Biochem - 9.5 notes (book + lect)
1/121
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
122 Terms
Q: What is pyruvate and how is it metabolized?
A: Pyruvate, the final product of glycolysis, is metabolized in one of three ways depending on the organism and oxygen availability.
Q: What happens to pyruvate under aerobic conditions?
A: Under aerobic conditions, pyruvate is metabolized in the mitochondria to CO₂ and H₂O by the citrate cycle and electron transport chain.
Q: How much ATP is generated from complete aerobic oxidation of glucose?
A: Complete oxidation of 1 mole of glucose by aerobic metabolism generates a net yield of 32 ATP.
Q: What happens to pyruvate under anaerobic conditions in muscle cells?
A: Under anaerobic conditions, pyruvate is converted to lactate by the enzyme lactate dehydrogenase.
Q: What is Lactococcus lactis and what does it do?
A: Lactococcus lactis is a bacterium used in the dairy industry to make yogurt and cheese. It converts pyruvate to lactate under anaerobic conditions.
Q: How does yeast (Saccharomyces cerevisiae) metabolize pyruvate?
A: Yeast converts pyruvate to CO₂ and ethanol using pyruvate decarboxylase and alcohol dehydrogenase.
Q: What is a critical function of pyruvate metabolism?
A: To regenerate the oxidized form of NAD⁺, maintaining glycolysis through glyceraldehyde-3-P dehydrogenase activity.
Q: How is NAD⁺ regenerated in lactate and ethanol production?
A: NAD⁺ is regenerated in the cytoplasm, allowing glycolysis to continue under anaerobic conditions.
Q: What happens when pyruvate is fully oxidized in the mitochondria?
A: When pyruvate is transported into the mitochondrial matrix and fully oxidized to CO₂ and H₂O, NAD⁺ regeneration requires a mitochondrial shuttle system linked to the citrate cycle.
Q: Why is NAD⁺ essential for glycolysis?
A: NAD⁺ is required for the enzyme glyceraldehyde-3-phosphate dehydrogenase to sustain ATP production via glycolysis.
Q: What happens when lactate dehydrogenase is defective?
A: A defect causes lactate dehydrogenase deficiency, preventing sufficient NAD⁺ regeneration for glycolysis, leading to ATP shortage and exercise intolerance.
Q: What is lactate dehydrogenase deficiency?
A: A recessive genetic disorder where lack of functional lactate dehydrogenase impairs ATP production during anaerobic exercise.
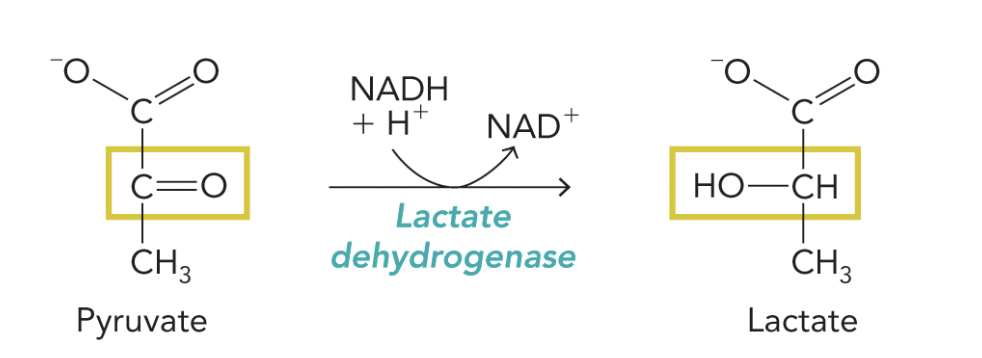
Q: What is shown in Figure 9.50?
A: The lactate dehydrogenase reaction converts pyruvate → lactate, regenerating NAD⁺ for glycolysis.
Equation:
Pyruvate + NADH + H⁺ ⇌ Lactate + NAD⁺
Q: What happens when lactate dehydrogenase isn’t fully functional?
