Lesson 3: Bonding
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
List the three main types of chemical bond.
Metallic, Ionic and Covalent
What happens when atoms combine ?
they form a chemical bond
The atoms react to do what?
achieve a stable electron arrangement
What happens in the formation of a metallic bond?
The positively charged nuclei of the atoms attract the electrons.
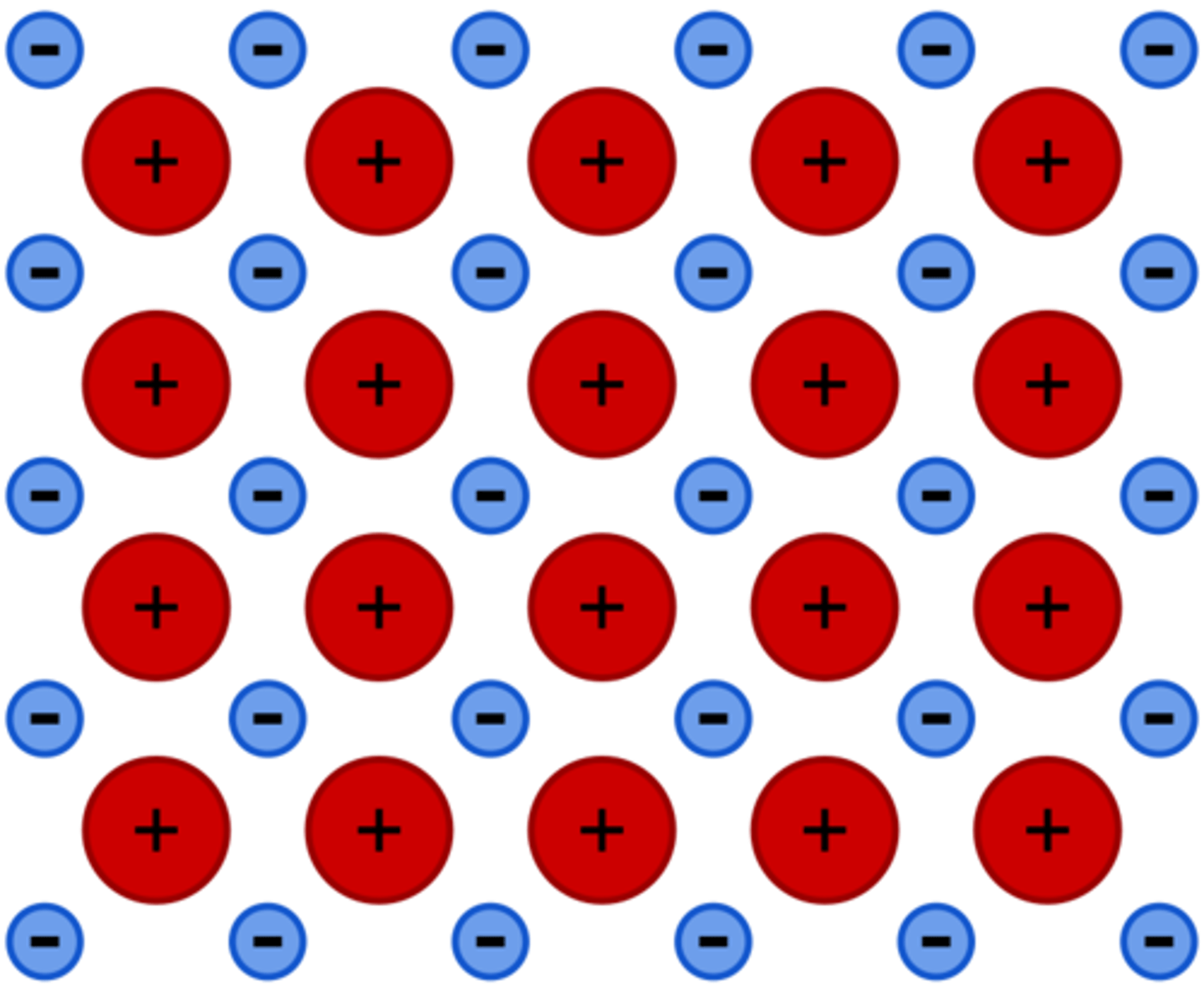
What are the properties of a metallic bonded substance (metal)
- High mp/bp
- Giant metallic lattice structure
-Conducts electricity
-Insoluble in most solvents
Define a covalent bond.
the attraction of the two positive nuclei for the shared pair of negative electrons.

