SN1, SN2, E1, E2
1/140
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
141 Terms
SN1
polar solvent, HX nucleophile
SN1
tertiary halogenoalkanes react in this method; first the halogen breaks off then the nucleophile attaches onto the positively charged carbon
SN1
The mechanism that forms a carbocation in the rate-determining step is the _____ mechanism.
SN1
addition of an acid, carbo-cation formation, addition of nucleophile
SN1
reaction type that needs highly substituted substrate and any nucleophile
SN1
Involves two steps. Step 1: The dissociation of a molecule into a carbocation and a good leaving group. Step 2: Combination of the carbocation with a strong nucleophile. The slowest step is the formation of the carbocation. Structural factors and solvent effects may accelerate the formation of the carbocation. Result is a loss of optical activity. First order kinetics; K=[A].
SN2
CH3X>primary>secondary, 1 step, nucleophile attachs as good LG leaves
SN2
Primary halogenoalkanes follow the _______ mechanism of nucleophilic substitution.
SN2
secondary halide + weakly basic nucleophile + polar aprotic solvent
SN2
The mechanism that forms a transition state with a high activation energy is the _____ mechanism.
SN2
1 step has no carbo-cation
E1
reaction type that needs a highly substituted substrate and any base
E1
2 steps, good LG leaves, electron is moved to form double bond
E1
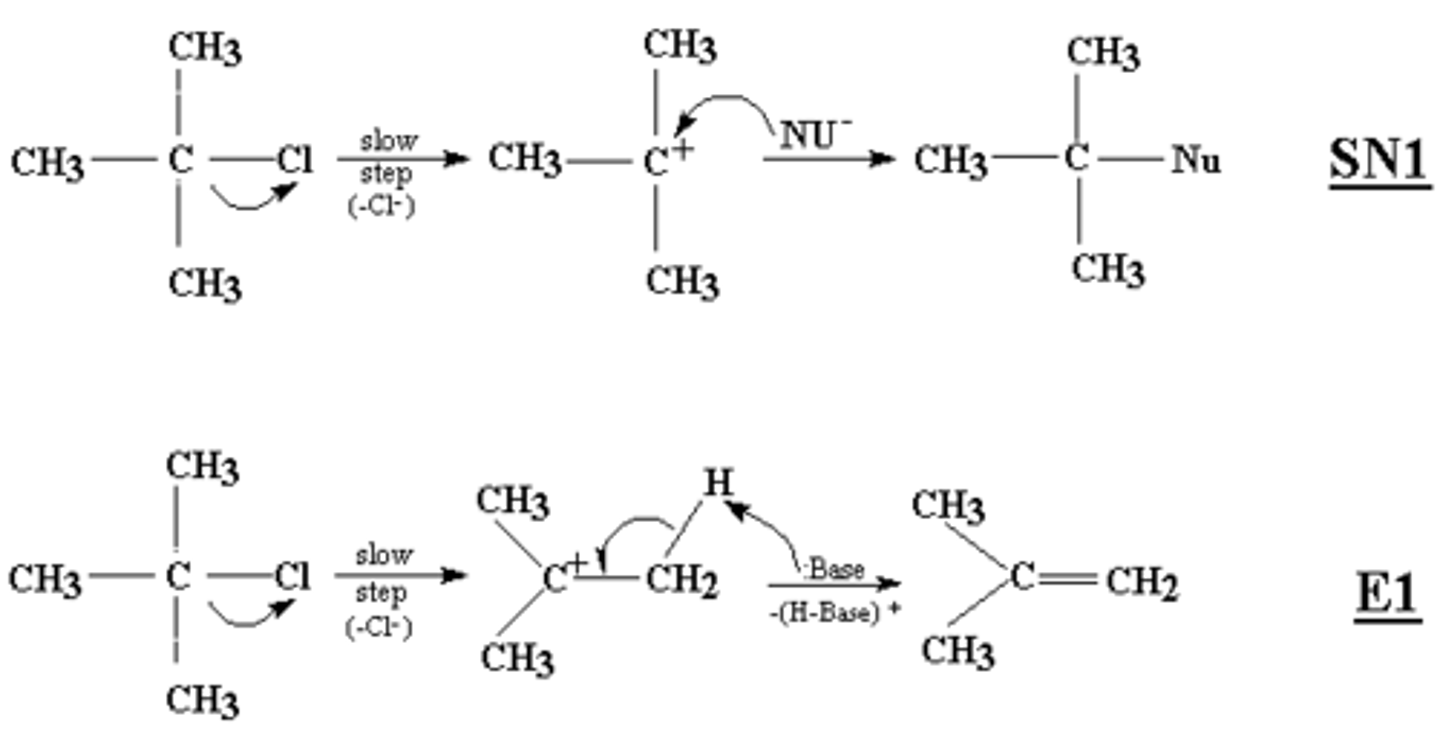
Two step process proceeding through a carbocation intermediate. Rate of reaction dependent on substrate. Elimination of a leaving group and a proton results in the production of a double bond. Step1: Leaving group departs, producing a carbocation. Step 2: Proton is removed by a base.
