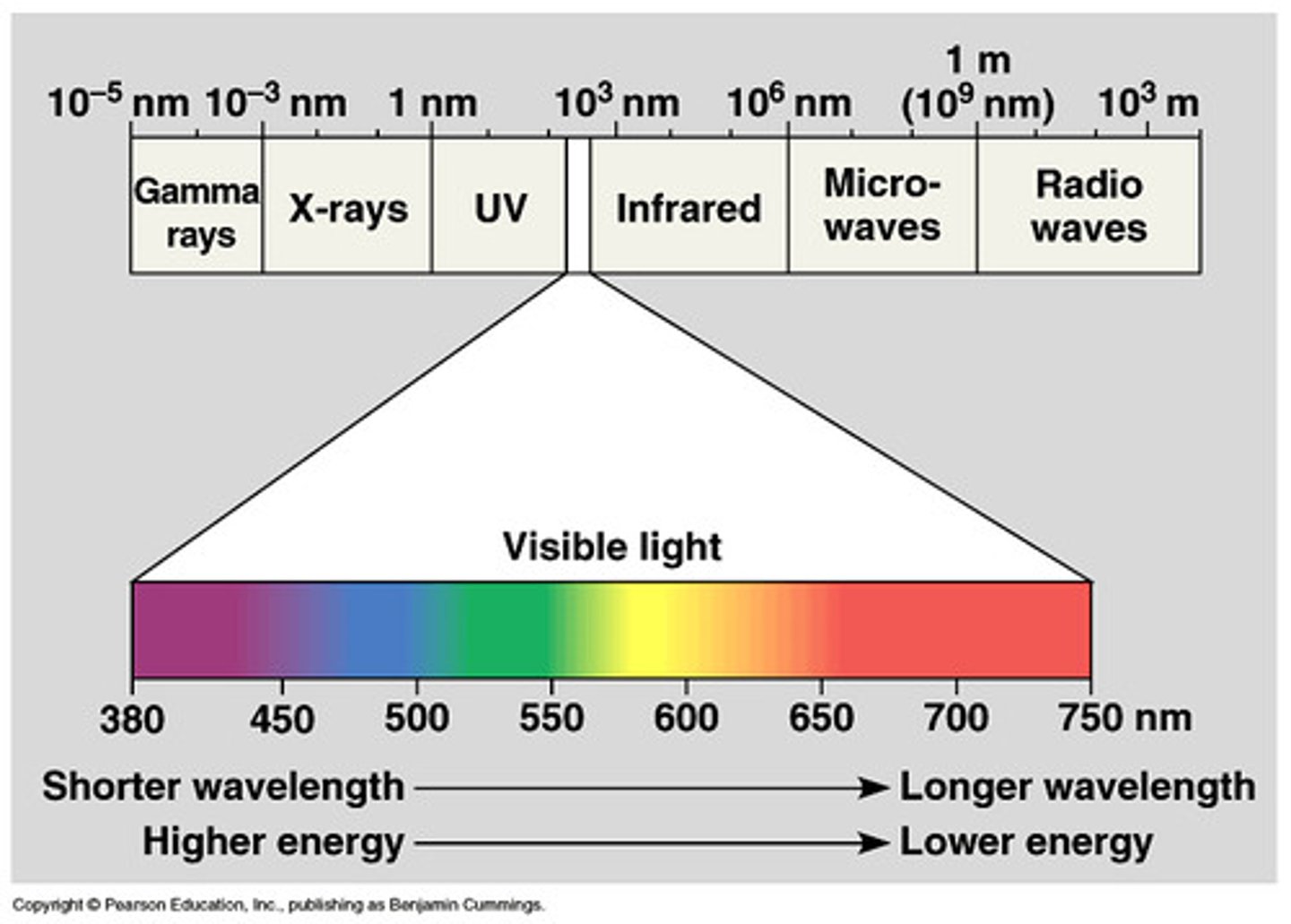
radio waves
Electromagnetic waves with the longest wavelengths and lowest frequencies
-(frequency range: 1 Hz-109 Hz; wavelength range: 1 m-109 m or 109 nm-1018 nm)
-ex: Audio signals, cell phone frequencies, and Wi-Fi communications, MRI
microwaves
the radiation that is primarily associated with heating up food items.
