Metabolism - ATAR Human Biology Yr 11
1/84
Earn XP
Description and Tags
Chapter 3
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
Metabolism
. the total of all the chemical processes that take place in the body
. these chemical processes convert the food you eat into the energy + materials needed for all life processes
. 2 types: anabolic and catabolic
Catabolic reactions
. destructive metabolic processes during which complex substances are broken down into simpler ones
. involve the breakdown of a larger molecule into smaller components
. exergonic: reactions release energy when bonds are broken
. EG: cellular respiration (oxidative breakdown of fuel molecules such as glucose) and digestion (different enzymes breakdown large food molecules so they can be absorbed by small intestine)
Describe what occurs in a catabolic reaction
. enzymes involved in catabolic reactions can cause a single substrate molecule to be drawn into the active site
—> chemical bonds are broken, causing the substrate molecule to break apart to become 2 separate molecules
What reactions does catabolism involve
. oxidation or hydrolysis (water is consumed) reactions
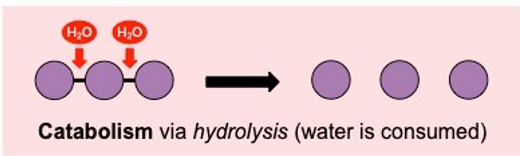
Anabolic reactions
. synthesises complex substances from simpler ones
. synthesis of larger molecules - smaller molecules are joined to form larger ones
. endergonic: uses energy to construct new bonds
. EG: protein synthesis (build up of polypeptides from peptide units), DNA synthesis (building DNA molecules from nucleotides), photosynthesis (builds complex molecules like glucose from simpler ones like CO2 and H2O using energy from sunlight)
What reactions does anabolism involve?
. involves reduction or condensation (water is produced) reactions
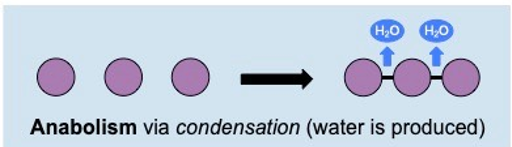
Describe what occurs in an anabolic reaction
. enzymes involved can cause 2 substrate molecules to be drawn into the active site
—> new chemical bonds are formed resulting in the formation of a single molecule
Enzymes
. protein molecules (made up of amino acids) that catalyse reactions without getting used up or altered in the reaction themselves
. are substrate-specific (although specificity varies) - binds specifically to a substrate according to lock and key hypothesis
. have an active site where the substrate locks on
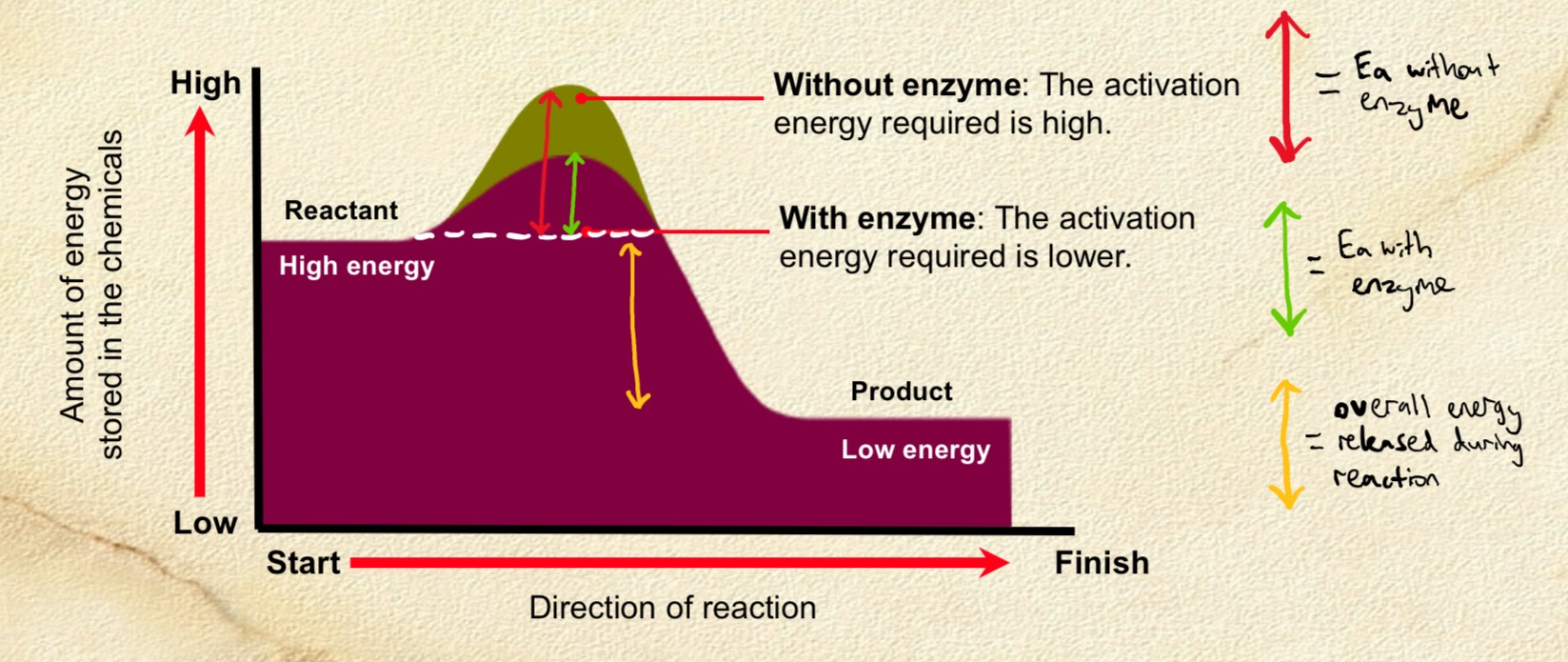
. provide an alternative reaction pathway with a lower activation energy = biological catalysts which speed up reactions
What is an enzyme’s function dependent on
. enzyme function is dependent on the environment
—> pH, temp, cofactors and coenzymes, concentration
Why does the shape of an enzyme matter and what feature of enzymes does this lead to?
. shape is very important as it has a direct effect on how enzyme catalyses a reaction
—> enzymes are specific = only molecules with exactly the right shape will bind to enzyme and react
—> the complexity of the active site is what makes each enzyme so specific
Why do enzymes speed up reactions?