Define the term "molecule"
A relatively small group of atoms held together by covalent bonds.
What is a molecule that only contains two atoms called?
Diatomic
How many diatomic elements are out there?
7
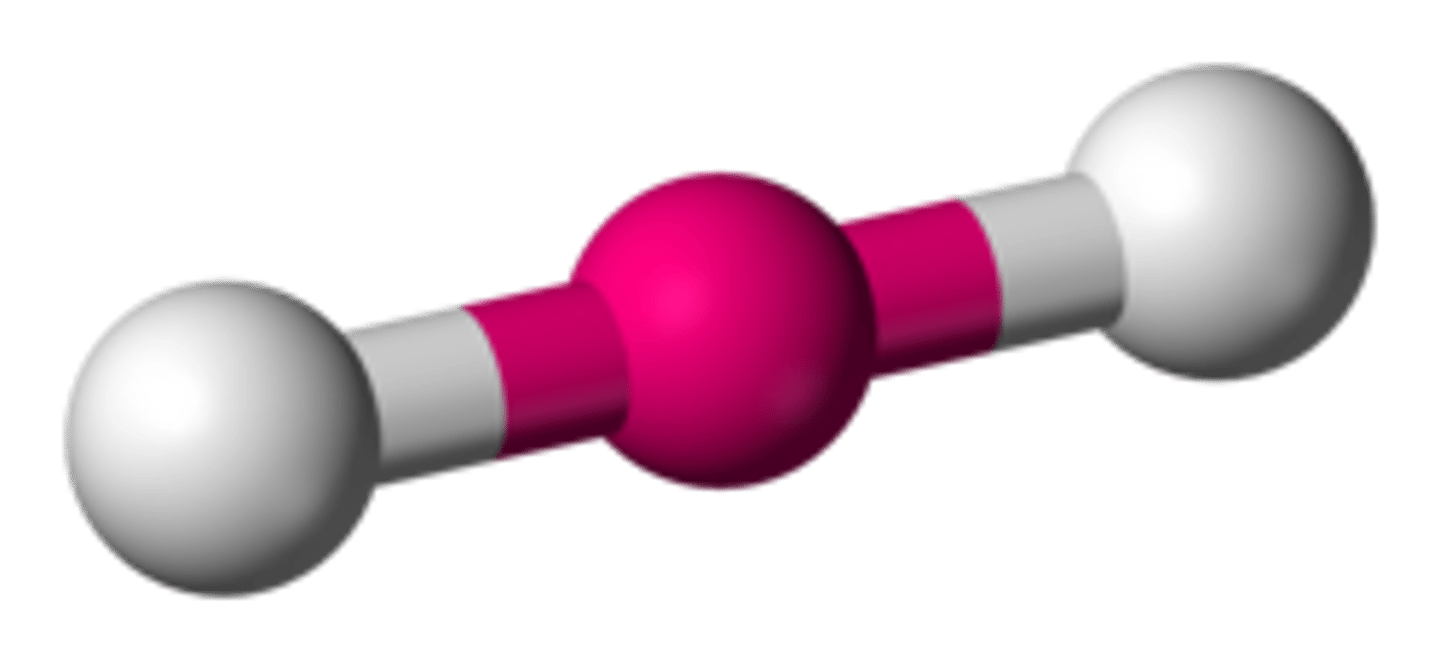
What is the shape of a molecule with one bond/ two atoms called?
Linear
What is the shape of a molecule with two bonds/ three atoms called?