-(frequency range: 108 Hz-1011 Hz; wavelength range: 10-3 m-1 m or 106 nm-109 nm)
-ex: microwave
Infrared
IR light is the energy associated with any warm object, such as the heat that emanates from an electric stovetop or a light fixture
-(frequency range: 1011 Hz-1015 Hz; wavelength range: 10-6 m-10-3 m or 103 nm-106 nm)
-ex:anything that emits heat
Visible region of the electromagnetic spectrum
400-700 nm humans can detect with eyes
-(frequency: ~1015 Hz; wavelength range: 10-7 m-10-6 m or 100 nm-1000 nm)
-ex: ROYGBIV
UV light
invisible light that lies beyond violet. Has higher energy and shorter wavelengths than visible light does.
-(frequency range: 1015 Hz-1017 Hz; wavelength range: 10-8 m-10-7 m or 10 nm-10-2 nm)
-ex: sunlight, tanning booths
X-rays
Electromagnetic radiation having a very short wavelength; can penetrate substances such as skin and muscle.
-(frequency range: 1017 Hz-1019 Hz; wavelength range: 10-11 m-10-8 m or 10-2 nm-10 nm)
-ex: x-ray
gamma rays
Electromagnetic waves with the shortest wavelengths and highest frequencies
-(frequency range: 1019 Hz-1024 Hz; wavelength range: 10-15 m-10-11 m or 10-6 nm-10-2 nm)
-ex: can be used to induce nuclear reactions
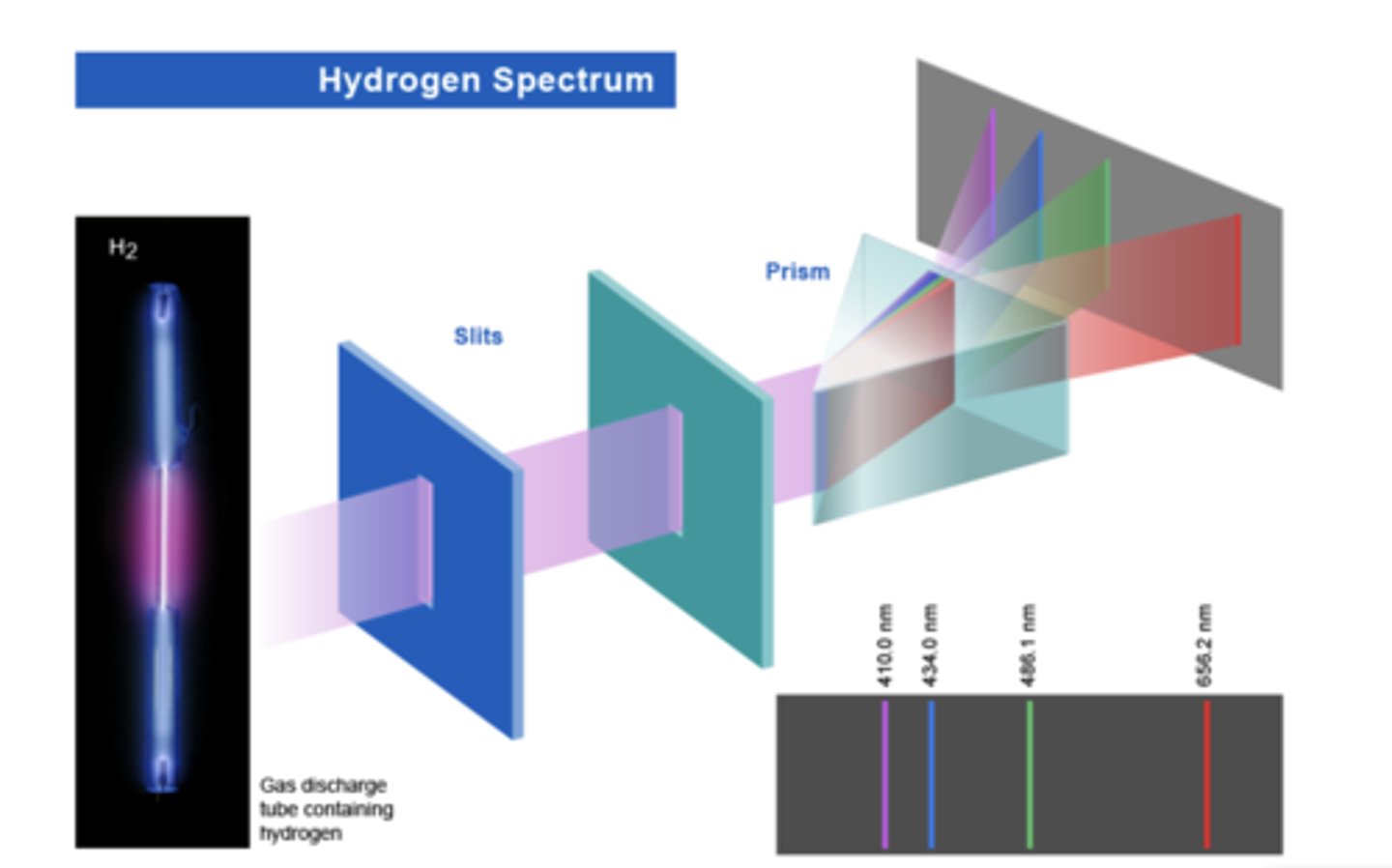
photoemission spectrum
When the vapor of an element absorbs incident energy it gives off a unique color, the emitted light is passed through a prism, which can then be used to identify the element, by the colors that are now visible
bohr model of an atom
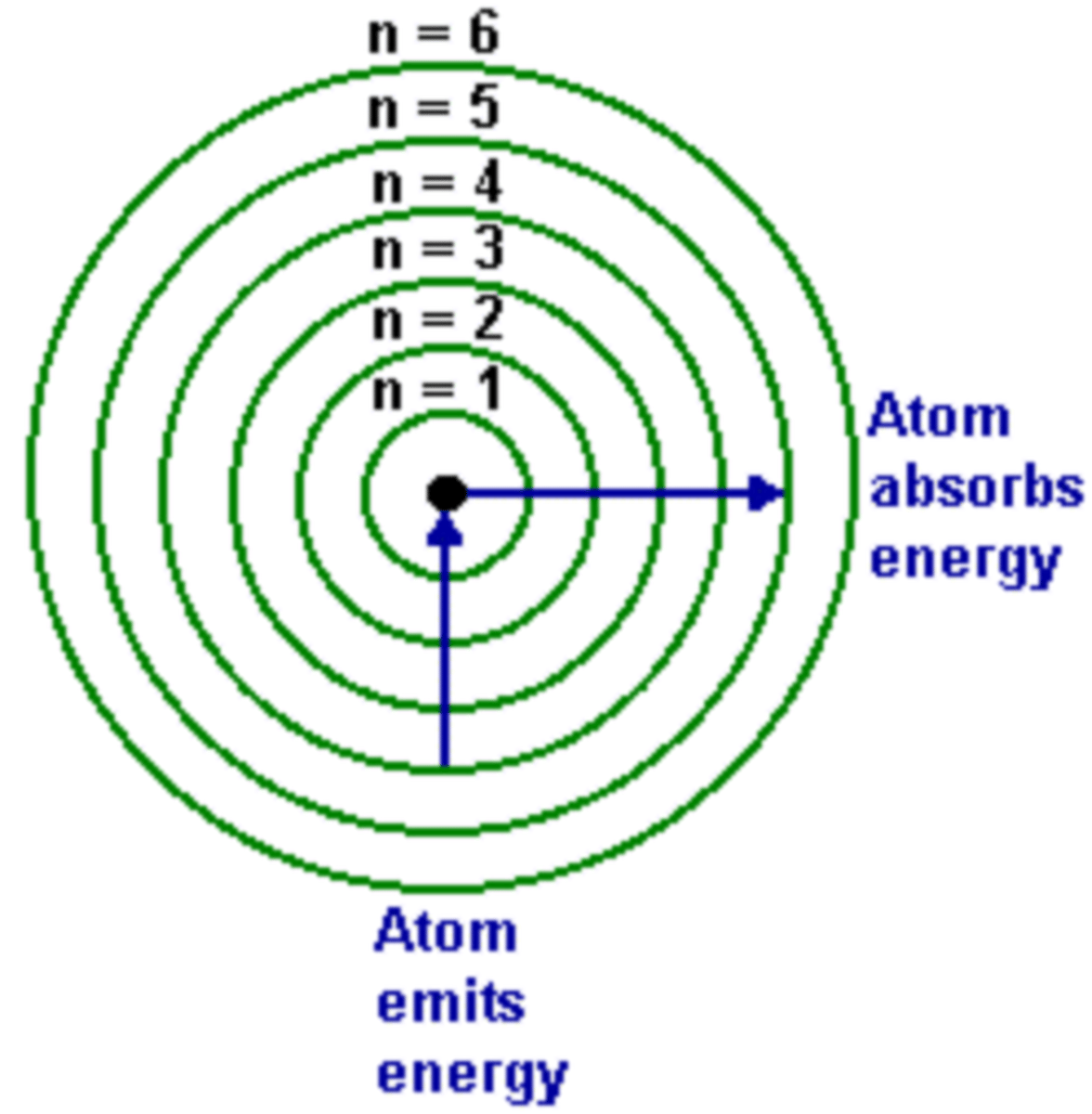
what is n in the bohr model
When n =1, Bohr's equation computes the lowest possible energy state for the hydrogen atom, called the ground state
what is the excited state in bohr model of an atom?
All states n > 1 are known as excited states
-As n increases, the excited state becomes higher and the further away the electron is from the nucleus.
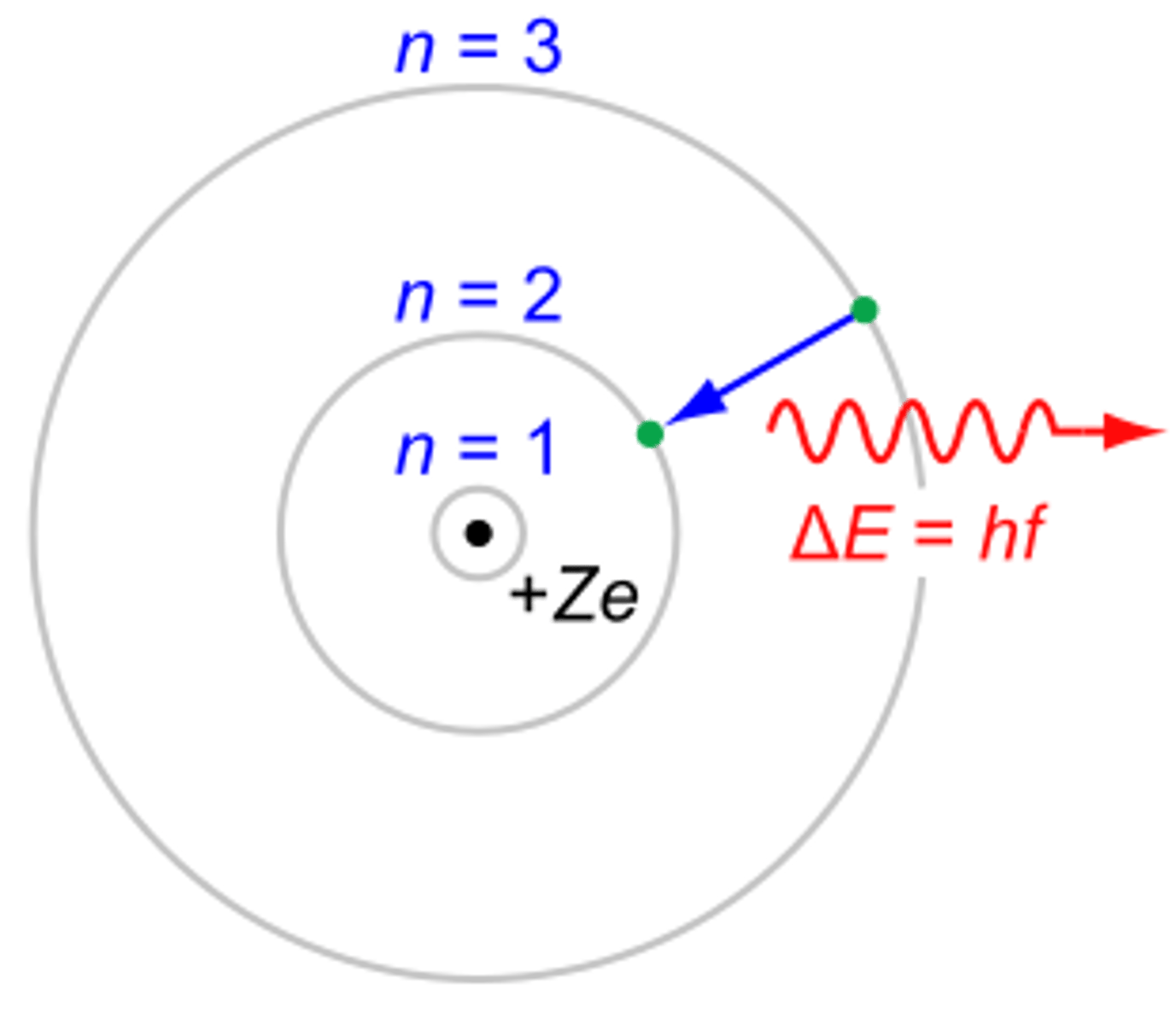
Bohr frequency condition
an equation derived from the Bohr model of the atom to explain the absorption and emission lines observed from atomic hydrogen.