. by lowering the activation energy (Ea) of a reaction (the energy needed to start a reaction)
—> different reactions have different activation energies
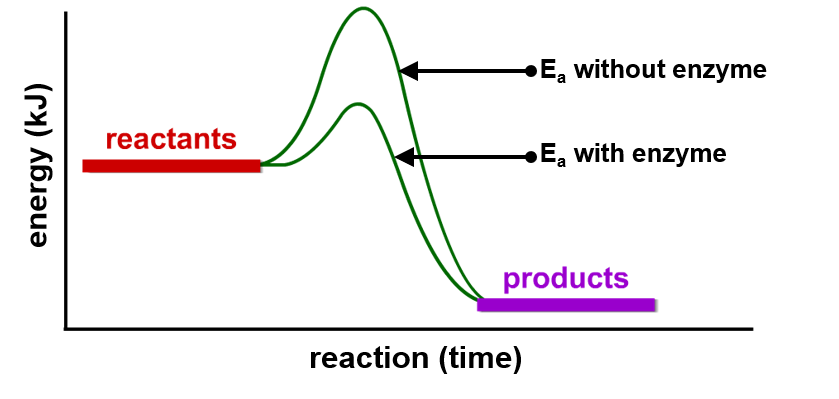
Active site
. part of the enzyme to which the substrate binds
. the specific region of an enzyme where the substrate binds + where catalysis occurs
. is usually a cleft or pocket at the surface of enzyme
. substrate modification occurs here
. contains both binding + catalytic regions
. substrate is drawn to the enzyme’s surface and the substrate molecule(s) are positioned in a way to promote a reaction - either joining 2 molecules together or splitting up a larger one
Optimal temperature for enzymes to work
37 degrees celsius - human body temp
Lock and key model
. an analogy to explain the specific action of an enzyme with a single substrate
—> proposes the substrate is simply drawn into a closely matching cleft on the enzyme molecule - only that substrate would fit the cleft like a key in a lock
—> the shape of the enzyme is always complementary to the shape of the substrate, therefore the two will fit exactly to form an enzyme-substrate complex
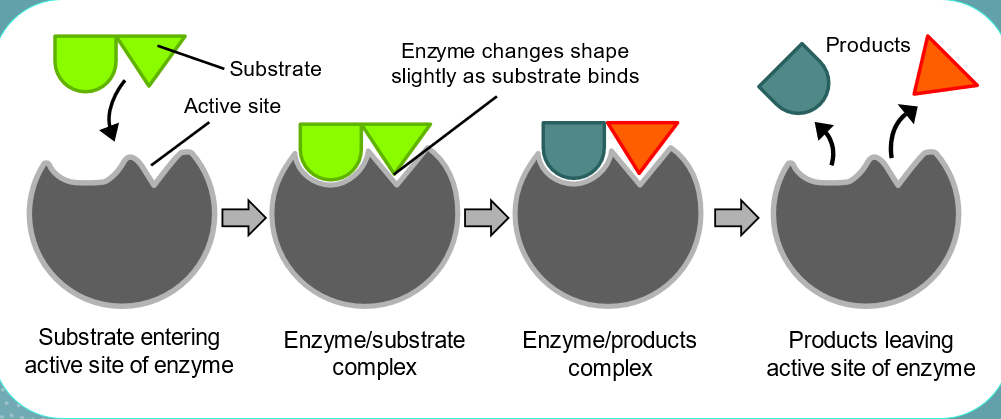
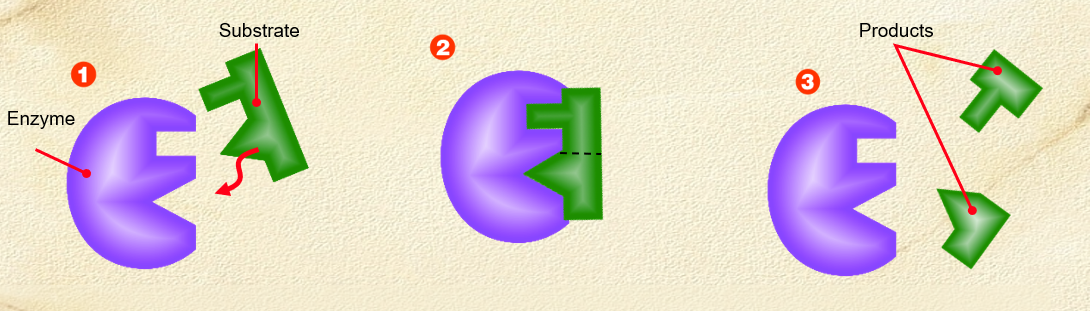
Label a simple Lock and Key model
a substrate is drawn into the active sites of the enzyme
substrate shape must be compatible with enzyme’s active site to fit + be reacted upon
Enzyme modifies substrate, breaking apart substrate, releasing 2 products
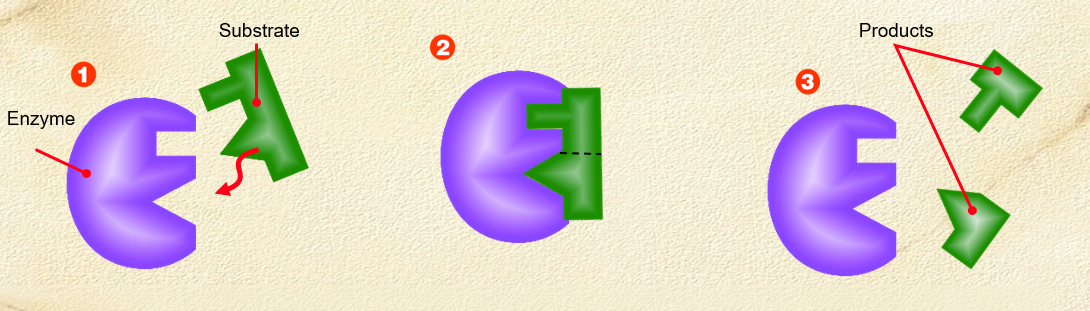
What happens when an enzyme denatures?
. bonds between amino acids in enzyme have broken apart
. changes the shape of the active site, which means it can no longer combine with the substrate
. structure of enzyme changes with extremes of temp + pH
Briefly explain the relationship between pH and temperature with enzymes
. each enzyme has an optimum pH or temperature (30-40 degrees) that it works best at to catalyse a chemical reaction
—> if temp + pH changes sufficiently beyond an enzyme’s optimum, the shape of enzyme irreversibly changes
—> affects the shape of active site + means enzyme will no longer work
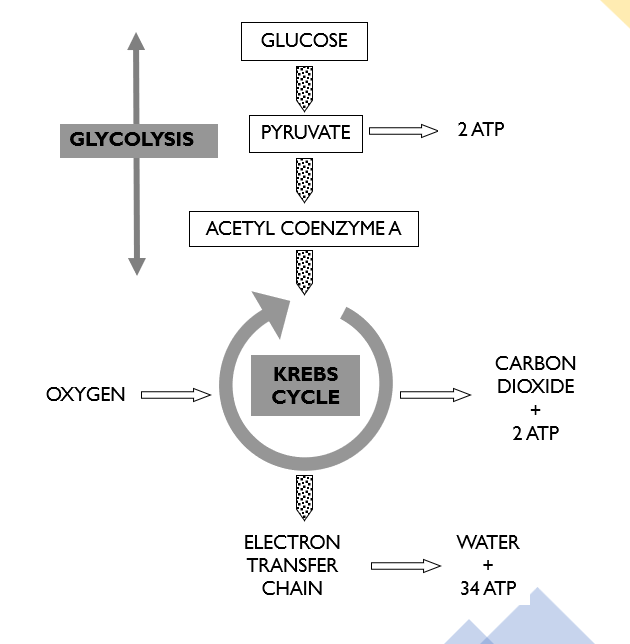
Outline Cellular Respiration
. the process by which organic molecules are broken down in cells to release energy for cell activities.
. releases energy from glucose, amino acids, fatty acids + glycerol
. cells convert chemical energy stored in food molecules (like glucose) into a usable form of energy called ATP
. can occur in presence or absence of oxygen
. energy is consumed by body in chemical form of ATP
Why are there many steps in cellular respiration
. there’s much energy stored in glucose molecule - must be released in small controlled steps
. if all energy from glucose were released at once, most of it would be lost as heat
. involves 20+ reactions that all occur in a series
. each step is catalysed by a different enzyme
. at each step a little energy is released, thus release of energy is controlled
cellular respiration equation
. is catabolic reaction:
glucose + oxygen → water + carbon dioxide + energy (ATP + heat)
C6H12O6+6O2 → 6H2O+6CO2+energy
Biological uses of energy
. 60-80% of energy produced by breakdown of ATP is heat energy, which maintains body temp
. muscle contraction
. active transport
. synthesis of large molecules needed for growth + repair
. transmission of nerve impulses
. cell division
Where does cellular respiration take place?