Angular
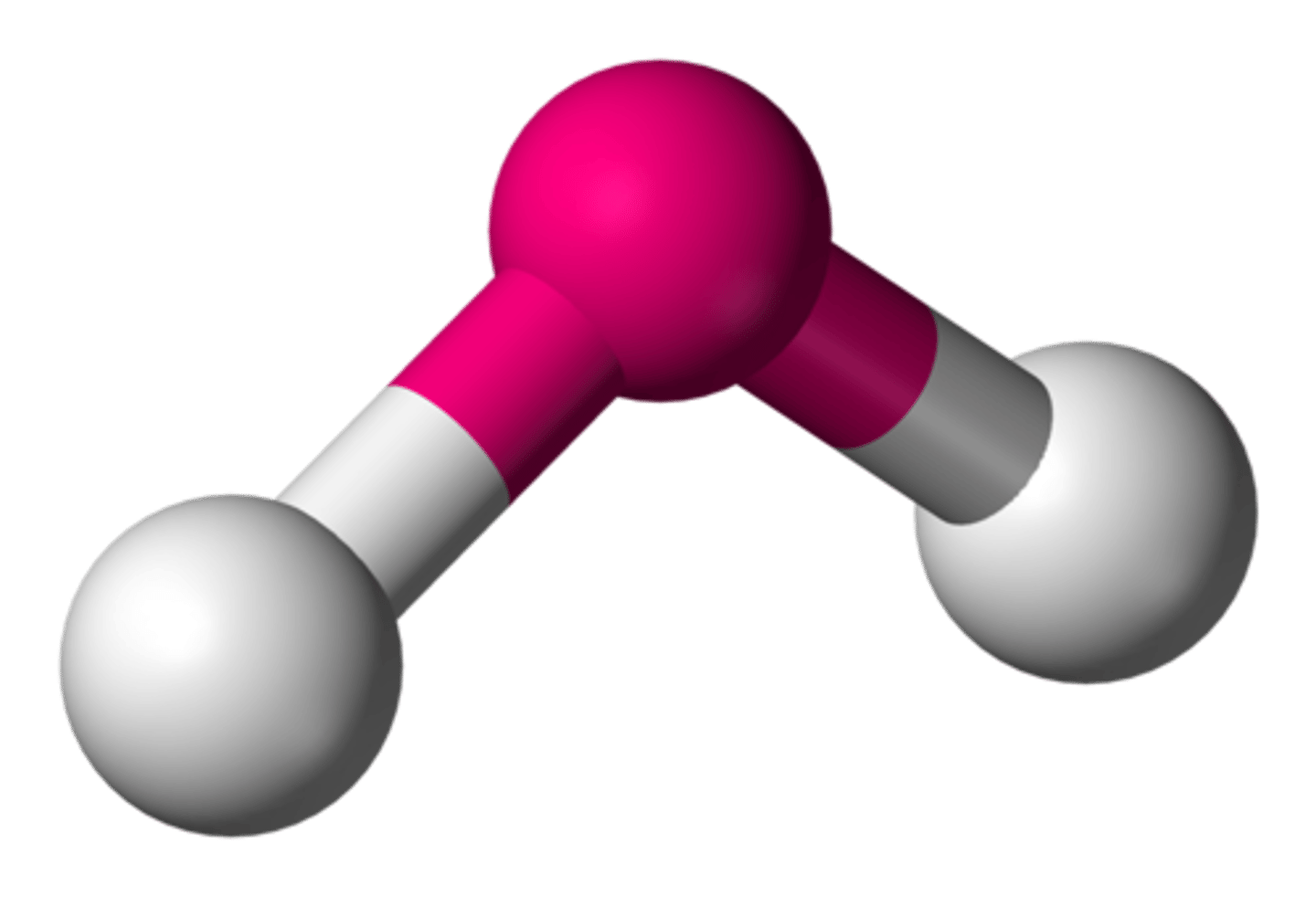
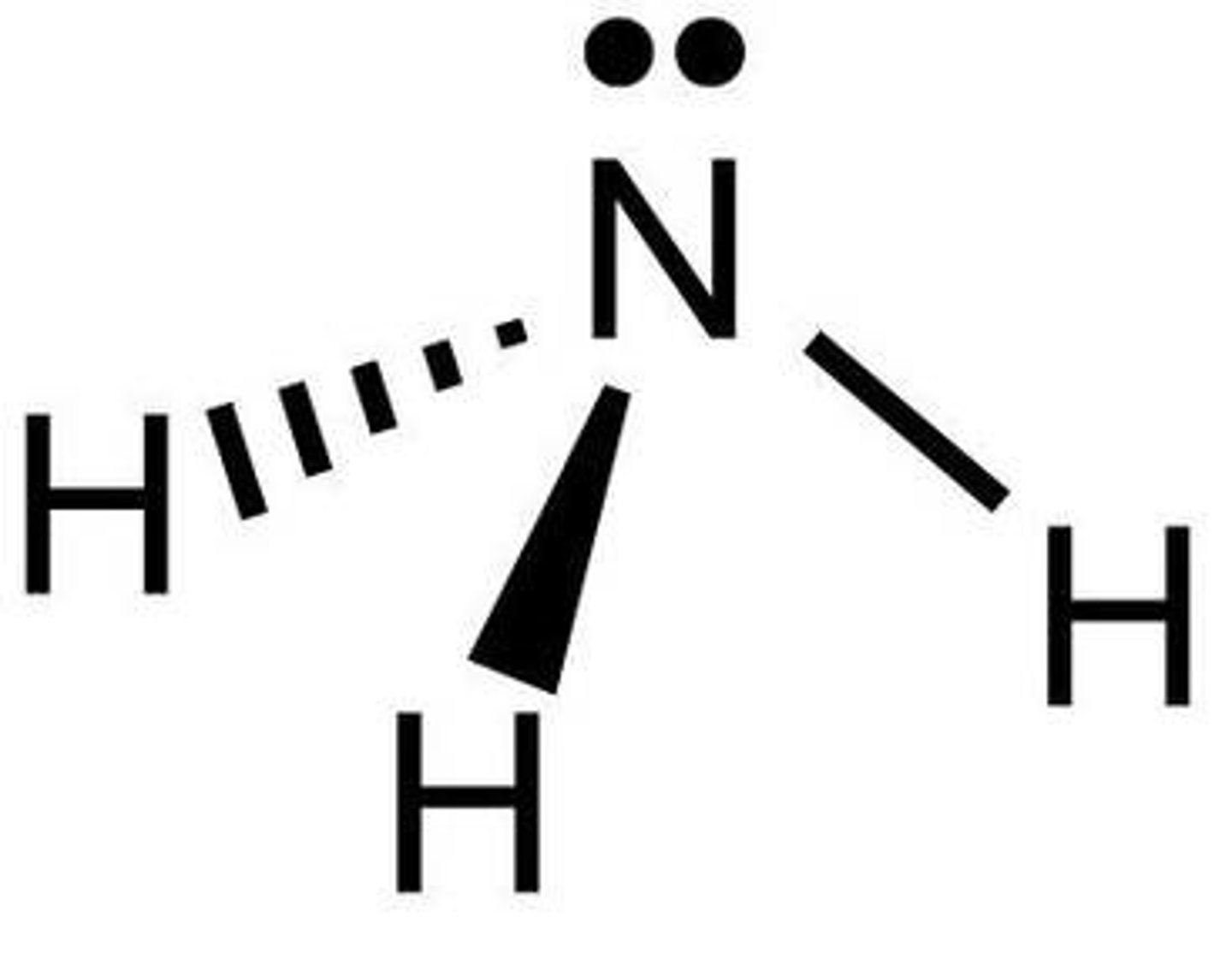
What is the shape of a molecule with three bonds/ four atoms called?
Trigonal Pyramidal
What is the shape of a molecule with four bonds/ five atoms called?
