VESPR Theory
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
EG- Linear MG- Linear
2 regions, 180 angle, 0 lone pairs
EG- trigonal planar, MG- trigonal planar
3 regions, 120 angle, 0 lone pairs
EG- Trigonal planar, MG- Bent/ V shaped
3 regions, <120 angle, 1 lone pair
EG- tetrahedral, MG- Tetrahedral
4 regions, 109.5 angle, 0 lone pairs
EG- tetrahedral, MG- Trigonal pyramidal
4 regions, <109.5 angle, 1 lone pair
EG- Tetrahedral, MG- Bent/ V shaped
4 regions, <109.5, 2 lone pairs
EG- Trigonal Bipyramidal, MG- Trigonal Bipyramidal
5 regions, 90 and 120, 0 lone pairs
EG- trigonal bipyramidal, MG- Seesaw
5 regions, 90 and <120, 1 lone pair
EG- trigonal Bipyramidal, MG- T shaped
5 regions, 90 and 180, 2 lone pairs
EG- trigonal Bipyramidal, MG-linear
5 regions, 180, 3 lone pairs
EG- Octahedral, MG- Octahedral
6 regions, 90, 0 lone pairs
EG- Octahedral, MG- Square Pyramidal
6 regions, 90, 1 lone pairs
EG- octahedral, MG-square planar
6 regions, 90 degree angle, 2 lone pairs
EG- Octahedral, MG- T shaped
6 regions, 90 and 180, 3 lone pairs
EG- Octahedral, MG- Linear
6 regions, 180, 4 lone pairs
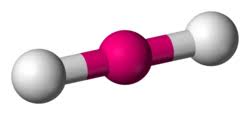
What is this?
Linear
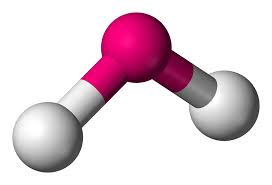
What is this?
Trigonal Planar
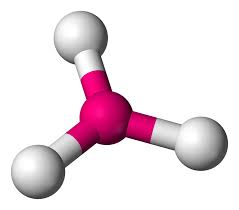
What is this?
Bent (V-shaped)
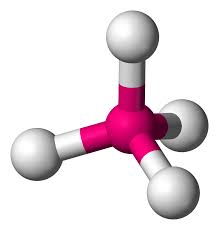
What is this?
tetrahedral
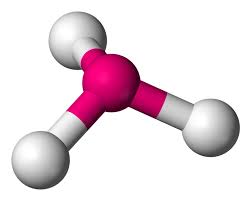
What is this?
Trigonal Pyramidal
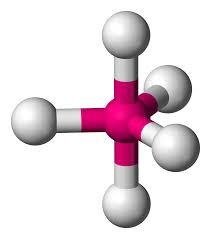
What is this?
Trigonal Bipyramidal
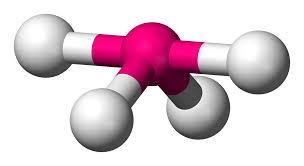
What is this?
Seesaw
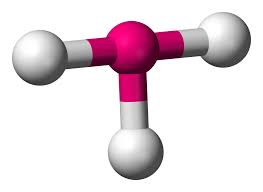
What is this?
T-shaped
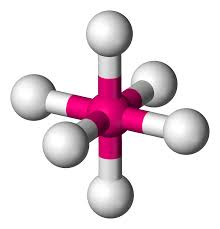
What is this?
Octahedral
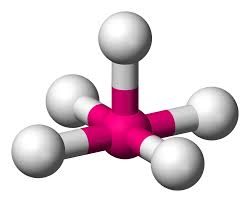
What is this?
Square Pyramidal
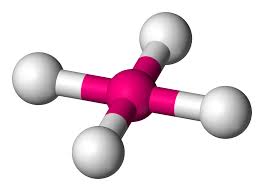
What is this?
square planar
Electronic Geometry
Looks at all pairs of electrons, lone pairs and those involved in bonding
Molecular Geometry
Only looks at pairs of electrons involved in bonding, does NOT include lone pairs
The more lone pairs
the smaller the bond angles will be