. 1st stage (anaerobic) in cytosol
. Aerobic stages in mitochondria in the cristae
Stage 1 in Cellular respiration
. Glycolysis (in cytosol):
. the process in which 1 molecule of glucose is oxidised to produce 2 molecules of pyruvic acid
. produces 2 molecules of ATP
. requires NO oxygen (anaerobic)
. when glucose is broken down, around 60% of energy created is released as heat
—> cells can’t use heat but it’s important in maintaining body temp as body heat is constantly being lost to the environment
. breaks down 1 molecule of glucose to produce 2 molecules pyruvic acid
What occurs after glycolysis if no oxygen is readily available
. pyruvate is converted to lactic acid
. lactic acid is taken to liver in bloodstream to be combined with oxygen to form glucose, which will later become glycogen
. during anaerobic respiration, an oxygen debt is accumulated which must be repaid by heavy breathing (recovery oxygen)
What occurs in anaerobic respiration
. if no oxygen present, pyruvate from glycolysis is converted into lactic acid
. Glucose → pyruvate → lactic acid = anaerobic respiration (respiration without oxygen)
. the energy released during glycolysis is enough to convert ADP to ATP so anaerobic respiration allows cells to produce energy without oxygen
. enzymes for anaerobic respiration are in cytosol, thus glycolysis and pyruvate to lactic acid conversion happens in cytosol
Why does anaerobic respiration occur?
. during intense + vigorous exercise, our respiratory + circulatory systems can’t provide enough oxygen to our working muscles
. anaerobic respiration fills in the gaps by providing this extra energy
. cost of this is production of lactic acid which is responsible for causing muscle cramps/pain
—> after vigorous exercise, the lactic acid is taken to the liver where it will recombine with oxygen to form glucose (later glycogen)
—> is why you continuously breathe heavy after exercise - your body is in oxygen debt, needing to repay the oxygen to convert the lactic acid
What occurs after glycolysis if oxygen is present?
. aerobic respiration steps can follow in the mitochondria
—> krebs cycle
—> electron transport system
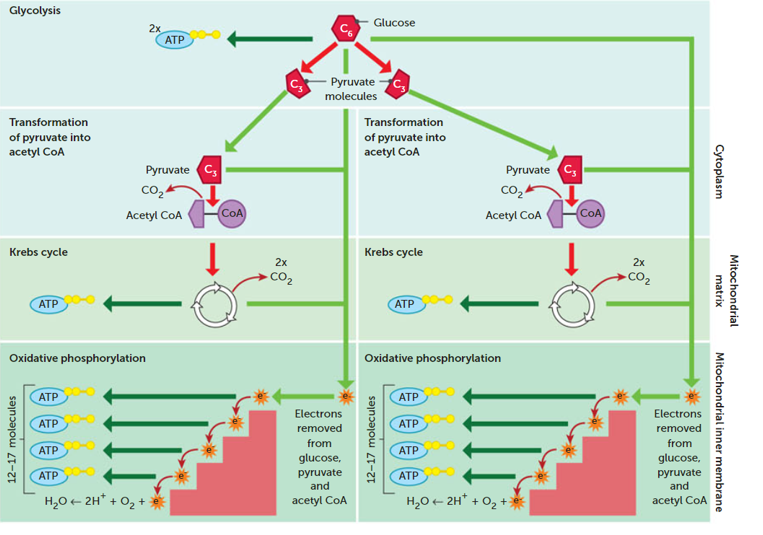
Describe the process of aerobic respiration
. requires presence of oxygen + takes place within mitochondrion
. 1st step is glycolysis which occurs in cytoplasm: glucose (6 carbon molecule) gets broken down into 2 molecules of pyruvate (3 carbon molecule)
—> pyruvate then converted into acetyl coenzyme A, which enters the Krebs cycle (link reaction)
. CO2 is produced in Krebs cycle
. throughout glycolysis + krebs cycle, electrons are released which then enter the oxidative phosphorylation stage (ETC)
. water is produced from oxidative phosphorylation
. throughout this process, a large amount of ATP is also produced (~32-38 molecules)