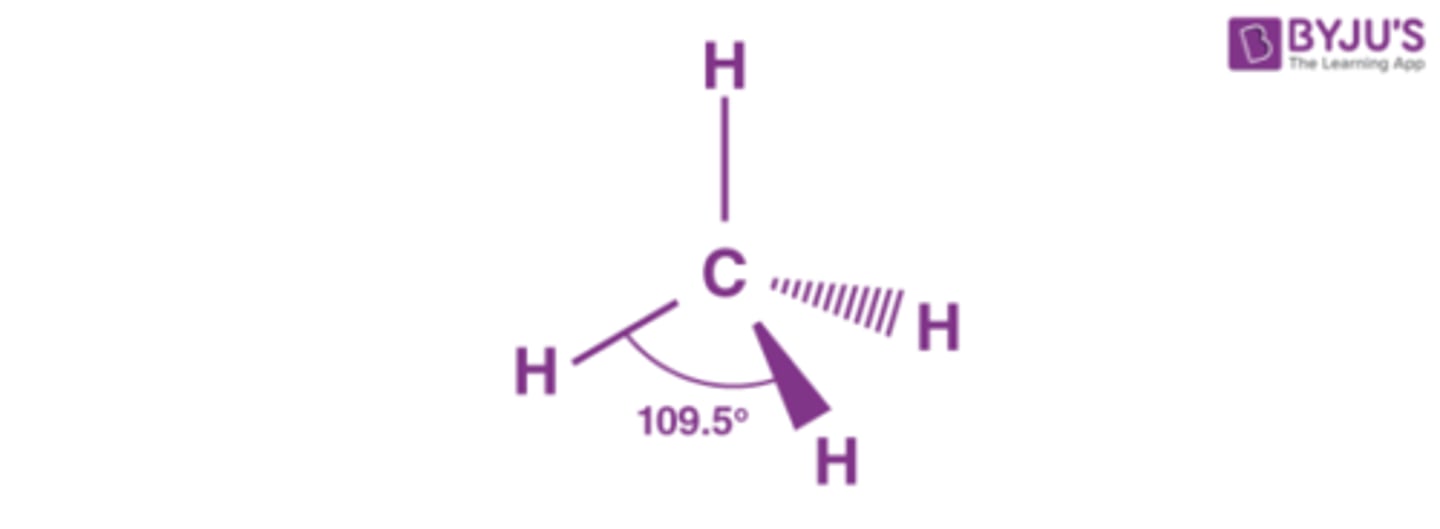
Tetrahedral
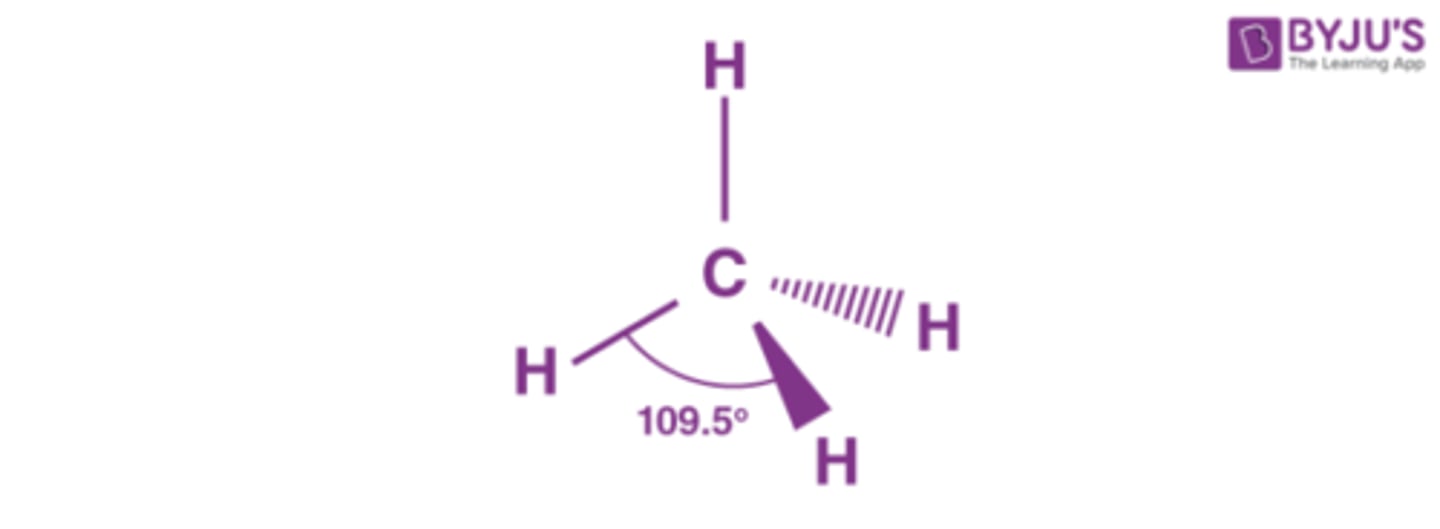
What are the two types of covalent structures?
Covalent molecular and covalent network
What are the properties of simple molecular substances?
- Low mp/bp
- Simple covalent structure
- Insoluble in water
-Does not conduct electricity
What are the properties of covalent network substances?
-high mp/bp
- Giant lattice structure
-Insoluble in water
- Does not conduct electricity
Why does simple molecular substance have a low mp/bp?
when these substances change state only weak forces of attraction are broken and requires small quantities of energy
Why does covalent network substance have a high mp/bp?
when these substances change state the strong covalent bonds have to be broken requiring large quantities of energy.