How does wavelength correspond to length of arrow in Bohr model?
the shortest wavelength is the highest energy, or longest arrow
ultraviolet catastrophe
the failed prediction of classical physics that the energy radiated by a blackbody at extremely short wavelengths is extremely large and that the total energy radiated is infinite
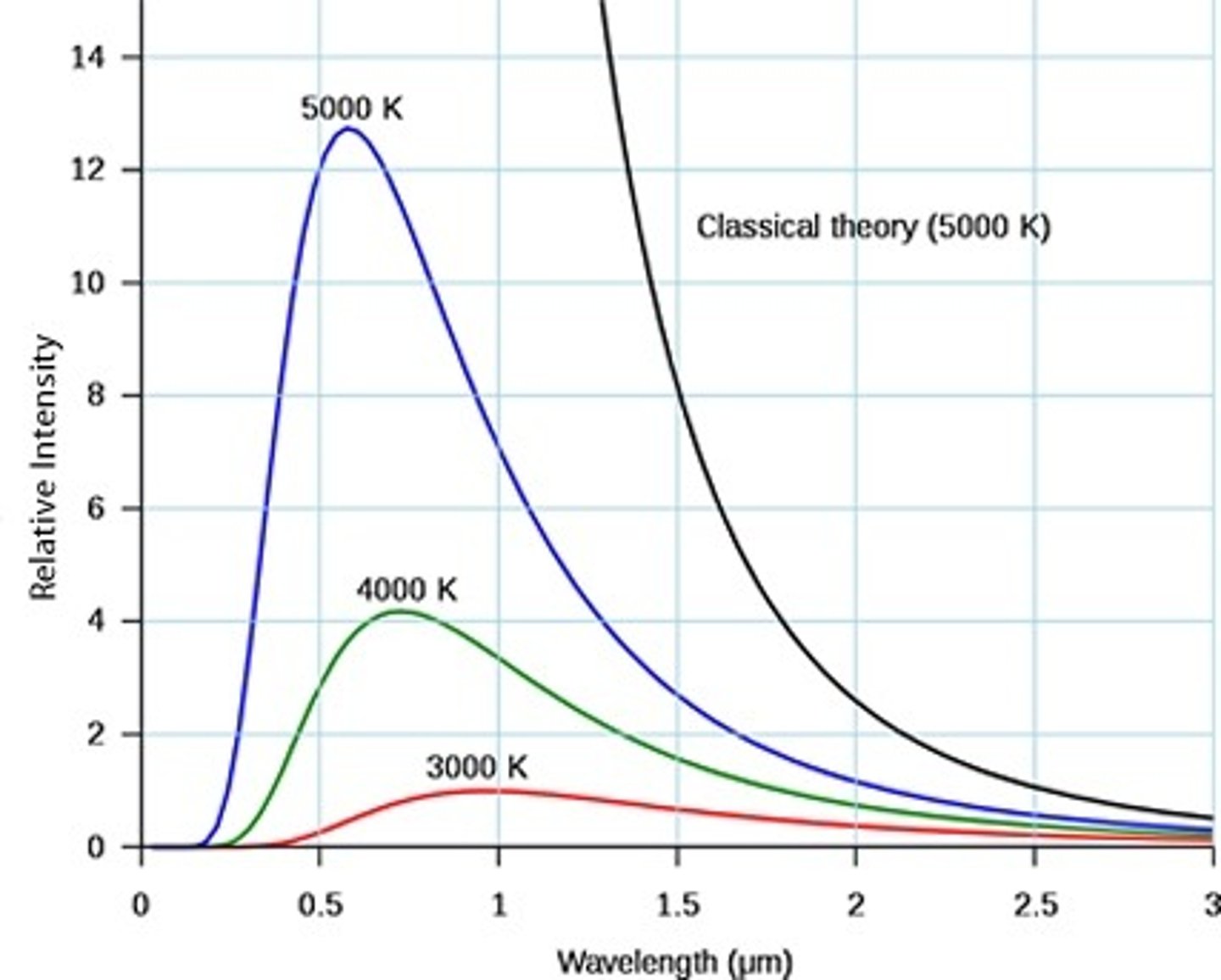
classic wave theory
(photoelectric effect) According to the classical wave theory of light, the intensity of the light determines the amplitude of the wave, increasing the intensity of light (amplitude of the wave) should result in electrons gaining more energy and being ejected from a metal surface with greater kinetic energy in the photoelectric effect
What is the photoelectric effect?
Electrons sitting on the surface of a metal are bound by a binding energy, and light needs to reach a threshold frequency to remove an electron from the metal.