. aerobic respiration typically begins with glycolysis in carbs (glycolysis is an anaerobic process and occurs in cytoplasm)
. aerobic respiration consists of the link reaction, Krebs cycle (citric acid cycle) and oxidative phosphorylation (ETC) which takes place in mitochondria
Stage 2 in Cellular respiration
. Krebs cycle (in the mitochondrion):
. pyruvic acid is completely broken down
. requires oxygen (aerobic)
. occurs on inner membrane of mitochondria ‘cristae’ = high SA
. pyruvate converted to acetyl coenzyme A (acetyl CoA) by removing CO2 molecule
. acetyl CoA goes through cycle where carbon atoms are released in form of CO2
. produces 2 ATP
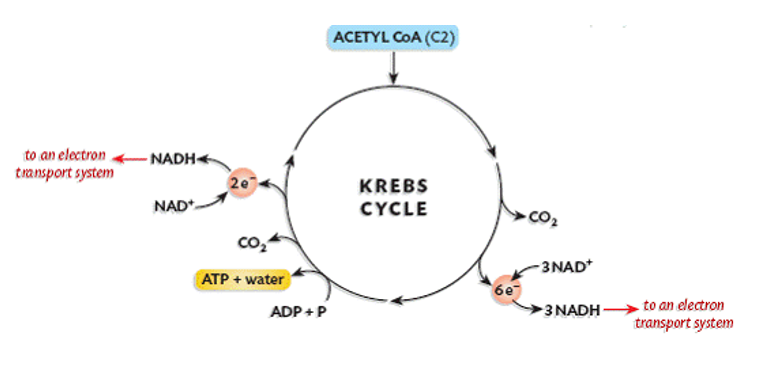
Stage 3 in Cellular respiration
Electron transport system:
. aerobic + in mitochondria
. uses oxygen - called oxidative phosphorylation
. electrons available for oxygen + hydrogen molecules to form water
. movement of protons (H+) through ATP synthase pumps gives energy and is where the ADP is joined with the P to form ATP
. produces 34 ATP
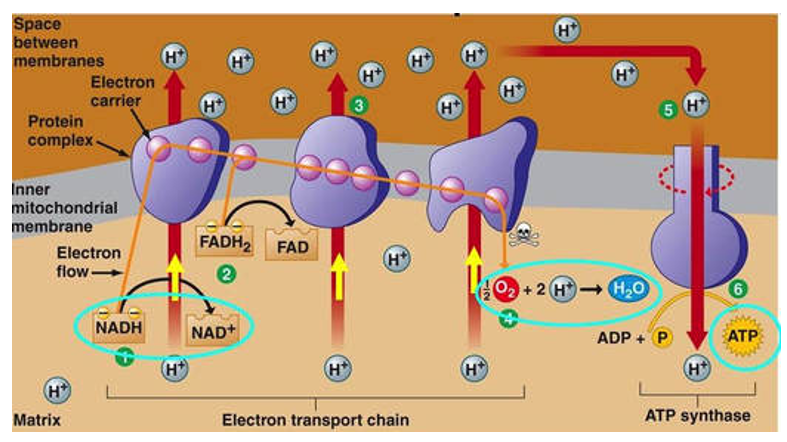
Separate into its 3 main stages and label a diagram of cellular respiration
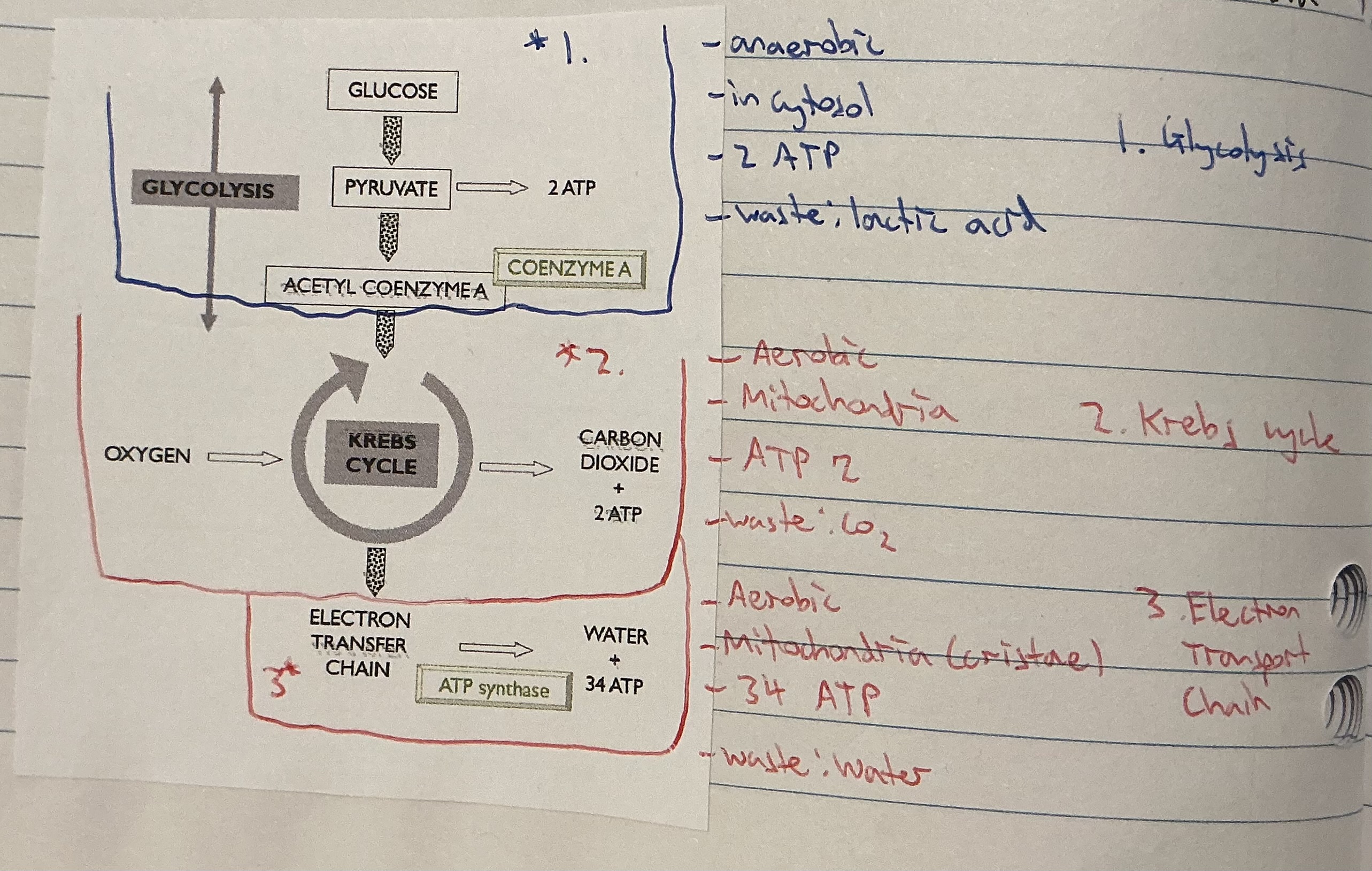
Compare aerobic and anaerobic respiration in terms of how much ATP is produced, whether oxygen is required, and their formulas
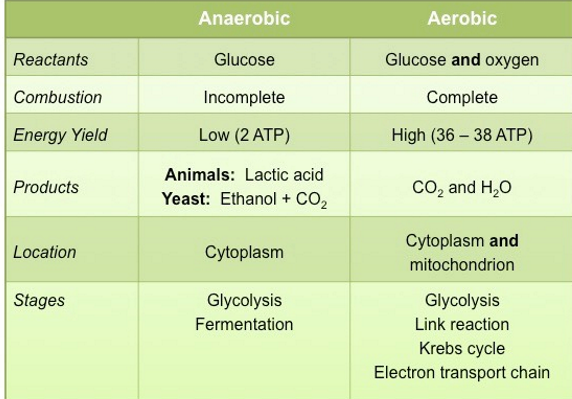
Aerobic respiration:
. produces ~38 ATP molecules
. requires oxygen
. C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP
. glucose + oxygen → carbon dioxide + water + energy
Anaerobic respiration:
. produces ~2 ATP molecules
. produces toxic lactic acid
. C6H12O6 → 2C3H6O3 + energy
. glucose → lactic acid + energy
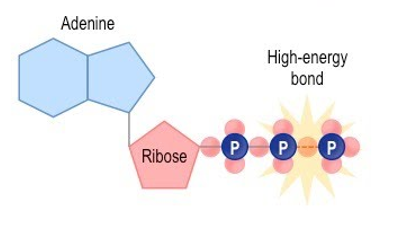
ATP and its formation
. adenosine triphosphate - energy rich
. a complex organic chemical that provides energy to drive many processes in living cells, e.g. muscle contraction, nerve impulse propagation, and chemical synthesis
. energy from the Krebs cycle is used to convert ADP to the energy rich compound ATP
. energy is stored in cells as ATP
Structure of ATP
. Adenine: a nitrogen base
. Ribose: a 5-carbon sugar
. A chain of 3 phosphate groups —> bonds between groups increase from closest to ribose to furthest
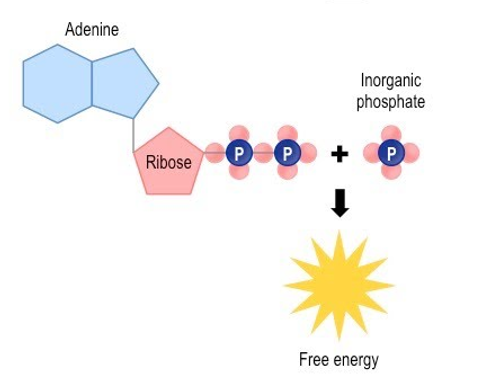
ADP
. adenosine diphosphate
. energy poor
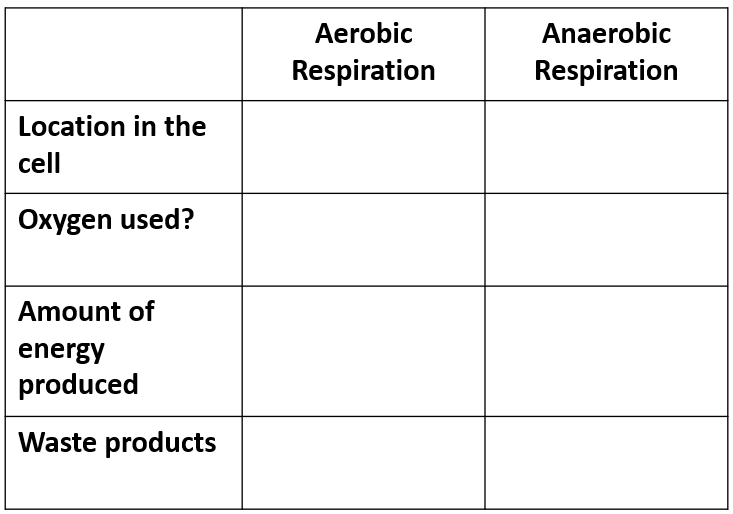
Aerobic vs Anaerobic respiration table comparison
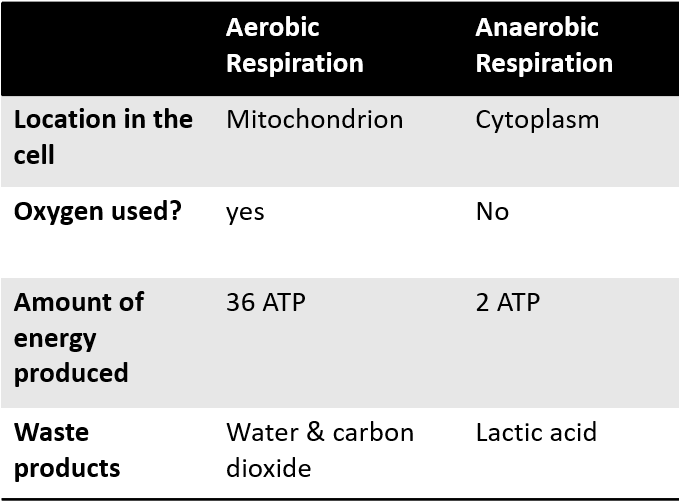
Outline the 4 main biological macromolecules, including their building blocks, functions, and examples
Nutrients
. any substance required for metabolism (used for growth, repair, maintaining the body)
Organic compounds
. molecules that have a carbon chain and lots of hydrogen atoms
—> carbohydrates
—> lipids
—> proteins
Inorganic compounds
. often don’t have carbon atoms
—> water, vitamins, minerals
Outline the nutrient: Water
