Gibbs Free Energy
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
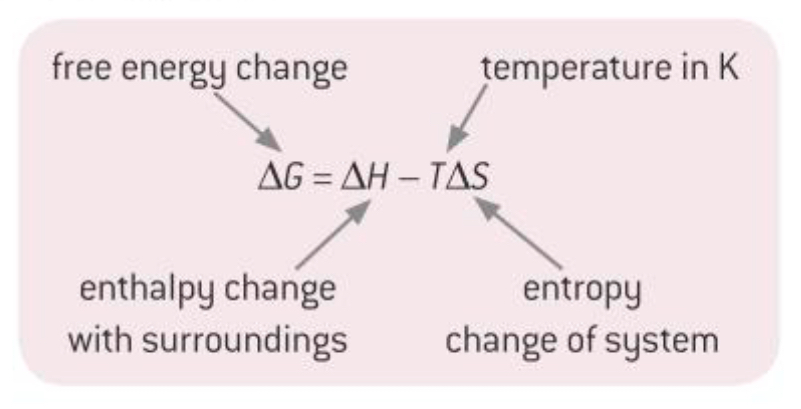
What is the free energy change ΔG?
The overall change in energy during a chemical reaction
Free energy change is made up of what 2 types of energy?
The enthalpy change
The entropy change at the temperature of the reaction
What is the Gibbs equation?
What is Gibbs free energy?
The free energy measured at constant pressure
What is feasible?
Feasible means a reaction CAN take place (although it might not)
What does it mean if ΔG < 0?
Reaction is feasible
What does it mean if ΔG > 0?
Reaction is not feasible
What are the limitations of predictions made for feasibility?
It can only be used to predict the feasibility of a reaction under standard conditions
Just because a reaction is feasible, doesn’t mean it will occur at an observable rate
It doesn’t take into account the kinetics of the reactions (the rate of the reaction)
There may be a large energy barrier (activation energy) which may need to overcome
The gibbs equation can be rearranged to give what?
Y = mx + c
Where -ΔS is the gradient
ΔH is the y intercept
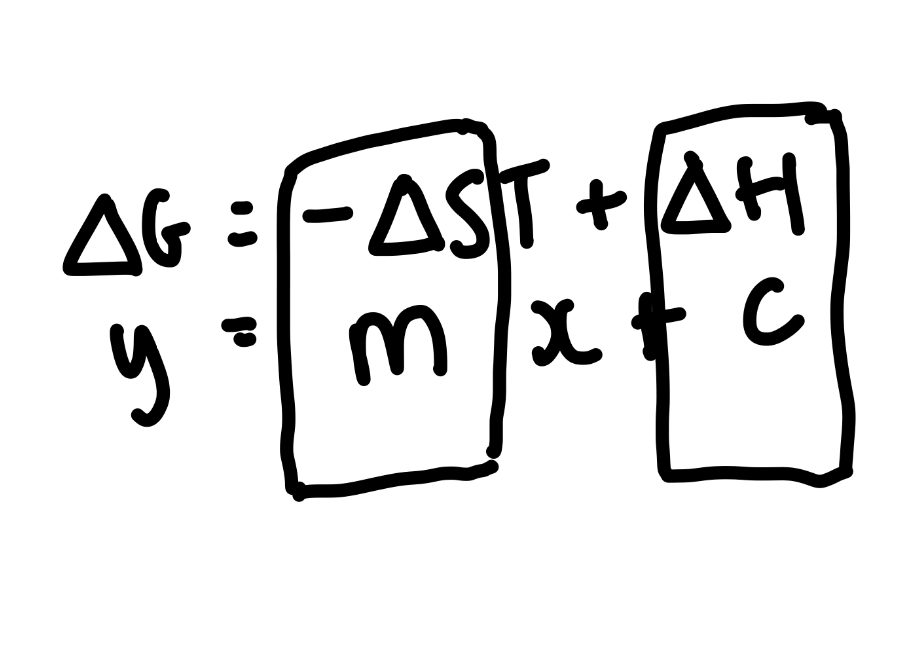
What does the coordinate where the line crosses the X axis mean?
Temperature at which feasibility changes
How do you calculate the minimum temperature at which a reaction is feasible?
ΔH / ΔS
What are the units of the entropy when doing calculations?
kJ / K
convert to kilojoules!!