GEN CHEM 1: EXAM I
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
Chemistry
the study of matter
matter
the stuff that makes up material things
mass
the measure of the quantity of matter (grams)
physical change
a process in which the chemical identity of the materials involved do not change
chemical change
a process in which the chemical identity of the materials involved change
law of conservation of mass
Matter is neither created nor destroyed
element
Atom
a basic unit of matter
chemical compound
pure substance
mixture
substances that can be separated further by physical changes
homogenous mixture
a mixture in which the components are completely mixed down to the level of molecules and ions
heterogeneous mixture
a mixture in which the components are not completely mixed down to the level of molecules and ions
Celcius
K-273.15
Farenheit
9/5C+32
True or False: 1cm^3= 1mL
true
volume of a circle
4/3πr³
What does x symbolize?
elemental symbol
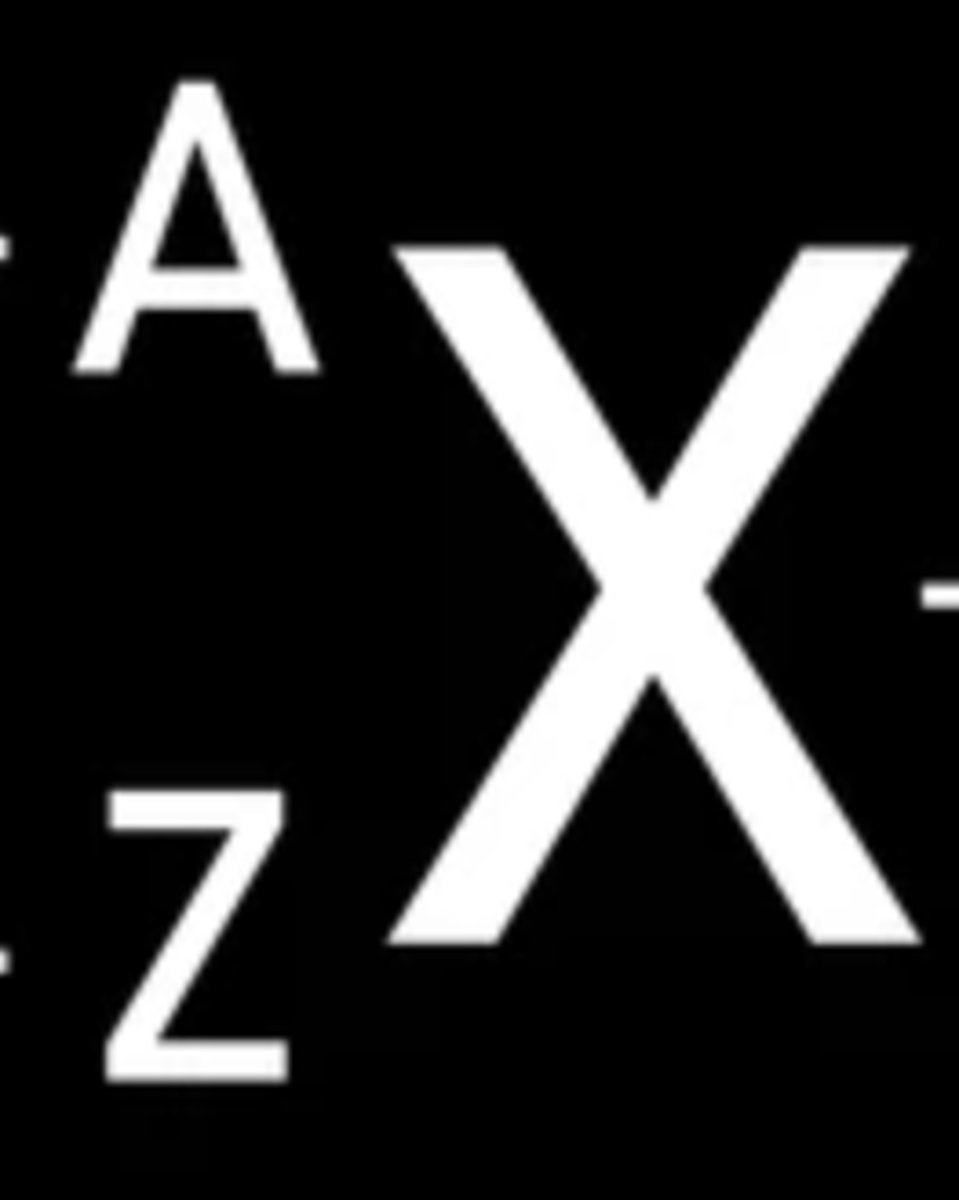
What does Z symbolize?
