Electromagnetic Radiation Principles
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Wave Model of EMR
Relationship between wavelength and frequency is inverse
i.e., gamma rays are the shortest and they have a high frequency
Wavelength that are shorter have the most amount of energy while the longest have to lowest energy
EMR was conceptualized as an electromagnetic (EM) Wave by James Clerk Maxwell in 1860s
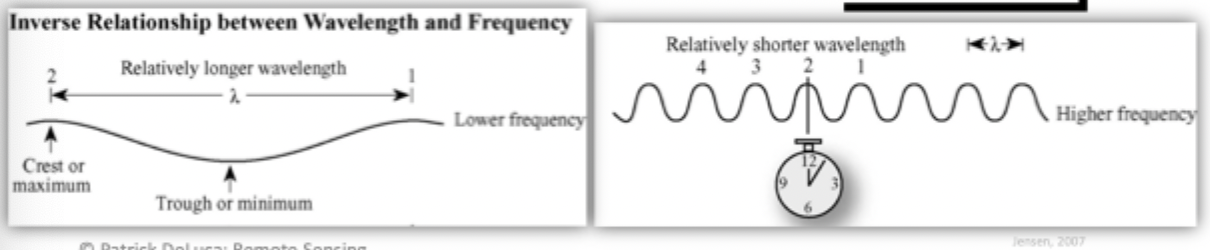
Wavelength
of EMR depends on length of time that a charged particle is accelerated
Mean distance between maximums(or minimums) or a roughly periodic pattern, normally measured in micrometers(micrometers or nanometers)
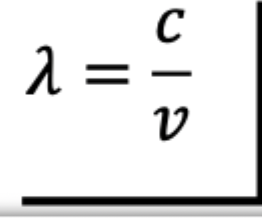
Frequency
or EMR depends on number of accelerations per second(typically periodic)
Number of wavelength that pass a point unit time.
a wave that sends one crest every second(1 complete cycle) is said to have a frequency of one cycle per second or one hertz, abbreviated 1 Hz
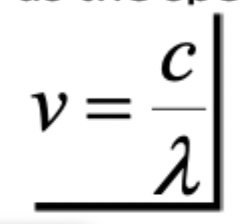
What produces EM energy?
all objects above absolute zero(-273°C or 0K)
The sun produces a continuous spectrum of EMR ranging from a very short, extremely high frequency gamma waves to long, very low frequency radio waves
We can think of the Sun as a 6000 K blackbody
Stefan-Boltzmann Law
The total emitted radiation (Mwavelength) from a blackbody is proportional to the fourth power of its absolute temperature.
σ is the Stefan Boltzmann constant:
5.6697 × 10-8 W m-1 K-4
it determines how much radiation is emitted based on its temperature
energy emmited is a function of temperatures
part of the wave model
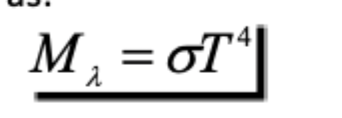
Wien’s Displacement Law
the peak wavelength of radiation emitted by a blackbody is inversely proportional to its absolute temperature
meaning that as an object gets hotter, the wavelength of its peak emitted light becomes shorter.
can determine the dominant wavelength(Where is the spectrum the radiation is most intense, what wavelength is the object emitting most?)
Wavelengthmax
***where “k” is a constant equaling 2898 μm K and T is the absolute temperature in Kelvin
i.e., if the sun approximates a 6000K blackbody, its dominant wavelength is :
0.483 μm = 2898 μm K/ 6000K
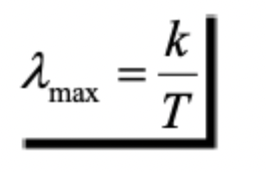
What is the Suns Dominant Wavelength?
0.48μm(blue-green light)
What is the Earths Dominant Wavelength?
9.66μm
EMR and Spectral Resolution
In remote sensing, spectral resolution is the ability of a sensor to detect distinct ranges of wavelengths within the EMR
Sensors divide the electromagnetic spectrum into several "bands", "channels" or “regions”. Each channel captures information within a specific wavelength range.
Spectral signatures: Different materials (like healthy vegetation, soil, or water) absorb and reflect EMR in unique ways at different wavelengths.