Why can't covalent substances conduct electricity?
they do not have delocalised electrons
What covalent substance is the only exception and can conduct electricity and why?
Carbon in the form of graphite because it has delocalised electrons
An ionic bond is generally formed between ________ and ______ ______ atoms
metal, non metal
What is an ionic bond?
The attraction between oppositely charged Ions
What is the structure of an ionic bond?
Giant Ionic lattice
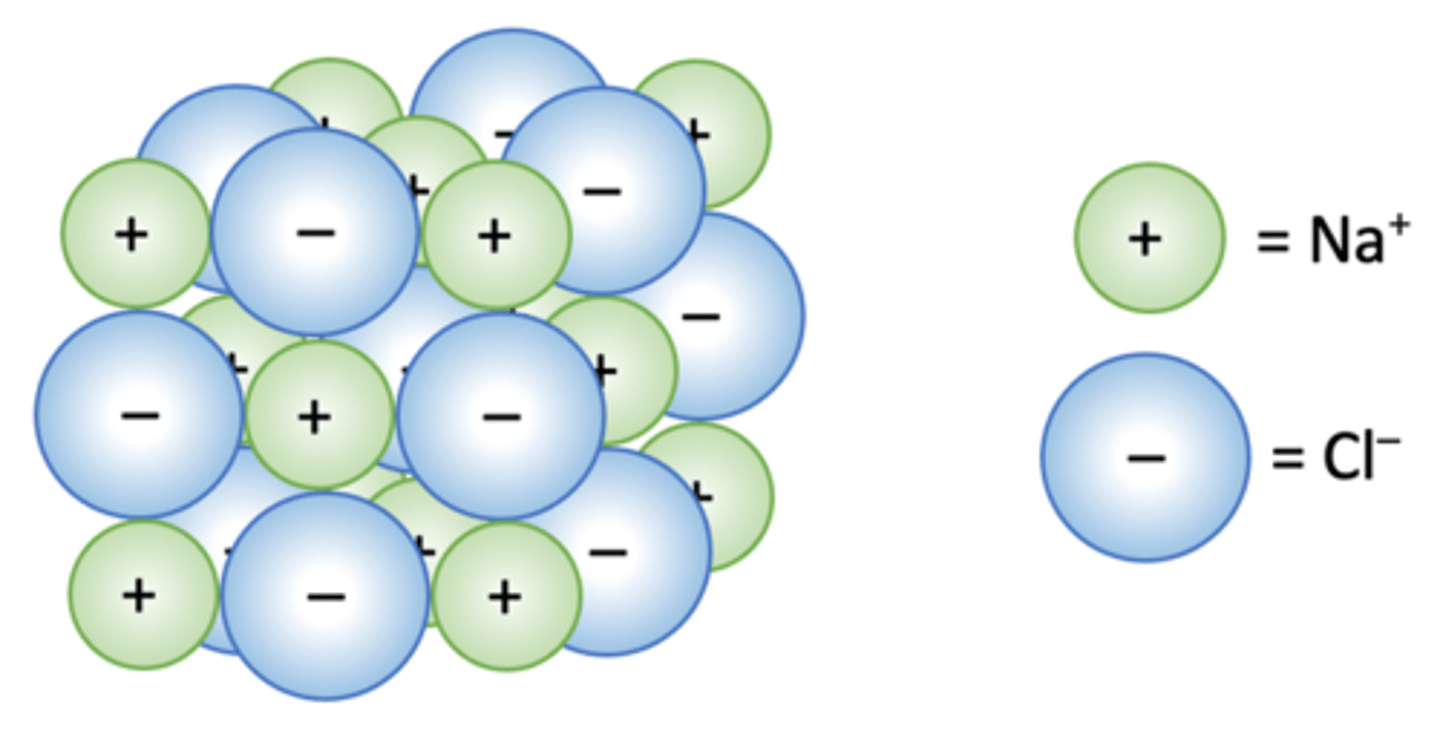
ionic compounds have low mp/bp (True / False)
False
Why don't SOLID ionic compounds conduct electricity ?
They do not contain free ions
Are Ionic compounds soluble in water?
Yes
In what 2 states can Ionic compound conduct electricity?
In molten or liquid state
What is meant by the term electrolyte?
Electrically conducting solutions containing ions