atomic mass
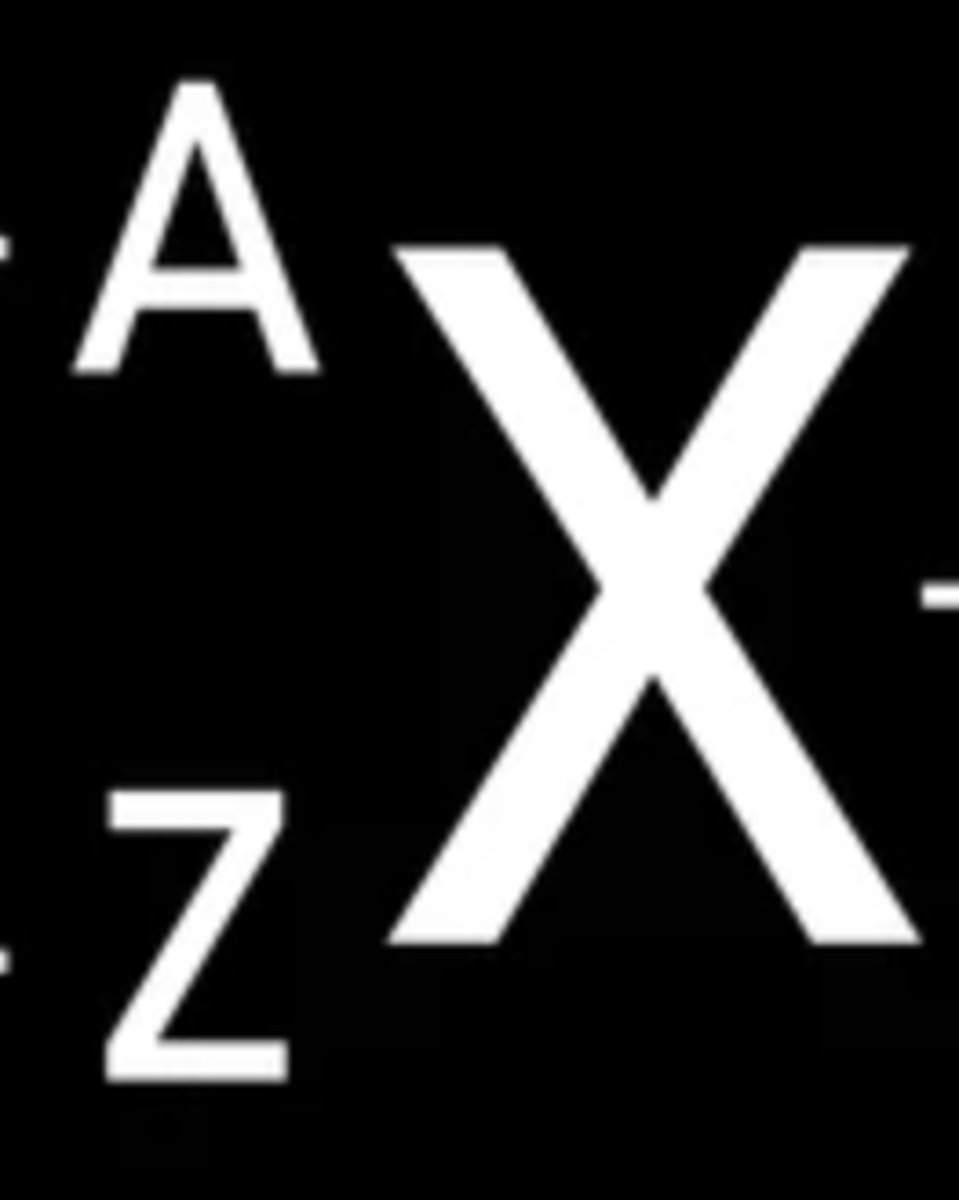
What does A symbolize?
atomic number
Isotopes
Atoms with the same number of protons but different numbers of neutrons
atomic weight
Average mass of a particular atom
ion
A charged atom
cation
A positively charged ion
anion
A negatively charged ion
Carbonate
CO3 2-
Sulfite
SO3 2-
Sulfate
SO4 2-
Phosphate
PO4 3-
dihydrogen phosphate
H2PO4-
Cyanide
CN-
Nitrate
NO3-
Hydroxide
OH-
Permanganate
MnO4-
Chlorate
ClO3-
Hypochlorite
ClO-
Hydrogen Carbonate
HCO3-
Hydrogen Sulfite (bisulfite)
HSO3-
Hydrogen Sulfate (bisulfate)
HSO4-
Hydrogen Phosphate
HPO4 2-
Chromate
CrO4 2-
Acetate
C2H3O2-
Nitrite
NO2-
Dichromate
Cr2O7 2-
Perchlorate
ClO4-
Chlorite
ClO2-
Ammonium
NH4+