Solvent Extraction
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
In solvent extraction, what is the mobile phase used for the TLC analysis?
3:7 Ether:Chloroform mixture is the mobile phase in the TLC
What is the stationary phase used for the TLC anaylsis?
Silica gel
What is the purpose of adding sodium sulfate (Na2SO4)?
Sodium sulfate dries the organic layer
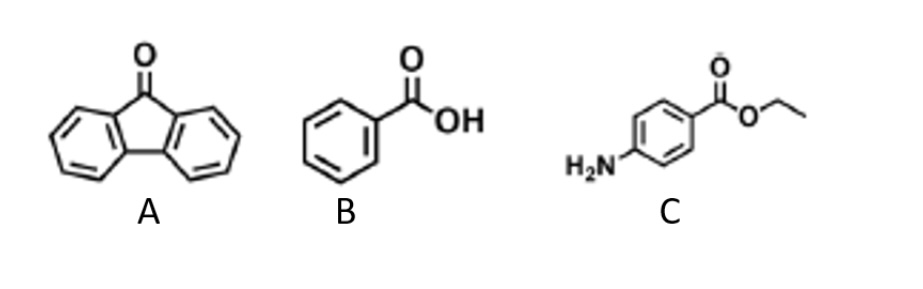
If a solid mixture of the three aromatic compounds are placed in 3 MHCl, which is likely to dissolve?
C: ethyl 4-aminobenzoate
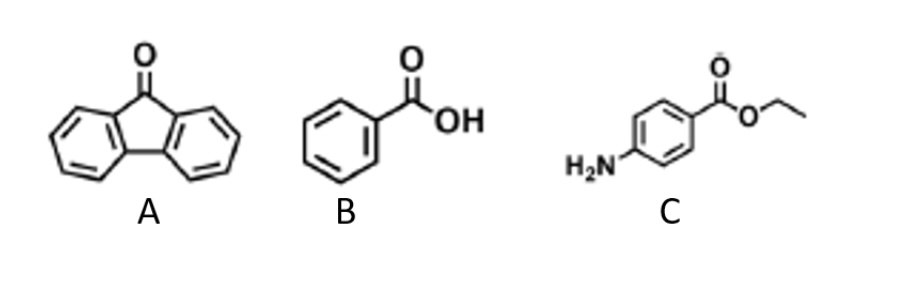
Whatis compound B?
B: Benzoic Acid
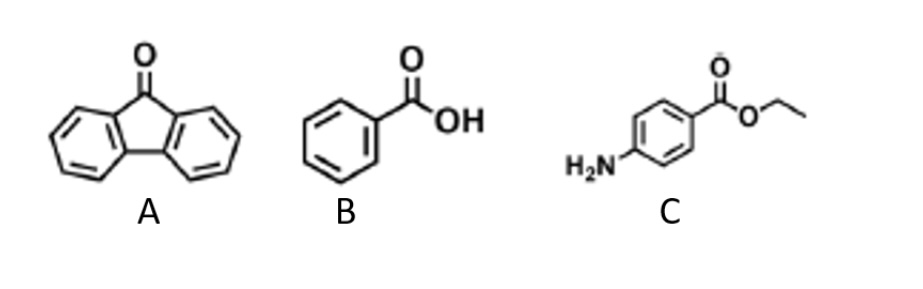
What is compound A?
9-fluorenenone
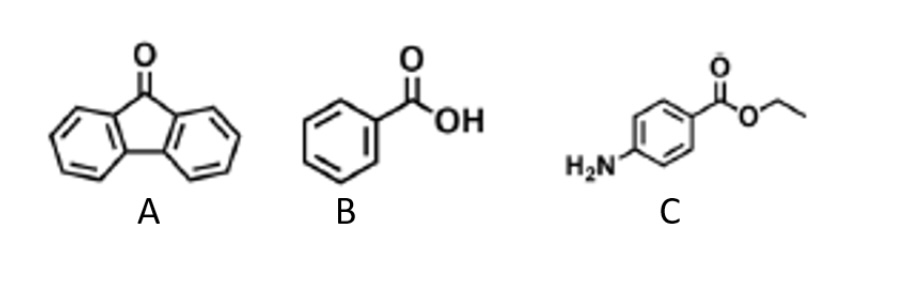
What solvent can dissolve all 3 aromatic compounds in the mixture?
