Unit 8 - Covalent Bonding
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Covalent bonds
formed when a non-metal shares electrons with another non-metal; can be solid, liquid, or gas
Ex: CO2
Covalent compounds physical properties
Solid, liquid, or gas at room temp
Low melting points
A few are network solids - substance made up of an array of repeating covalently bonded atoms (like a diamond or quartz)
Non-electrolytes
When a covalent bond is put into water…
it dissolves; C6H12O6(s) —> C6H12O6(aq)
7 diatomic molecules
H₂, N₂, F₂, O₂, I₂, Cl₂, Br₂ (examples of non-polar covalent bonds)
Prefixes for naming
mono
di
tri
tetra
penta
hexa
hepta
octa
nona
deca
Common covalent compounds
CH4 - methane
H2O - water
NH3 - ammonia
Double bonds just count as…
"1 bonding area"
HONC Rule
explains the number of times each atom is connected to other atoms
Hydrogen and halogens form 1 bond
Oxygen and sulfur form 2 bonds
Nitrogen and Phosphorus form 3 bonds
Carbon and silicon form 4 bonds
Intermolecular forces
Dispersion - weakest, caused by temporary dipoles, both polar and nonpolar, found in gas at STP
Dipole–Dipole - medium strength, formed due to permanent dipoles, polar molecules only, found in liquid at STP
Hydrogen Bonding - strongest attraction and therefore highest boiling point, hydrogen bonded to F, O, or N, found in solid at STP