Max Planck(1900)
describe spectral distribution of radiation
proposed that energy is emitted or absorbed in discrete amounts, or quanta
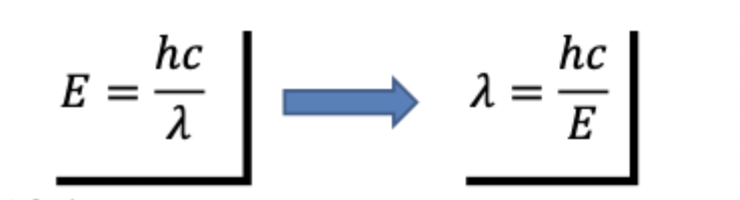
Plancks Radiation Law: E = hv
E= energy of Photon
h = Planck constant
v = frequency
Albertas Input on Plancks Law
proposed light consists of energy packets or particles, each carrying energy(E=hv)
the particles were named photons
each reacts with matter like a particle, but also have wave like properties
Quantum Theory of EMR
posits that EMR energy is not continuous but comes in discrete packets called photons
the relationship between the frequency of radiation is expressed by wave theory and the quantum theory is : E= hv
where E is the energy of a quantum measured in Joules h is the Planck constant (6.626 × 10-34 J·s), and v is the frequency of the radiation
Energy of a quantum is inversely proportional to its wavelength(i.e., the longer the wavelength involved the lowers ints energy content
** it is more difficult to detect longer wavelengths compared to shorter visible wavelengths
Creation of Light From Atomic Particles
photon of light is emitted when an electron drops from a higher energy state to a lower energy state
Add energy = electrons will jump up and go up levels for 10 ^-8? Seconds then it will go back down to the next available level and release the enregy it had
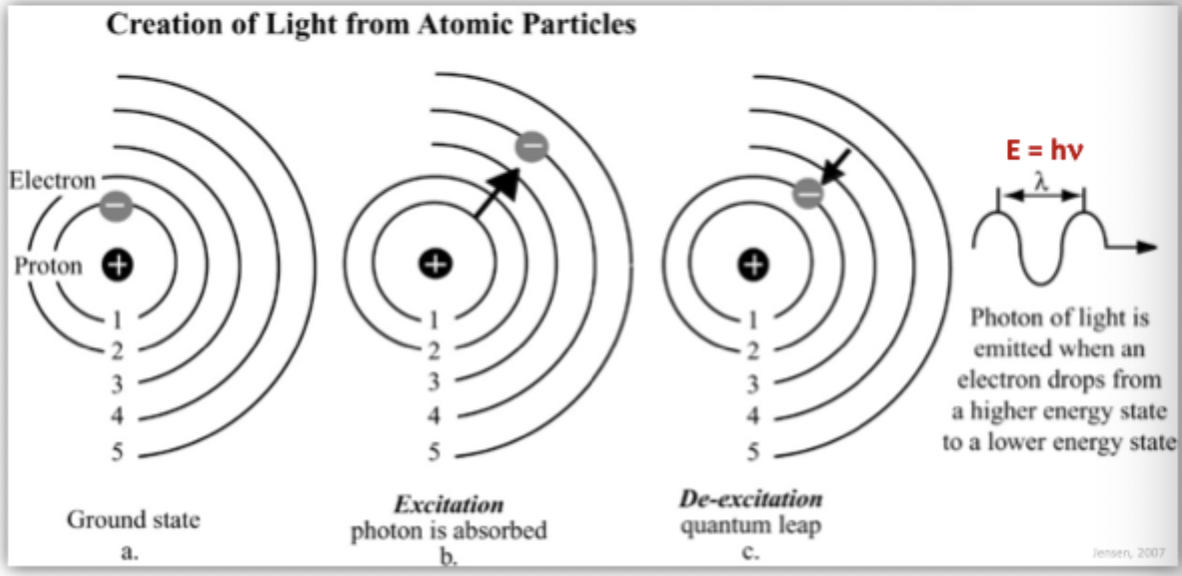
Photo Electric Effect