. fluid in which other substances are dissolved
. chemical reactions in the cell occur in water
. water molecules take part in some reactions
Outline the nutrient: Carbohydrates
. main source of energy for cells
. complex carbs are broken down (catabolised) to simple sugars (eg glucose) which can be broken down in cellular respiration to release energy
. all carbohydrates contain atoms of carbon, hydrogen and oxygen - there are double the amount of hydrogen to oxygen on the molecule
Outline the nutrient: Lipids
. important energy source
. lipids catabolised into fatty acid and glycerol
. glycerol can enter the glycolysis pathway + broken down to release energy
. functions = structural support for cell, energy storage and cell signaling
. normally non-polar in nature + don’t interact with water but conditions apply
Outline the nutrient: Proteins
. organic compounds made of many amino acids
. broken down into amino acids
. amino acids are anabolised into proteins
. important proteins made are enzymes = type of protein that influences metabolism by controlling the chemical reactions that occur in body
. can be used as a source of energy (only if inadequate supplies of carbs + lipids)
Amino acid molecules
. contains amino group + carboxylic group
. 2 amino acids bond together = peptide bond releasing H2O
. DNA determines the type + order as each protein consists of 100+ amino acids
. Proteins characteristic shapes → folding of the chain
. Dipeptides = shorter lengths with 2 amino acids joined
. Polypeptides = 10 or more amino acids bonded together
Outline the nutrient: Minerals
. could be: part of enzyme, cofactors for enzymes, part of ATP
Outline the nutrient: Vitamins
. co-enzymes for many chemical reactions of metabolism
Describe role of vitamins and minerals with enzymes in general
. serve as cofactors + coenzymes, which are necessary for the proper-functioning of enzymes involved in metabolism
. EG: B vitamins are essential for energy metabolism, while minerals like iron are necessary for oxygen transport in blood
Describe how ATP becomes ADP
. when a phosphate molecule is stripped from ATP, stored chemical energy is released and ATP becomes ADP
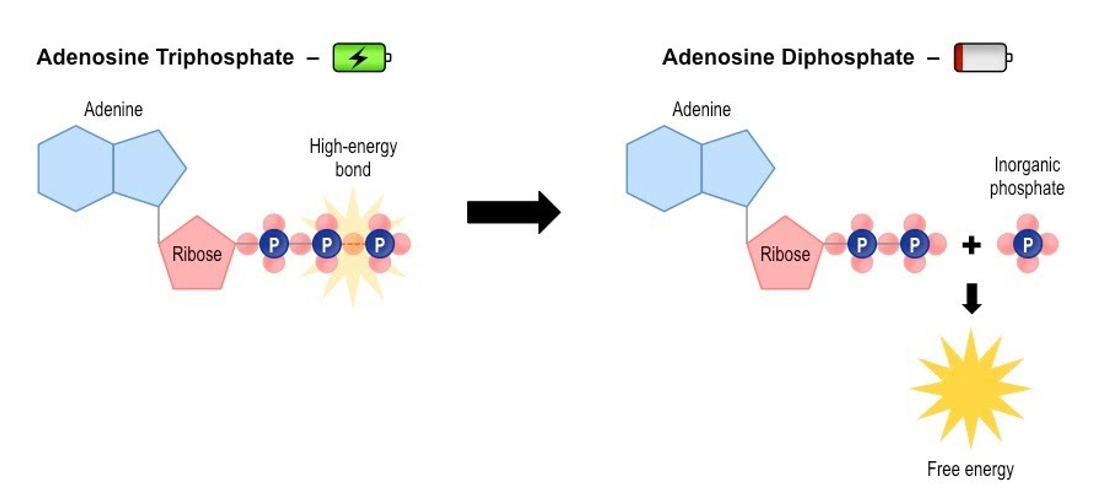
Draw a diagram to summarise the relationship between ATP and ADP
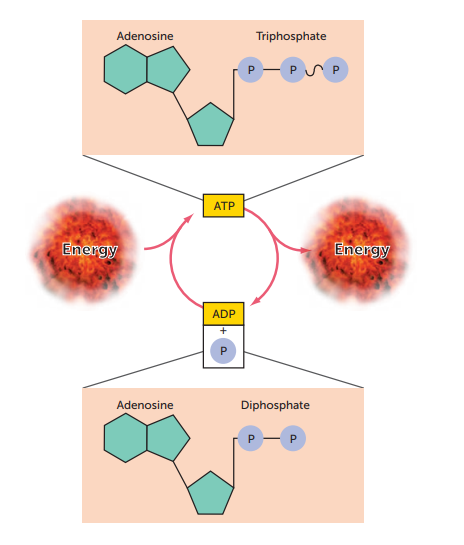
Visualise the ATP-ADP cycle
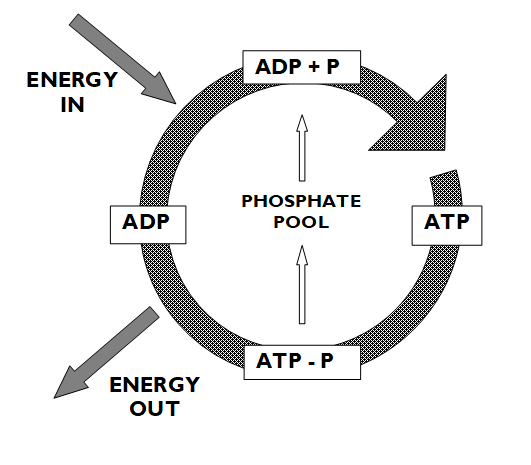
Draw the steps in aerobic respiration
Enzyme affect on reaction rate
. are catalysts that speed up reactions by influencing the stability of bonds in the reactants
. may also provide an alternative reaction pathway, lowering activation energy needed for reaction to take place
Role of enzymes
. molecules that act as catalysts to speed up biological reactions
. not consumed during the biological reaction
. can break a single structure into smaller components or join 2 or more substrate molecules together