A: NADH oxidation to regenerate NAD⁺ slows, glycolysis halts, ATP runs out quickly, causing fatigue or muscle damage under anaerobic conditions.
Q: How does Saccharomyces cerevisiae (brewer’s yeast) produce energy anaerobically?
A: Under anaerobic conditions, yeast uses glycolysis to produce ATP and metabolizes pyruvate by alcoholic fermentation to regenerate NAD⁺.
Q: What carbohydrate source is used in beer production?
A: Cereal grains like barley, which germinate to express degradative enzymes such as α-amylase and maltase.
Q: What is “mashing” in beer production?
A: Crushing germinated barley to release enzymes, then adding water to produce the wort for fermentation.
Q: What occurs during incubation of wort?
A: Wort is incubated at an optimal temperature to activate catabolic enzymes that break down carbohydrates and proteins into glycolytic metabolites.
Q: What happens after boiling the wort with hops?
A: Boiling adds flavor; after cooling, yeast is added to start aerobic growth before switching to anaerobic fermentation.
Q: What happens during fermentation in beer production?
A: Yeast ferments remaining sugars under anaerobic conditions, producing ethanol and CO₂.
Q: How is beer carbonated in commercial production?
A: Beer is aged, filtered, and carbonated by injection of CO₂ before bottling.
Q: How do microbreweries carbonate beer?
A: They often use a second fermentation at bottling, adding yeast to produce natural carbonation and freshness.
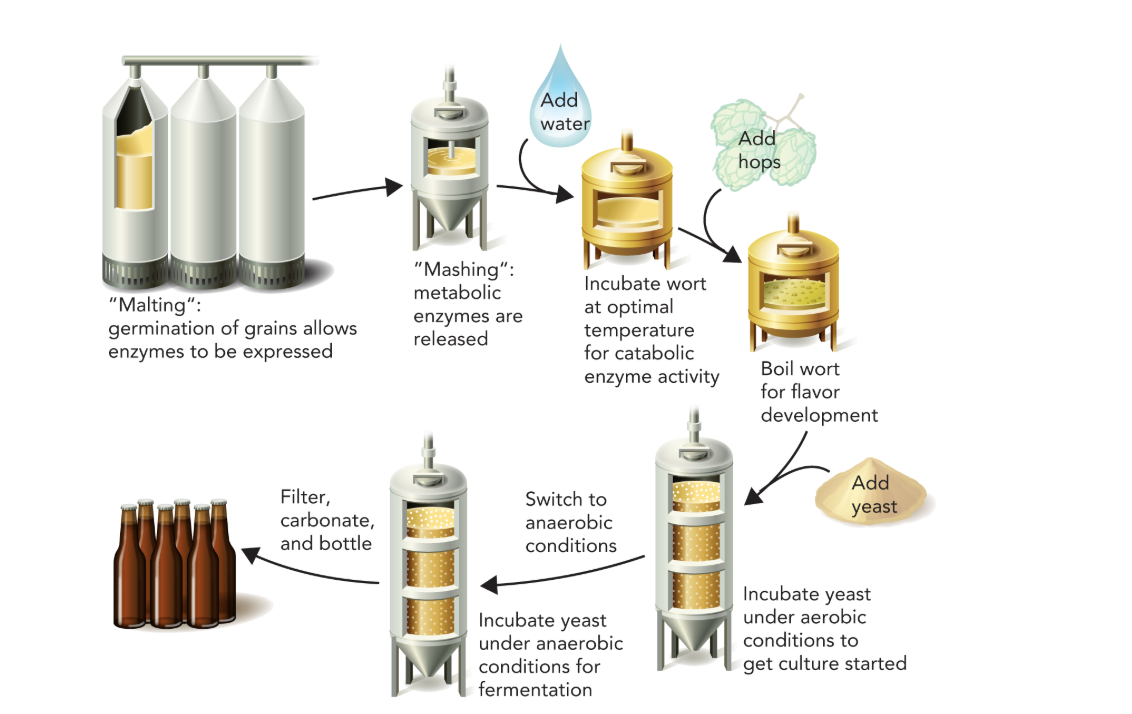
Q: What concept is illustrated by Figure 9.51?