Diethyl ether can dissolve all aromatic compounds
SiO2 is a lewis ______
SiO2 is a lewis acid and electrophile
What is more soluble in water: hexane or glucose?
Hexane
Compounds that interact more strongly with the silica gel will travel:
not as quickly on the TLC plate
What is the purpose of this experiment (solvent extraction)?
The purpose of the solvent extraction experiment was to seperate the mixture of 9-fluorenone (neutral), benzoic acid (acid), and ethyl 4-aminobenzoate (base) using liquid-liquid extraction. A TLC plate is then used to observe how pure the compounds were seperated
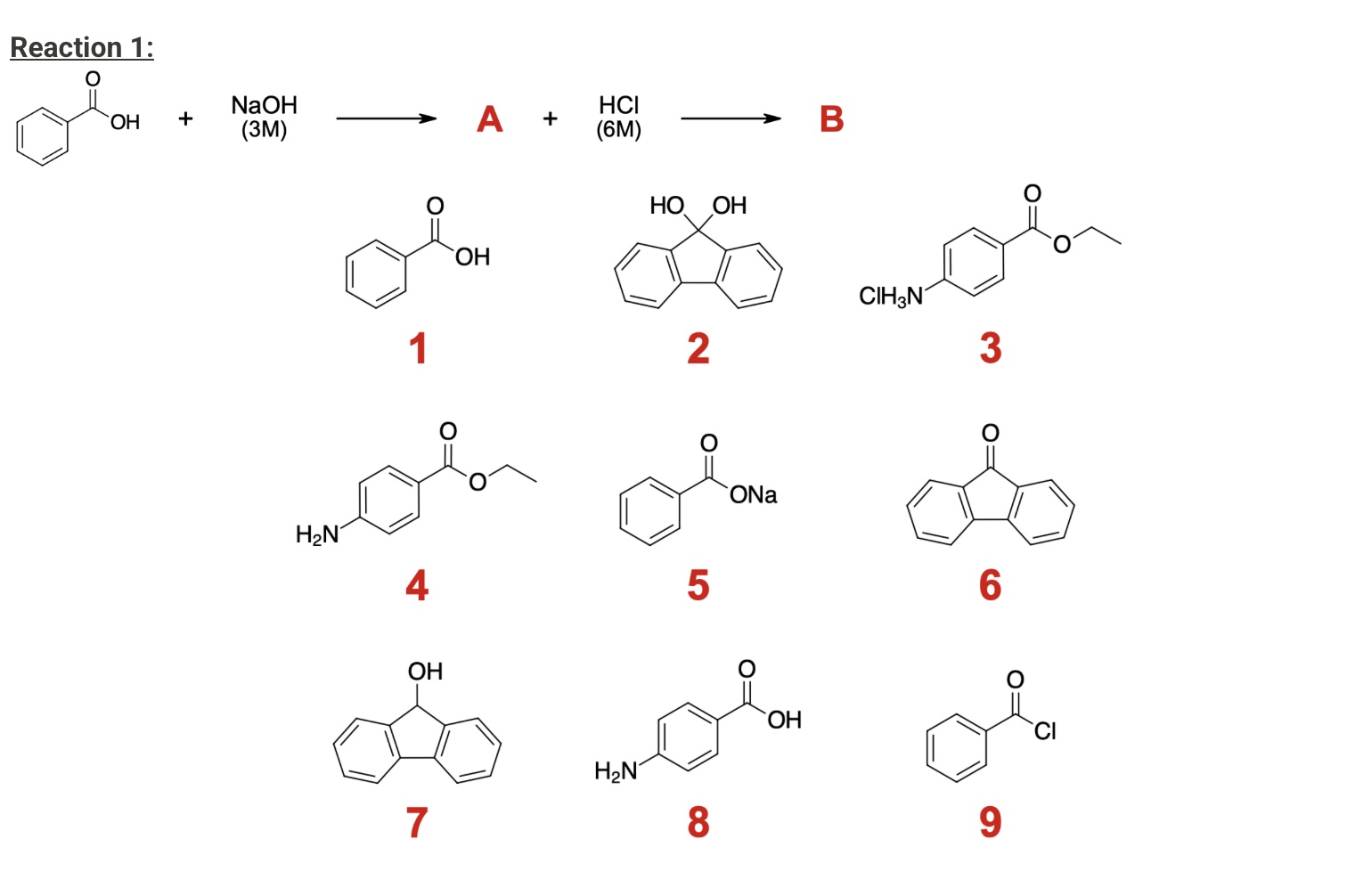
What will be the final products of A and B?