The substrate
. the compound on which an enzyme acts
. the chemicals that an enzyme acts on
. they’re drawn into the cleft of enzyme
Describe the role of pepsin and lactase
Pepsin: stomach enzyme used to break protein down to peptides - works at very acidic pH (1.5)
Lactase: a digestive enzyme that breaks lactose into glucose + galactose - low levels of lactase can result in lactose intolerance
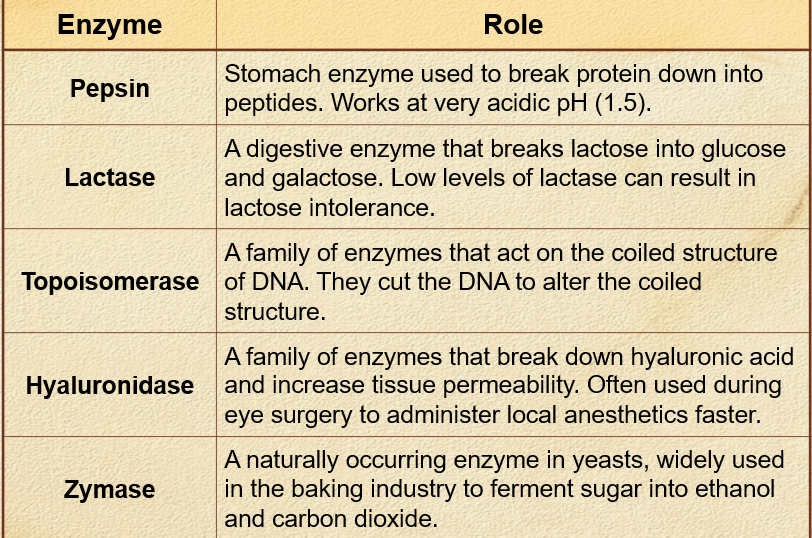
Describe the 2 types of enzyme substrate specificity
High specificity: enzyme will only bind with a single type of substrate
Low specificity: enzyme will bind with a range of related substrates (eg lipases, hydrolyse, any fatty acid chains)
Enzyme substrate complex formation
. when a substrate binds to an enzyme’s active site
Induced fit model
. when an enzyme and substrate join, they form weak bonds that cause the shape of the enzyme to change, creating complementary shapes
—> enzyme or reactants/substrate change their shape slightly
—> the reactants become bound to enzymes by weak chemical bonds
—> this binding can weaken bonds within the reactants themselves, allowing reaction to proceed easily
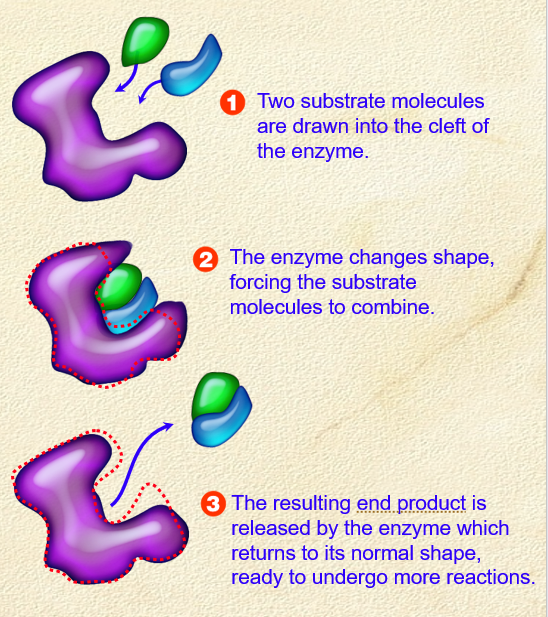
Describe the effect of temperature on enzyme activity
. for most plant + animal enzymes, little activity at low temps
. enzyme activity increases with temp until the temp is too high for enzyme to function
—> at this point, enzyme denaturation occurs and enzyme can no longer function
. temp increases to the optimum, the kinetic energy of the enzyme and substrate increases, causing more collisions between the enzyme and substrate.
—> causes the formation of more enzyme-substrate complexes, leading to an increase in enzyme activity.
. increase in temp beyond the optimum causes the enzyme’s active site to become denatured.
—> means the active site loses its important shape and can no longer form enzyme-substrate complexes, leading to a decrease in enzyme activity
Denaturation
. irreversible changes to structure and shape of a protein
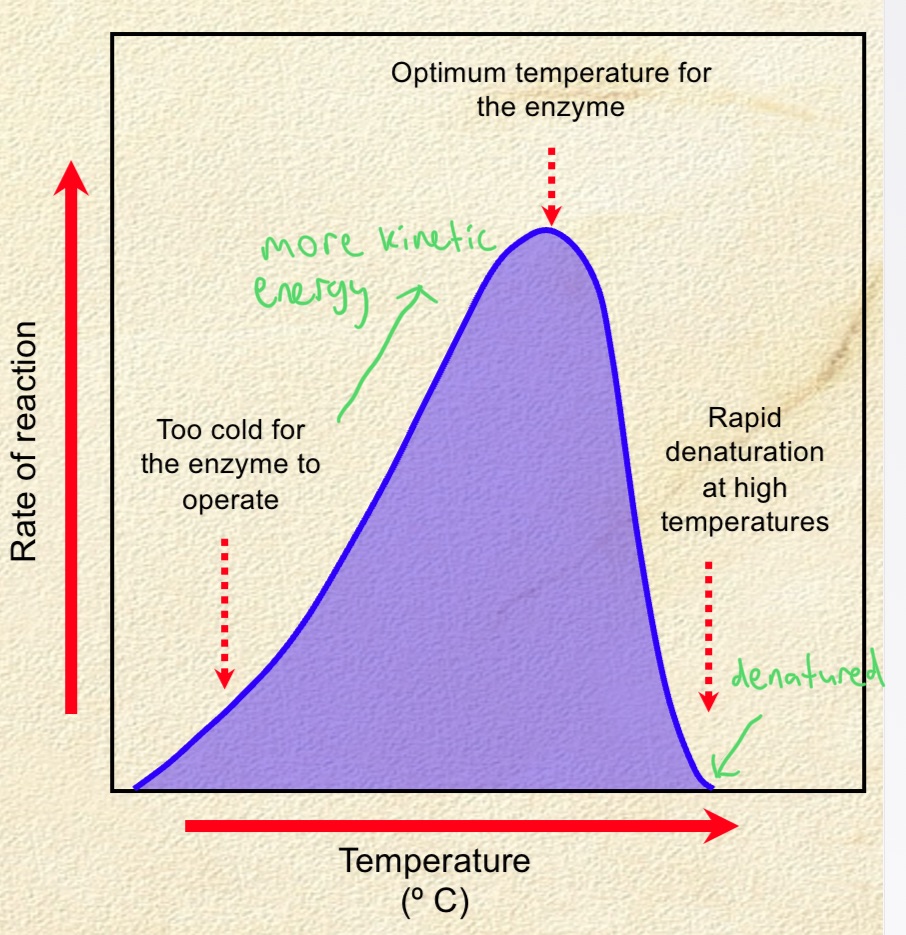
Effect of pH on enzyme activity
. extremes of pH away from enzyme optimum can result in denaturation
. enzymes are found in very diverse pH conditions (as they often work over a range of pH values) so they must be suited to perform in these specialist environments (but all enzymes have an optimum pH where their activity rate is fastest)
—> Pepsin = stomach enzyme and has an optimal working pH of 1.5 which is suited for the very acidic conditions of the stomach
—> urease = breaks down urea + has an optimal pH of near neutral
. Deviating from optimum pH (too high or too low) causes the enzyme’s active site to become denatured and the active site loses its important shape.
—> It can no longer form enzyme-substrate complexes, leading to a decrease in enzyme activity.