Electronegativity difference
The degree to which an atom attracts electrons in a chemical bond is described by electronegativity
Non-polar covalent (0.0-0.4)
Polar covalent (0.5-1.8)
Ionic (>1.8)
VSEPR
Valence Shell Electron Pair Repulsion, electrons repel each other, predicts shape of molecule
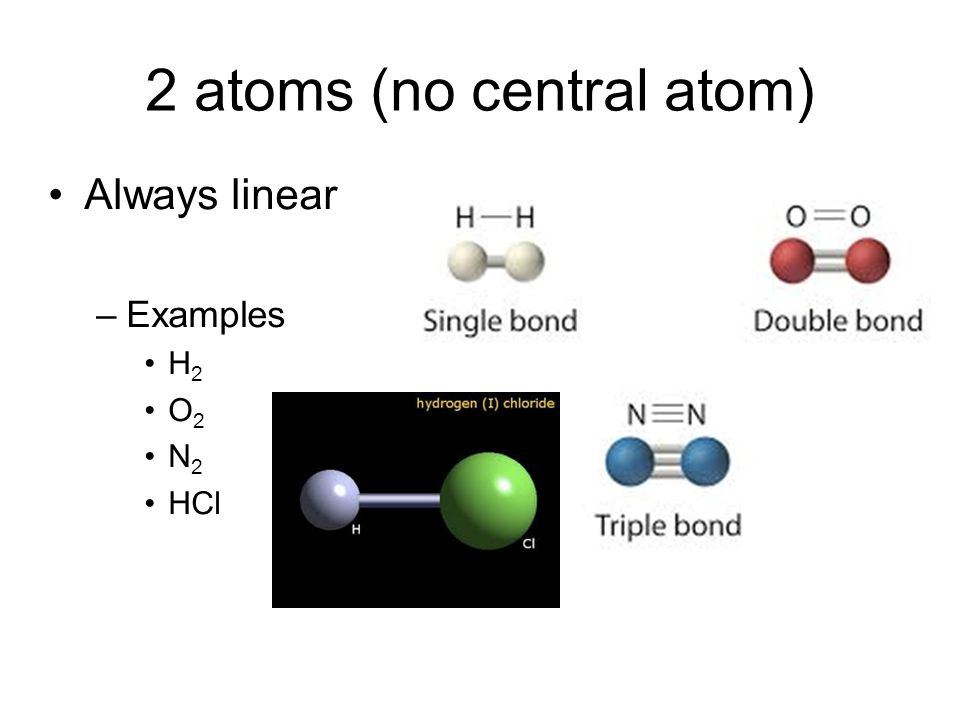
Linear (no central atom)
diatomic
No angle bc no central atom
non-polar if atoms are same (O2, N2, Br2)
polar if atoms are different (HCl)
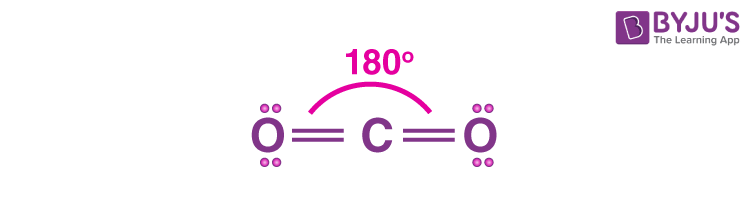
Linear
2 shared, 0 unshared
Double and triple bonds count as 1 shared pair
180˚
Non-polar if bonded atoms are the same (CO2)
Polar if bonded atoms are different (HCN)
Trigonal planar
3 shared, 0 unshared
120˚
Non-polar if bonded atoms are the same (BF3)
Polar if bonded atoms are different (HCOH)
Tetrahedral
4 shared, 0 unshared
Bc molecule is 3D, angle is 109˚
Non-polar if bonded atoms are the same (CH4)
Polar if bonded atoms are different (CH3F)
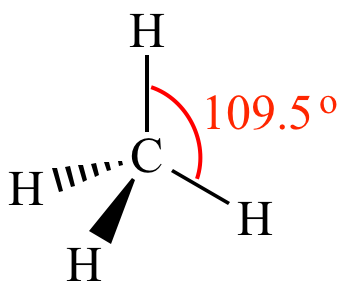
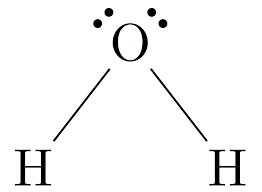
Bent
2 shared, 2 unshared
105˚
Not symmetrical so it is always polar (H2O or H2S)
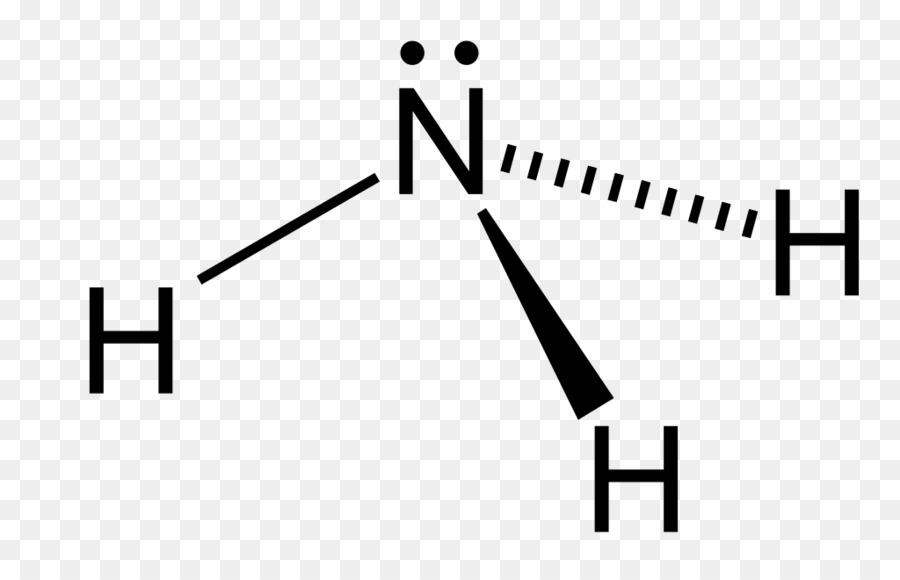
Pyramidal
3 shared, 1 unshared
107˚
Not symmetrical so it is always polar (NH3 or PH3)