A: compound 5
B: Compound 1
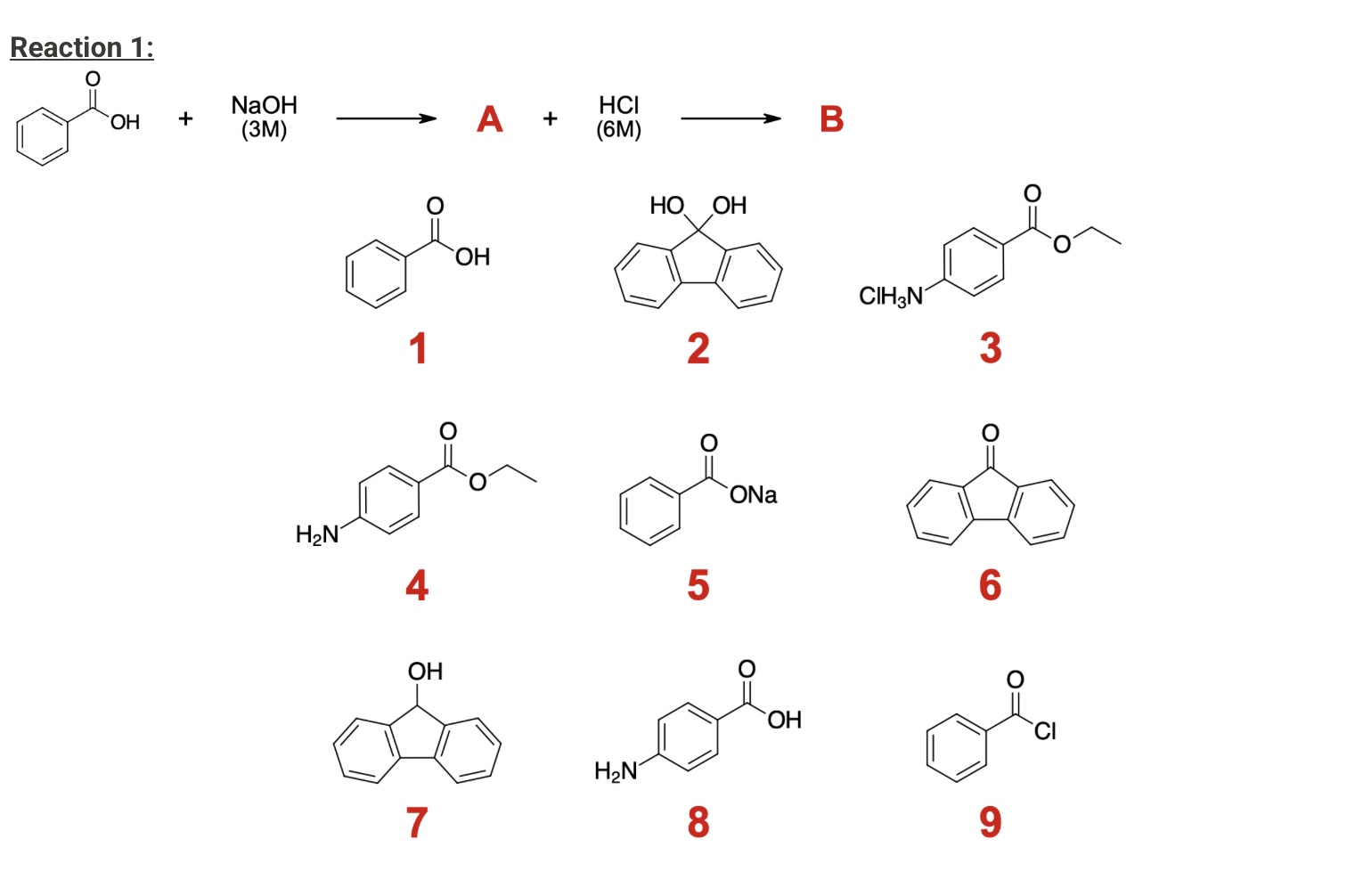
What will be the final products of reaction 2 C and D?