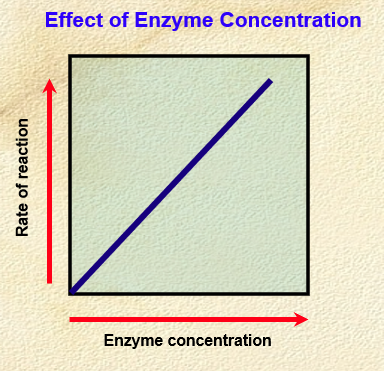
Effect of enzyme concentration on enzyme reaction rate
. rate of reaction continues to increase with increasing enzyme concentration
. directly proportional relationship
—> assumes non-limiting amounts of substrate + cofactors
. higher enzyme concentration = more enzymes there are to form enzyme-substrate complexes = increase in activity
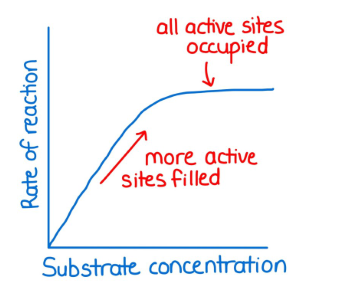
Effect of substrate concentration on enzyme reaction rate
. rate of reaction increases, then plateaus with increasing substrate concentration
—> assumes a fixed amount of enzyme
. higher substrate concentration = the more substrate there are to form enzyme-substrate complexes = increase in enzyme activity
—> happens up to a certain point - enzyme activity then levels off/plateaus as there aren’t enough enzyme molecules to react with the extra substrates (all active sites occupied)
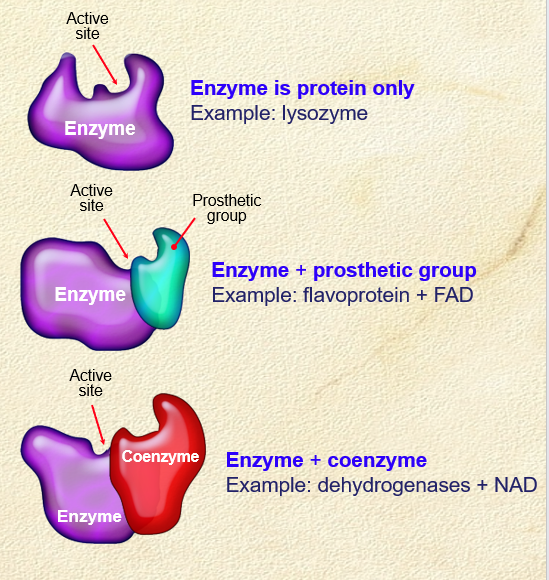
Enzyme cofactors
. a non-protein component of an enzyme, a chemical compound that’s bound to an enzyme, required for the enzyme’s biological activity
. some enzymes require cofactors to be active
What types of particles can cofactors be
organic molecules: coenzymes like vitamins
inorganic ions: Ca2+, Zn2+
How may cofactors be attached to enzymes
permanently attached: prosthetic groups
temporarily attached coenzymes: that detach after a reaction and may participate with another enzyme in other reactions
Coenzymes affect on enzyme activity
. presence of cofactors may be needed for enzymes to catalyse a reaction.
—> they change shape of active site so enzyme can bind to substrate.
—> without cofactor, enzyme is intact, but cannot function
. promotes enzyme action/increases rate of reaction
. Coenzymes are non-protein organic molecules/vitamins
. Alters shape of active site
. Making the enzyme and substrate complementary
Enzyme inhibitors effect on enzyme activity
. enzymes can be deactivated by enzyme inhibitors
—> substances that slow or stop an enzyme's activity.
—> may be used by cells to control reactions so products are produced in specific amounts
- e.g. penicillin is an inhibitor of an enzyme in bacteria that is involved in construction of the cell wall
Describe the 2 types of enzyme inhibitors
Reversible inhibitors:
. used to control enzyme activity
. there’s often an interaction between the substrate or end product and the enzymes controlling the reaction
Irreversible inhibitors:
. bind tightly + permanently to enzymes destroying their catalytic activity
. usually covalently modify an enzyme
. some heavy metals (native arsenic, mercury) are examples of poisons which act as this
What type of molecules are commonly enzyme inhibitors
. many drug molecules are enzyme inhibitors
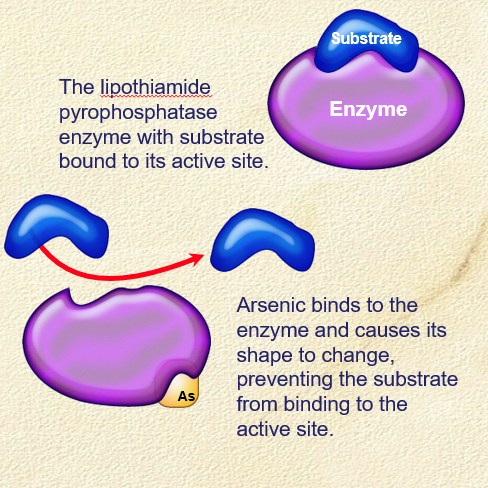
Irreversible enzyme inhibitors
. some heavy metals (Cd, As, Pb) act as
. bind strongly to the sulphydryl (-SH) groups of the protein, destroying its catalytic activity
. most heavy metals (arsenic) act as non-competitive inhibitors
. Mercury (Hg) is an exception - acts as a competitive inhibitor, binding directly to a sulphydryl group in the active site of the papain enzyme
. heavy metals are retained in the body + lost slowly
. poisons (such as As) act as irreversible enzyme inhibitors, binding to the lipothiamide pyrophosphate enzyme altering its shape so the substrate can’t bind
Efficient metabolism
. the biochemical processes within cells that convert nutrients into energy + building blocks for cellular functions and growth
Monosaccharides
. a simple sugar molecule/simplest forms of carbs
. glucose, fructose, galactose
Disaccharides
. 2 simple sugars joined together to form a larger molecule
. sucrose, maltose, lactose
. the sugar formed when two monosaccharides are joined by glycosidic linkage.
Polysaccharides
. many simple sugars formed together to create this larger carbohydrate molecule
. long chains of carbohydrate molecules, composed of several smaller monosaccharides
—> glycogen, cellulose, starch
For the body to use energy properly, which 6 nutrients are required?