A: Carbonated beer is made by yeast fermentation of sugars in barley; yeast metabolizes simple sugars from enzymatic degradation of germinated barley, generating pyruvate via glycolysis.
Q: What are the three metabolic fates of pyruvate?
A: Pyruvate can be metabolized aerobically to CO₂ and H₂O, or anaerobically to lactate or ethanol, depending on oxygen availability and organism type.
Q: Under what conditions is pyruvate metabolized to CO₂ and H₂O?
A: Under aerobic conditions, pyruvate enters mitochondria and is oxidized by the citrate cycle and oxidative phosphorylation.
Q: What happens to pyruvate when oxygen is limited?
A: It is converted to lactate or ethanol through fermentation, allowing NAD⁺ regeneration.
Q: How many total ATP molecules are generated from aerobic metabolism of one glucose?
A: A net 32 ATP—2 from glycolysis and 30 from mitochondrial reactions.
Q: How is the ATP from oxidative phosphorylation divided between glycolysis and mitochondria?
A: 2 ATP from substrate-level phosphorylation in glycolysis, and 30 ATP from mitochondria (2 from citrate cycle and 28 from ATP synthase).
Q: What key molecule links glycolysis to further energy pathways?
A: Pyruvate, the end product of glycolysis, links glycolysis to either aerobic or anaerobic metabolism.
Q: What happens to NAD⁺ and NADH during glycolysis and fermentation?
A: NAD⁺ is reduced to NADH during glycolysis; fermentation regenerates NAD⁺ from NADH so glycolysis can continue without oxygen.
Q: What process regenerates NAD⁺ in aerobic metabolism?
A: NADH donates electrons to the electron transport chain, where O₂ acts as the final electron acceptor, producing H₂O and regenerating NAD⁺.
Q: What are the main products of aerobic metabolism of pyruvate?
A: CO₂, H₂O, and ATP (produced by oxidative phosphorylation and substrate-level phosphorylation).
Q: How much ATP is generated by oxidative phosphorylation compared to glycolysis?
A: Oxidative phosphorylation yields 30 ATP, while glycolysis produces 2 ATP by substrate-level phosphorylation.
Q: What process generates ATP in glycolysis and the citrate cycle?
A: Substrate-level phosphorylation, which directly transfers a phosphate group to ADP to form ATP.
Q: What is the role of lactate dehydrogenase in anaerobic metabolism?
A: It catalyzes the reduction of pyruvate to lactate while oxidizing NADH to NAD⁺, allowing glycolysis to continue without oxygen.
Q: How does ethanol fermentation differ from lactate fermentation?
A: Ethanol fermentation (in yeast) converts pyruvate to ethanol + CO₂, while lactate fermentation (in muscles) converts pyruvate directly to lactate.
Q: Why does anaerobic metabolism produce less ATP than aerobic metabolism?
A: Without oxygen, the electron transport chain cannot function, so cells rely only on glycolytic ATP (2 ATP) instead of the 32 ATP from aerobic respiration.
Q: What is the overall fate of carbon atoms from glucose under aerobic conditions?
A: They are fully oxidized to CO₂ through the citrate cycle.
Q: What is the overall fate of hydrogen atoms from glucose under aerobic conditions?
A: They combine with oxygen to form H₂O during oxidative phosphorylation.