is physical mechanism insides optical detectors to convert photons to electric signals
photons hit a detector material
if their energy is above a threshold the free electrons
the freed electrons create an electrical signal that is crowded to digital
The Particle Model
Behaviour of EM radiation as photon
E=hv
used to show hoe photons interact with matter and how sensors detect them
energy quantization detection
EMR and the Atmosphere
EMR goes through space in at the speed of light in a vacuum
The atmosphere affects the speed, wavelength, intensity spectral distribution, and/or direction of EMR
refreation, scattering, and absorption happens before things hit the ground; when it does hit the ground more interactions happen again
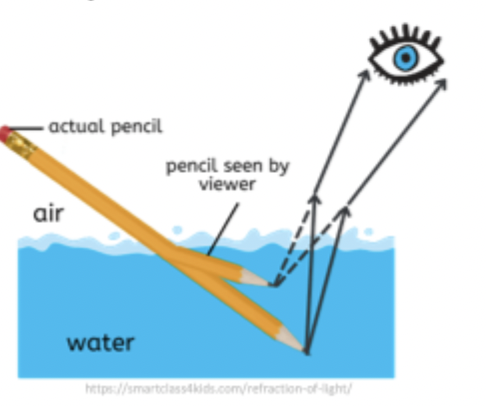
Refraction
occurs when EMR encounters substances of different density, i.e., air to water; space to atmosphere
Bending or light when it passes from one medium to another of different density
Index of Refraction, n
measure of the optical density of a substance
speed of light in a vacuum and how it changes in a substance
n is always >1
n of atmosphere = 1.0002926
n of water = 1.33

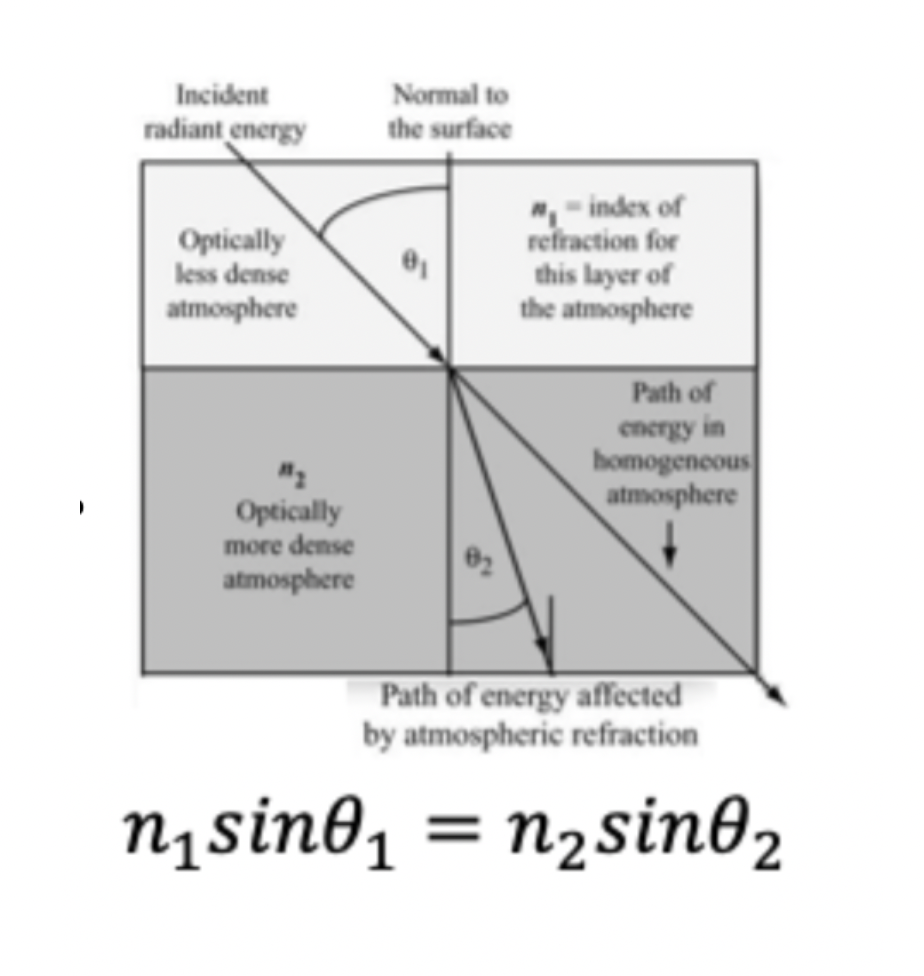
Snells Law
for a given frequency of light, the product of the index of refraction and the sine of the angle between the ray and a line normal to the interface is constant
If we know the insect of refraction we can find out how much it is?