Carbohydrates: sugar molecules composed of carbon, hydrogen and oxygen
Proteins: used to build muscle, composed of amino acids
Lipids: fat molecules, used to build cell membranes + hormones
Vitamins: made of carbon, act as coenzymes
Minerals: cofactors in enzyme reactions
Water: primary solvent in the body
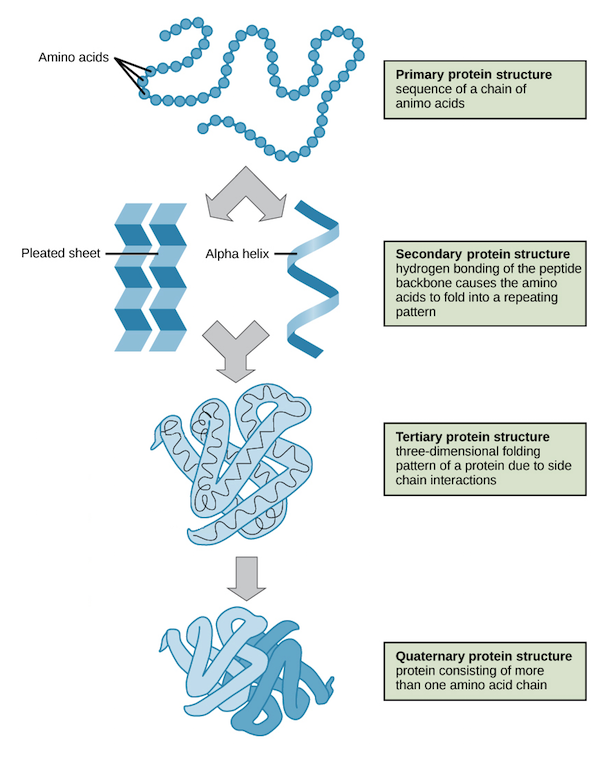
Identify the 4 types of protein structures
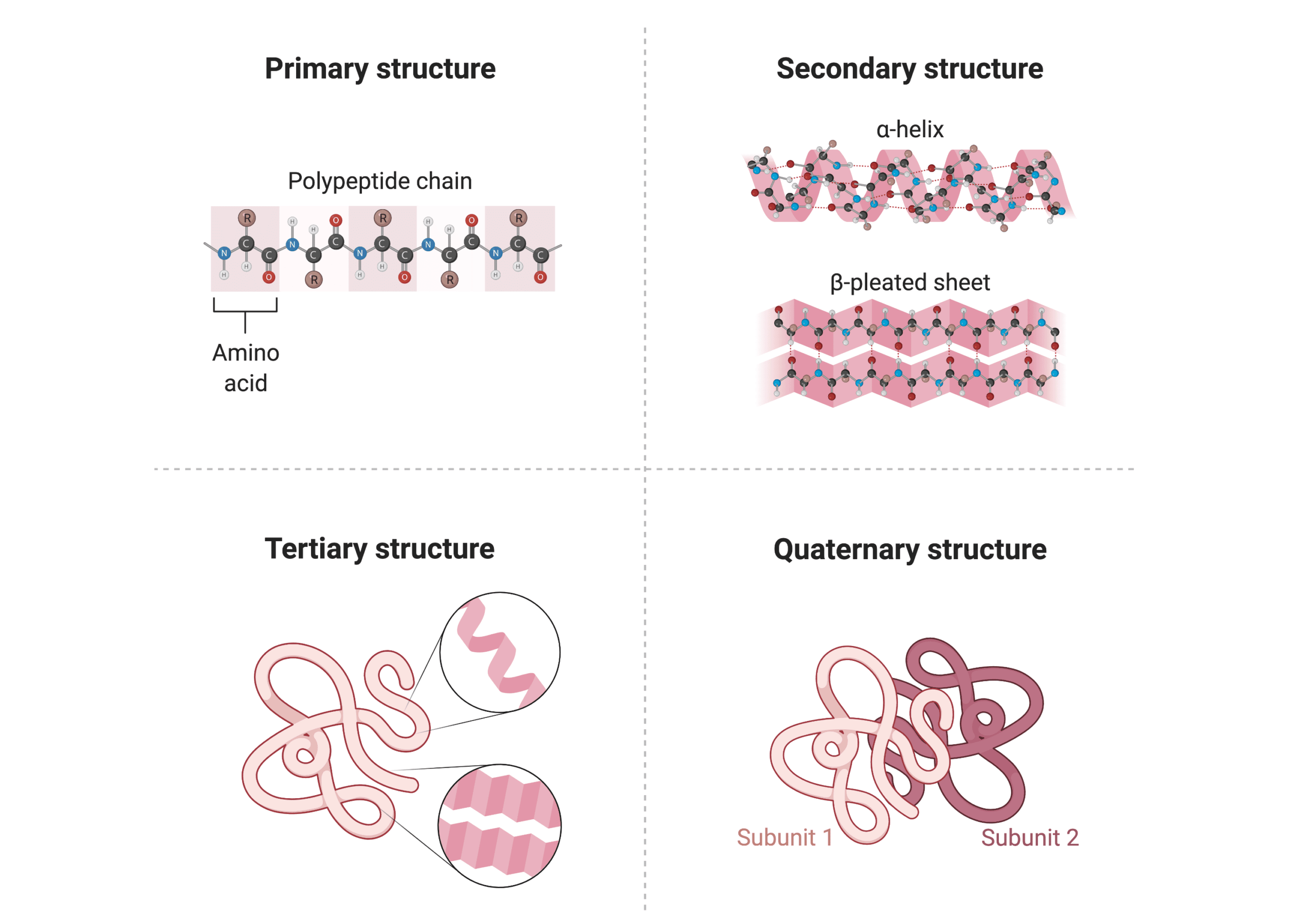
Describe the 4 different protein structures
Draw a complex diagram of cellular respiration
Glycogen
. polysaccharide/polymer of glucose (stored form of glucose in humans) primarily found in liver + muscles, acting as a readily available energy reserve
Difference between lock and key model and induced fit theory
LK theory: the substrate will fit precisely to enzyme to form an enzyme-substrate complex
IF theory: as the substrate joins the enzyme, the shape of the enzyme and substrate changes slightly so that they can fit together to form an enzyme-substrate complex
Draw the process of ATP releasing energy and turning into ADP + P
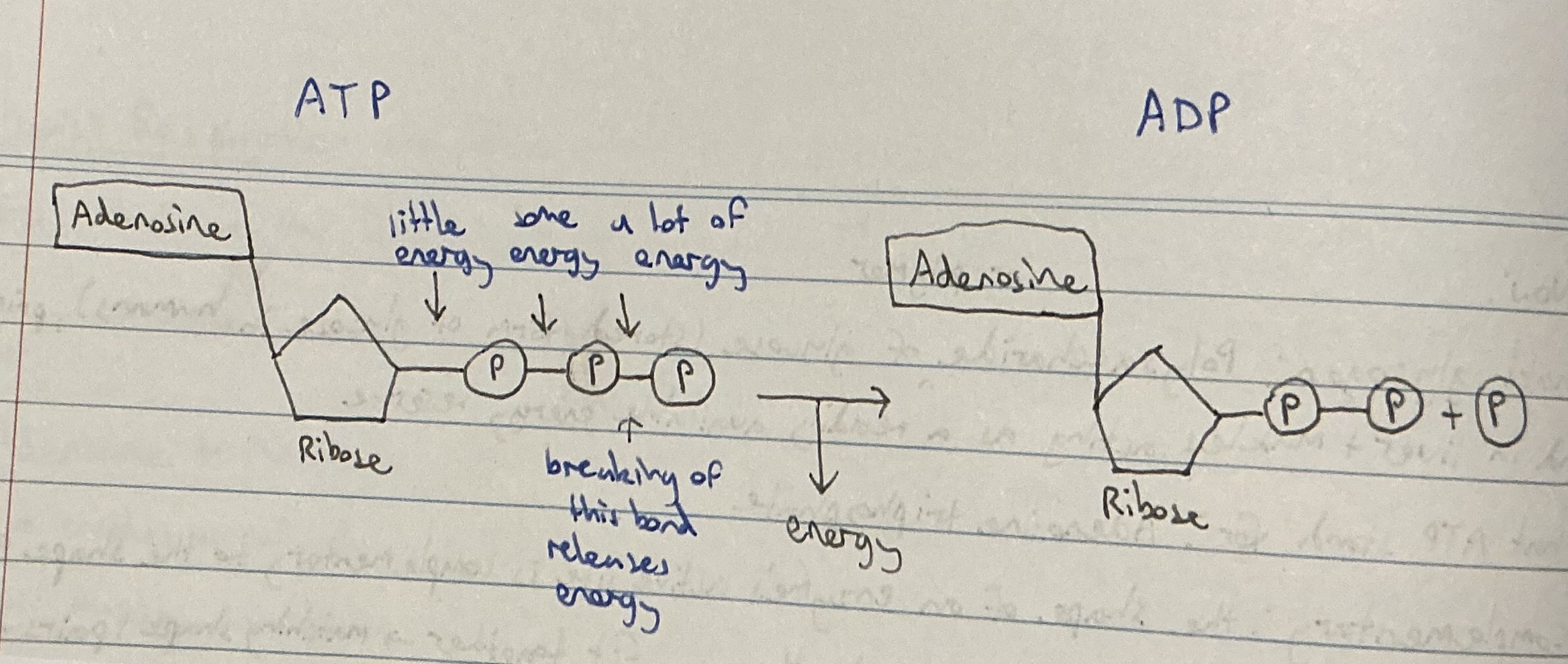
Aerobic respiration definition
. respiration requiring oxygen
Anaerobic respiration definition
. respiration that does not require oxygen