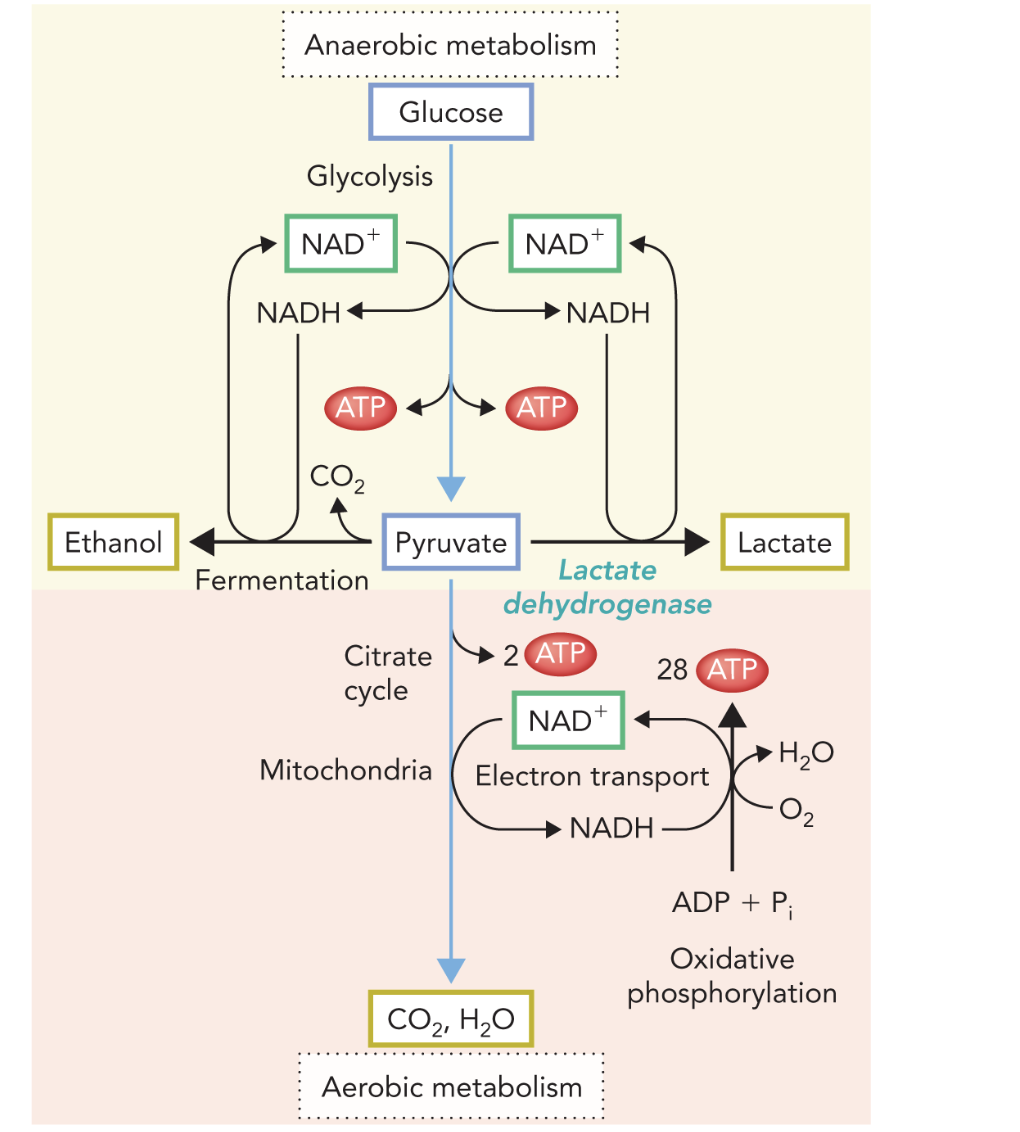
Q: In the diagram, what do the red arrows labeled “ATP” represent?
A: Sites of ATP production—2 ATP in glycolysis and ~30 ATP from mitochondrial processes.