C: Compound 3
D: Compound 4
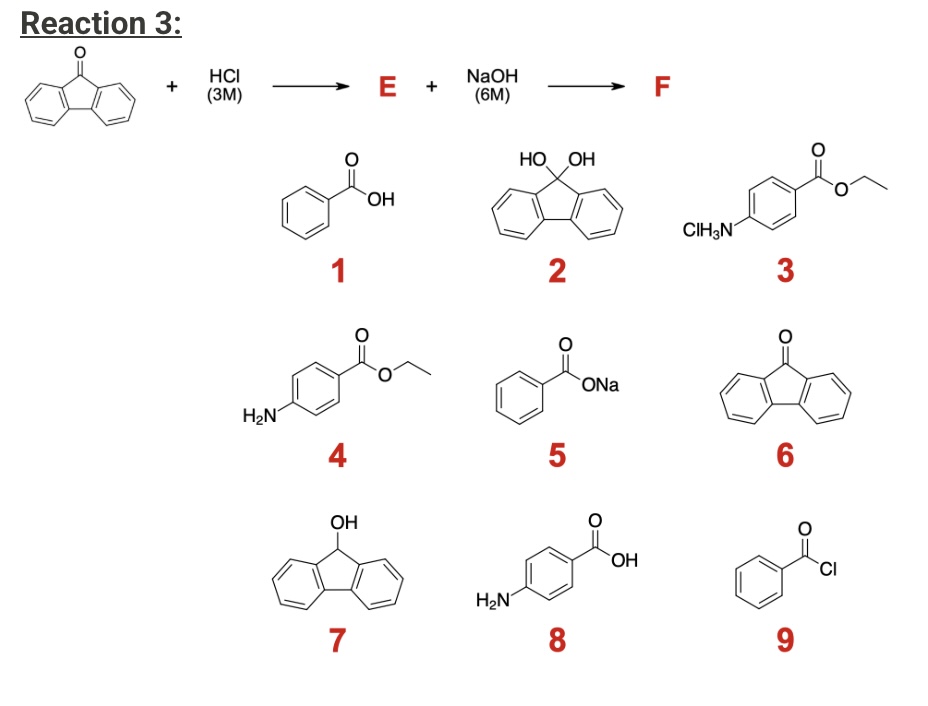
What will be the products for reaction 3 E and F?
E: Compound 7
F: Compound 6
If methylene chloride was used as the organic solvent in solvent extraction, instead of ether, what would the top layer be in the seperation?
Teh top layer would be water since it has a lower density than methylene
Which compound has the weakest interaction with the stationary phase? Why?
9-fluorenone will have the weakest interaction with the stationary phase since it is a non-polar compound and the stationary phase is polar
Where will benzoic acid, 9-fluorenone, and ethyl 4-aminobenzoate be discarded?
In the solid hazardous chemical waste
SiO2 has _______ indicator added
SiO2 has fluorescent indicator added