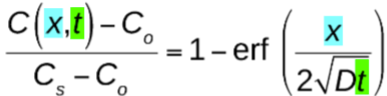Ch 6: Diffusion
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
how do gases and liquids diffuse?
through random (Brownian) motion
how do solids diffuse?
through vacancy diffusion and interstitial diffusion
what is interdiffusion?
diffusion of atoms from one material into another material
what is self diffusion?
migration of host atoms in pure metals
what is vacancy diffusion?
when atoms and vacancies exchange positions
random drifting around
what is interstitial diffusion?
when interstitial atoms move from one interstitial position to another
smaller atoms move between different spaces created by bigger atoms
interstitial diffusion is faster/slower than vacancy diffusion
faster
what is case hardening?
when the outer surface of a material is hardened by diffusing carbon atoms into the surface
example of interstitial diffusion
presence of C atoms makes iron (steel) harder
what is doping?
when very small concentrations of atoms (impurity) are diffused into a semiconductor silicon
what is the rate of diffusion expressed as?
flux (how much moves into a specific area over time)
ratio of mass diffused over the product of area and time
what is the equation of the rate of diffusion?
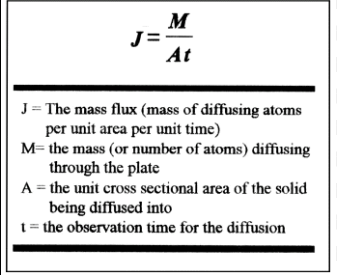
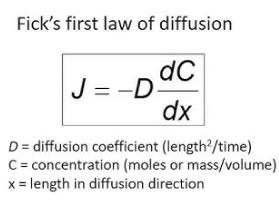
what is steady-state diffusion?
when flux is proportional to concentration gradient (rate of diffusion) → Fick’s First Law of Diffusion
there is a relatively even transition of matter
dC = C2 - C1
dx = x2 - x1
what is chemical protective clothing (CPC) an example of?
diffusion
how does temperature influence diffusion?
as temperature increases, diffusion coefficient increases
D and T have an exponential relationship!
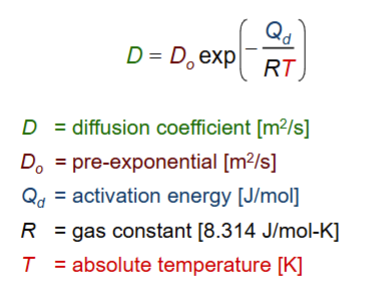
what equation relates diffusion coefficients at two temperatures?
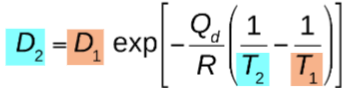
what law represents non-steady state diffusion?
Fick’s Second Law of Diffusion
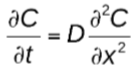
what does Fick’s Second Law assume?
that diffusion is independent of concentration
what is another equation for non-steady state diffusion that accounts for error?
error function → erf(z) = 0.8125