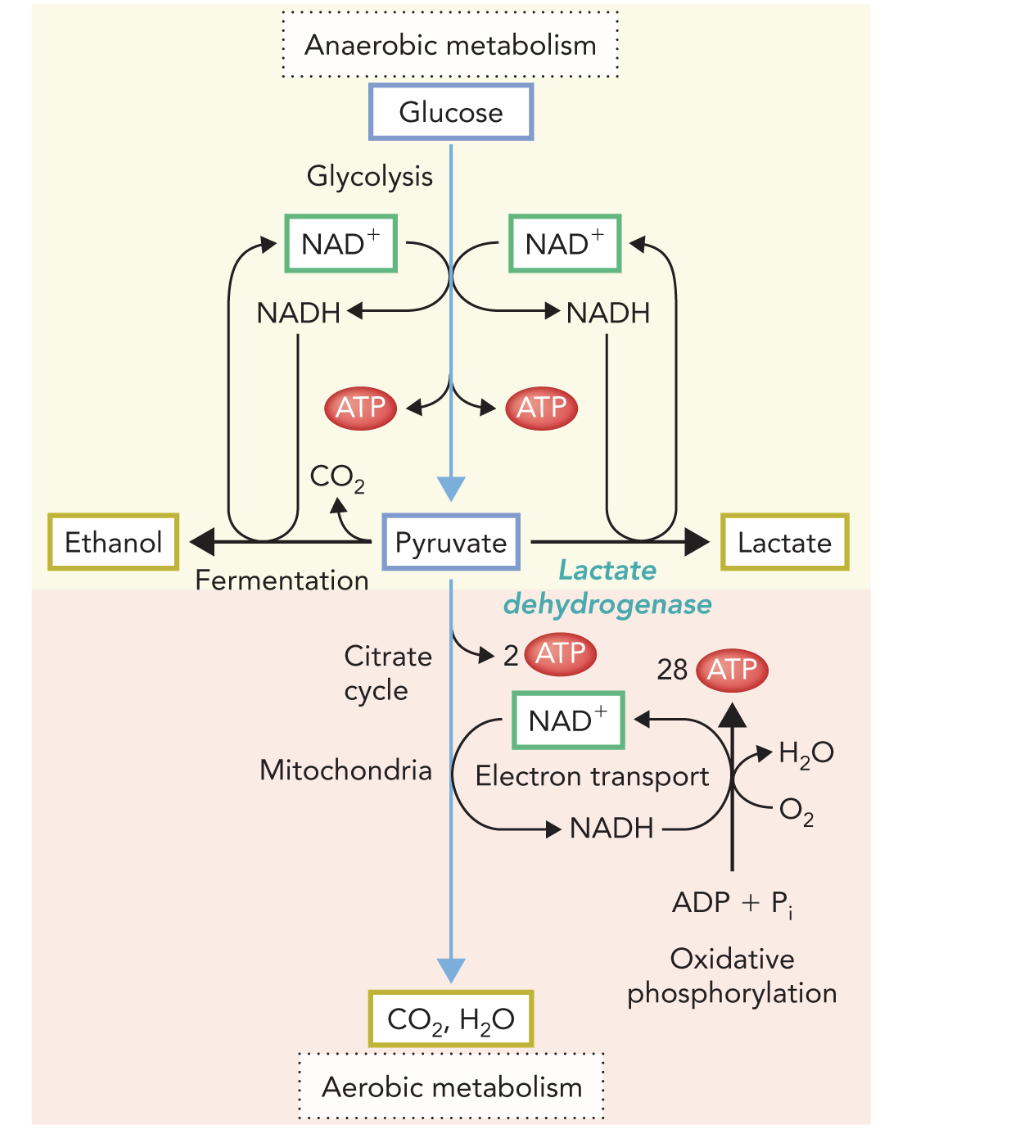
Q: In the diagram, what do the blue NAD⁺/NADH arrows represent?
A: The redox coupling between glycolysis, fermentation, and oxidative phosphorylation, maintaining the NAD⁺/NADH balance.
Q: Why is pyruvate metabolism considered a “metabolic branch point”?
A: It determines whether energy is produced via oxidative (aerobic) or fermentative (anaerobic) pathways based on oxygen availability.
Q: What enzyme reaction requires NAD⁺ regeneration for glycolytic flux?
A: The glyceraldehyde-3-phosphate dehydrogenase reaction in glycolysis.
Q: Why is NAD⁺ regeneration important in glycolysis?
A: It maintains the flux of glycolysis by allowing continuous oxidation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate.
Q: How is NAD⁺ regenerated under aerobic conditions?