What did the classical model propose about the photoelectric effect?
The classical model suggested that any light with enough intensity can remove an electron from a metal, which is not true in reality.
How does increasing intensity affect the photoelectric effect?
Increasing intensity beyond the threshold frequency ejects more electrons from the metal.
How does increasing frequency affect the photoelectric effect?
Increasing frequency beyond the threshold frequency increases the kinetic energy of the ejected electrons.
Why does each metal have a different threshold frequency in the photoelectric effect?
Each metal has a different threshold frequency because the binding energy varies between metals.
intensity in terms of photoelectric effect
refers to the number of packets of energy
threshold frequency
minimum light frequency necessary to eject an electron from a given metal
3rd Paradox of Physics (Bohr Model)
-Classical understanding: if you shine a light on an element then the element will absorb all light
-In reality: elements only emit light at certain wavelengths and frequencies
Bohr Model of Electron
-Bohr says that without doing anything to an electron it will sit in stationary (n=1) state
>each state (n=1,2,3,4...) have specific energy
-electron can only transition to one state it absorbs EXACT amount of energy
>then when it transitions back it releases exact amount of energy
Energy in Bohr Model
-When electron moves from lower numbered shell to higher number shell energy is absorbed (+)
-When electron moves from higher numbered shell to lower numbered shell energy is released (-)
-As distance traveled by electron increases, energy needed also increases
Higher value of n in Bohr Model
The further the shell is from the nucleus and the higher the energy associated with the shell
-as shell number (n) increases the distance between them decreases
What is the Heisenberg uncertainty principle?
It states that it is impossible to know exactly both the velocity and the position of a particle at the same time.