in RS understanding light behaviour can help explain distortion in imagery or discrepancy in measurement for distance and elevation(optical path calculations)
most atmospheric correction involves snells law
When is Refraction Most Important in RS
near the horizon at oblique angles there is noticeable bending
the more oblique the more the problem grows
high res. or long range systems
microwave RS affecting signal delays
Refraction Impact on RS
affects RS the least compared to the other interactions
causes geometric distortions: take longer to georeference
Positional error in platforms with low look angles
Impacts on calibration of RS instruments and
atmospheric correction of imagery
Atmospheric Scattering
is the unpredictable dispersal of radiation by particles in the atmosphere
It doesn’t change the energy’s wavelength, but it can change its direction, and therefore, what actually reaches the sensor
Different kinds of particles cause different types of scattering, depending on their size relative to the wavelength of the radiation
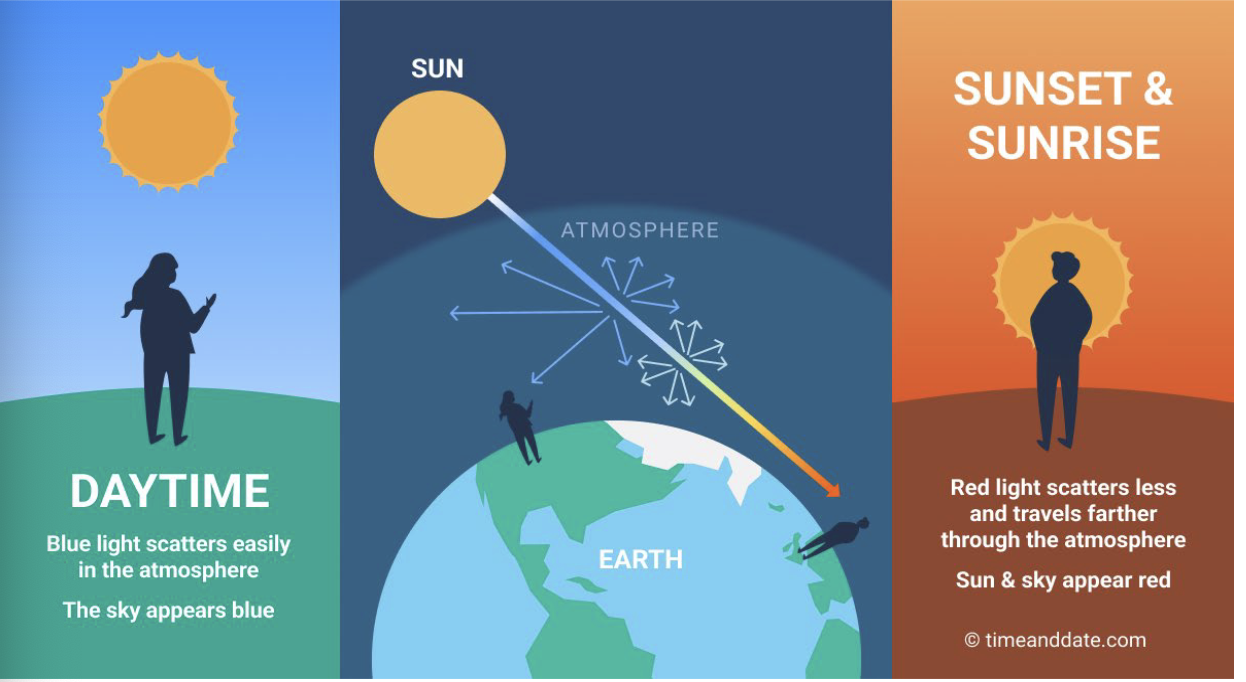
Rayleigh Scattering (aka molecular scattering)
Takes place in the atmosphere 2 to 8km above ground
Occurs when particles are very small compared to λ
Could be small specks of dust or N 2 and O2 molecules
Scattering amount inversely related to 4 th power of radiation's wavelength. e.g., blue (0.4 μm) scattered 9.4 times more than red (0.7 μm), i.e., (0.7/0.4)4 =9.4
Mie Scattering
takes place in the lower 4.5km of the atmosphere
Occurs when the particles are just about the same size as the wavelength of the incident EMR
The amount of scatter is greater than Rayleigh scatter and the wavelengths scattered are longer

Non - Selective Scattering
Occurs in the lowest portion of atmosphere
- Occurs when diameter of particles are larger than the
wavelength of radiation (> 10 times)
- Water droplets and large dust particles
- All wavelengths of light are scattered equally
Impacts of Scattering on Imagery
Reduced image clarity and contrast: scattered light adds
haze, especially in visible bands
Radiometric distortion: adds extra light to what a sensor
receives
Atmospheric path radiance: light reaching sensor
without ever touching the ground
Colour balance issues: uneven scattering across
wavelengths can alter colour balance.
Obscures or masks surface features: smoke, dust, haze
Increases need for atmospheric correction algorithms