A: Through mitochondrial shuttle systems that transfer reducing equivalents from cytosolic NADH into the mitochondria.
Q: What happens to pyruvate after it enters the mitochondria?
A: Pyruvate is fully oxidized to CO₂ and H₂O via the citrate cycle and oxidative phosphorylation.
Q: What role do mitochondrial shuttle systems play in metabolism?
A: They link cytosolic glycolysis and mitochondrial respiration, ensuring NADH from glycolysis can contribute to ATP production.
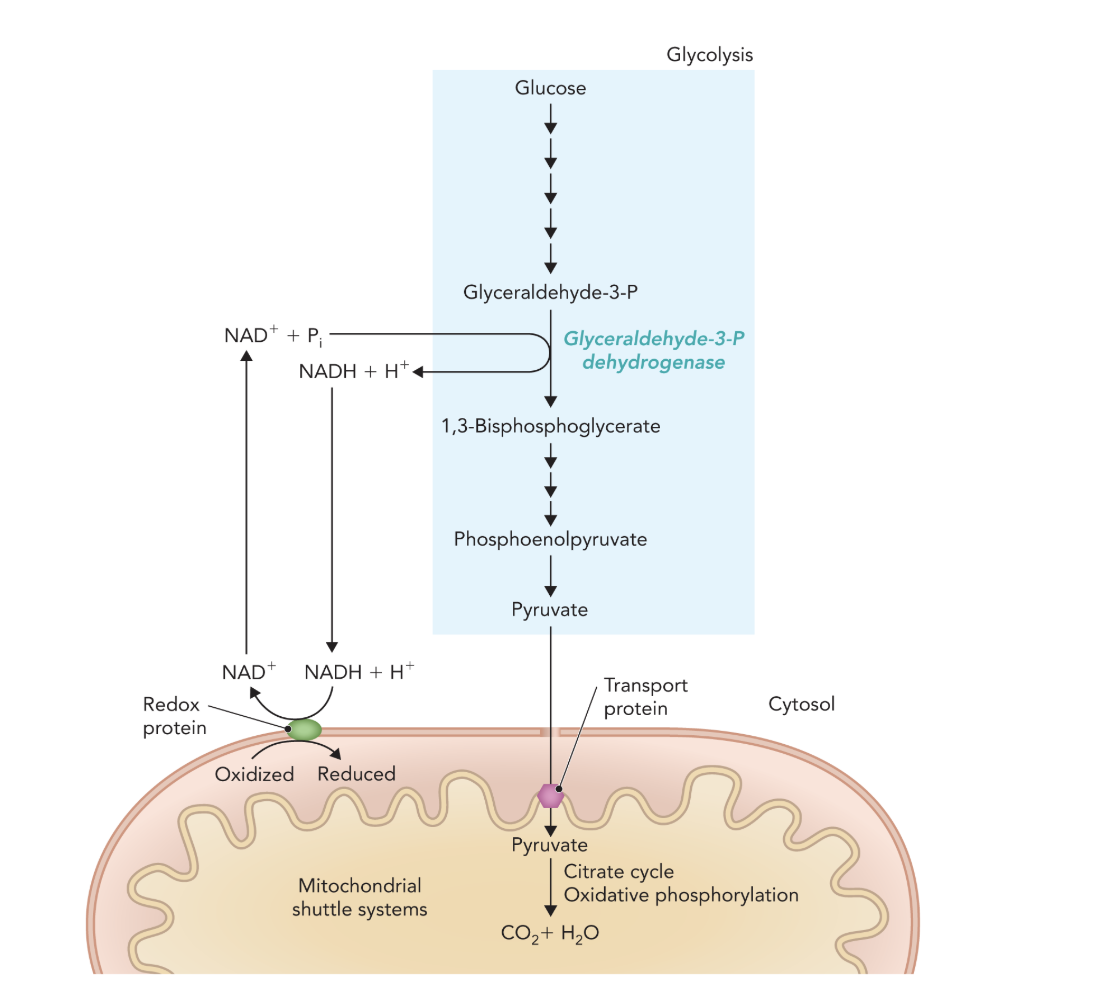
Q: What do the multiple short arrows in Figure 9.49 represent?
A: Individual enzymatic steps involved in pyruvate oxidation and electron transport.
Q: How does NADH generated in glycolysis contribute to ATP production?
A: NADH transfers its electrons into mitochondria via shuttles, fueling oxidative phosphorylation to generate ATP.
Q: What is the overall outcome of NAD⁺ regeneration through the mitochondrial shuttle system?
A: Continuous glycolysis in the cytosol and complete oxidation of pyruvate in mitochondria, yielding maximal ATP.
Q: What enzyme converts pyruvate to lactate?
A: Lactate dehydrogenase (LDH).
Q: What is the function of the lactate dehydrogenase reaction?
A: It converts pyruvate → lactate and regenerates NAD⁺ for continued glycolysis.
Q: Why is NAD⁺ regeneration important in anaerobic metabolism?
A: NAD⁺ is required for glyceraldehyde-3-phosphate dehydrogenase activity in glycolysis, enabling ATP production without oxygen.
Q: What happens to NADH in the lactate dehydrogenase reaction?
A: NADH is oxidized to NAD⁺, transferring electrons to pyruvate to form lactate.
Q: Under what conditions is the lactate dehydrogenase pathway most active?
A: Under anaerobic conditions (limited oxygen), such as in active muscle cells or certain microorganisms.
Q: What is the overall equation for the lactate dehydrogenase reaction?
A: Pyruvate + NADH + H⁺ ⇌ Lactate + NAD⁺
Why can about 1 in 500 adults have reduced lactate dehydrogenase (LDH) activity without showing symptoms?
Because LDH mainly functions under anaerobic conditions to regenerate NAD⁺ from NADH, reduced LDH activity has little effect under normal aerobic conditions, where pyruvate is fully oxidized in mitochondria. Heterozygous individuals retain enough LDH activity, so symptoms only appear during intense anaerobic exercise, which is rare.
What is the purpose of mashing in beer making, and what enzyme produces CO₂ in fermentation?
Mashing converts starches in grains into fermentable sugars using amylase enzymes. During fermentation, pyruvate decarboxylase releases CO₂ by converting pyruvate to acetaldehyde.
Q1: Why are membrane transport proteins essential for cellular function?
A1: They maintain cellular homeostasis and enable signaling by regulating the movement of molecules across cell membranes.
Q2: Why are membrane transport proteins important in medicine?
A2: They serve as good drug targets because pharmaceuticals can bind to and regulate the activity of these transporters.
Q3: Which transport protein is targeted by drugs to treat acid reflux?
A3: The Gastric H⁺–K⁺ ATPase.
Q4: Which transport protein is targeted to treat depression?
A4: The serotonin transporter (SERT).
Q5: Which transport protein is targeted to treat hypertension?
A5: The Na⁺–Cl⁻ co-transporter.
Q1: What is the main function of gastric proton pumps?
A1: They transport H⁺ ions into the stomach, lowering the pH of gastric juices to aid digestion.
Q2: What type of transporter is the gastric proton pump?
A2: It is a P-type primary active transporter.
A low ____ would be good for digestion
pH
Q3: What is the specific name of the gastric proton pump?
A3: H⁺–K⁺ ATPase.
Q4: How does the H⁺–K⁺ ATPase function?
A4: It exchanges K⁺ ions for H⁺ ions across the parietal cell membrane using ATP hydrolysis.
Q1: What do Proton Pump Inhibitors (PPIs) like omeprazole do?
A1: They treat excess gastric acid by inactivating H⁺–K⁺ ATPases in the stomach.
Q2: In what type of environment is omeprazole activated?
A2: In the acidic environment of the stomach.
Omeprazole is _________ inhibitor
irreversible
Q3: Into what intermediate is omeprazole converted in the stomach?
A3: It’s converted into a reactive sulfenamide intermediate.
Q4: How does the active form of omeprazole inhibit the proton pump?
A4: The sulfenamide intermediate covalently binds to a cysteine residue in the H⁺–K⁺ ATPase, blocking H⁺ transport.
Q5: What is the result of omeprazole binding to the gastric proton pump?
A5: The pump becomes inactive, preventing further acid secretion into the stomach.
Q1: What conditions are commonly treated with Proton Pump Inhibitors (PPIs)?
A1: Heartburn, gastroesophageal reflux disease (GERD), and peptic ulcers.
Q2: What was the first clinically approved PPI?
A2: Omeprazole (brand name: Prilosec).
Q3: How do PPIs reduce stomach acidity?
A3: By irreversibly inhibiting acid secretion through blocking H⁺–K⁺ ATPase activity in parietal cells.
Q4: Why do PPIs provide long-lasting relief from acid-related conditions?
A4: Because their inhibition of acid secretion is irreversible, so the effect lasts until new proton pumps are synthesized.
Q1: In synaptic signaling, where is serotonin released from?
A1: Serotonin is released from presynaptic neurons into the synaptic cleft.
Q2: What does serotonin do after being released into the synapse?
A2: It binds to receptors on postsynaptic neurons to mediate neural responses.
Q3: What is the role of the serotonin reuptake transporter (SERT)?
A3: SERT removes serotonin from the synapse and returns it to the presynaptic neuron, recycling the neurotransmitter.
SERT is the target
Q4: Why is serotonin reuptake important for neural signaling?
A4: It terminates the signal and allows serotonin to be reused, maintaining neurotransmitter balance.
Q1: How is serotonin cleared from the synaptic cleft?
A1: By serotonin reuptake transporters (SERTs), which remove serotonin from the synapse and return it to the presynaptic neuron.
Q2: What type of transporter is SERT?
A2: It is a Na⁺-coupled symporter that co-transports sodium ions with serotonin.
Q3: What type of transport mechanism does serotonin reuptake use?
A3: It uses secondary active transport, powered by the Na⁺ gradient maintained by the Na⁺–K⁺ ATPase.
Q4: What is the purpose of serotonin reuptake?
A4: It resets the synapse for future signaling and recycles serotonin for reuse.
Q1: What do SSRIs (Selective Serotonin Reuptake Inhibitors) do?
A1: They bind to SERTs and block serotonin reuptake into the presynaptic neuron.
Q2: How do SSRIs affect serotonin levels in the synapse?
A2: They prolong serotonin presence in the synaptic cleft by preventing reuptake.
Q3: What is the therapeutic effect of SSRIs?
A3: They enhance receptor signaling and improve mood in patients with depression.
Q4: What is the main target of SSRIs in neuronal signaling?
A4: The serotonin transporter (SERT).
Q1: What are SSRIs primarily used to treat?
A1: They are frontline antidepressants used to treat depression.
Q2: Why are SSRIs preferred for treating depression?
A2: Because they have high efficacy and relatively low side effects compared to other antidepressants.
Q3: What are two common examples of SSRIs?
A3: Prozac and Zoloft.
Q4: Besides depression, what other conditions can SSRIs treat?
A4: Anxiety disorders, obsessive-compulsive disorder (OCD), and post-traumatic stress disorder (PTSD).
Q5: How do SSRIs support mood regulation?
A5: By enhancing serotonin signaling, which stabilizes and improves mood.
Q1: What can cause high blood pressure (hypertension)?
A1: Excess sodium, which increases blood volume via osmosis.
Q2: How does sodium affect blood volume?
A2: Sodium draws water into the bloodstream by osmosis, increasing blood volume and therefore raising blood pressure.
Q3: What organ plays a key role in regulating sodium levels?
A3: The kidneys.
Q4: What are diuretics and how do they lower blood pressure?
A4: Diuretics are drugs that increase renal excretion of sodium and water, lowering blood pressure by reducing blood volume.
Q1: Where is the Na⁺–Cl⁻ co-transporter located in the kidney?
A1: In the distal convoluted tubule of the nephron.