E1
least likely mechanism out of sn1, sn2, e1, e2
E2
A one-step elimination reaction
E2
Primary halide + strong base +aprotic solvent
E2
reaction type that needs a strong base
E2
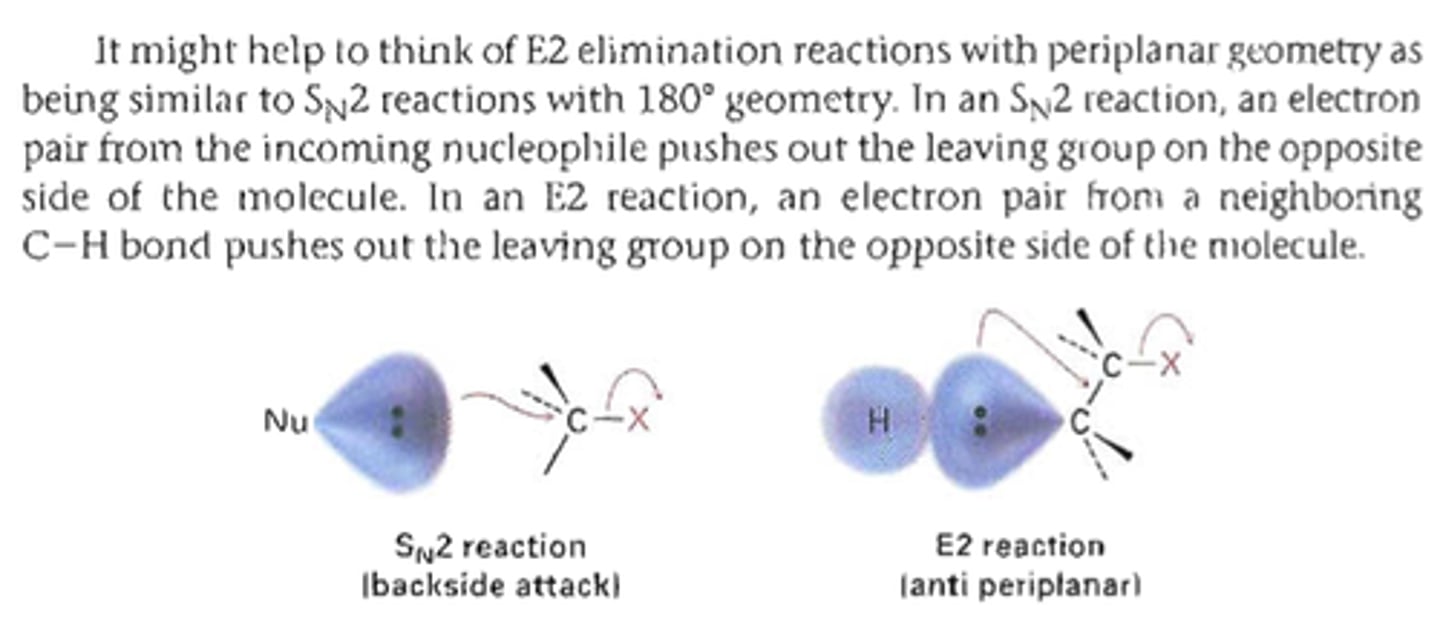
Occurs in one step. A strong base removes a proton, while a halide ion anti to the proton leaves. Steric hinderance does not greatly affect these reactions.
E2
bimolecular elimination, strong base remove proton, halide anti to proton leaves and makes db
What are the types of reactions on Test 2?
SN1, SN2, E1, E2
What is an alkyl halide?
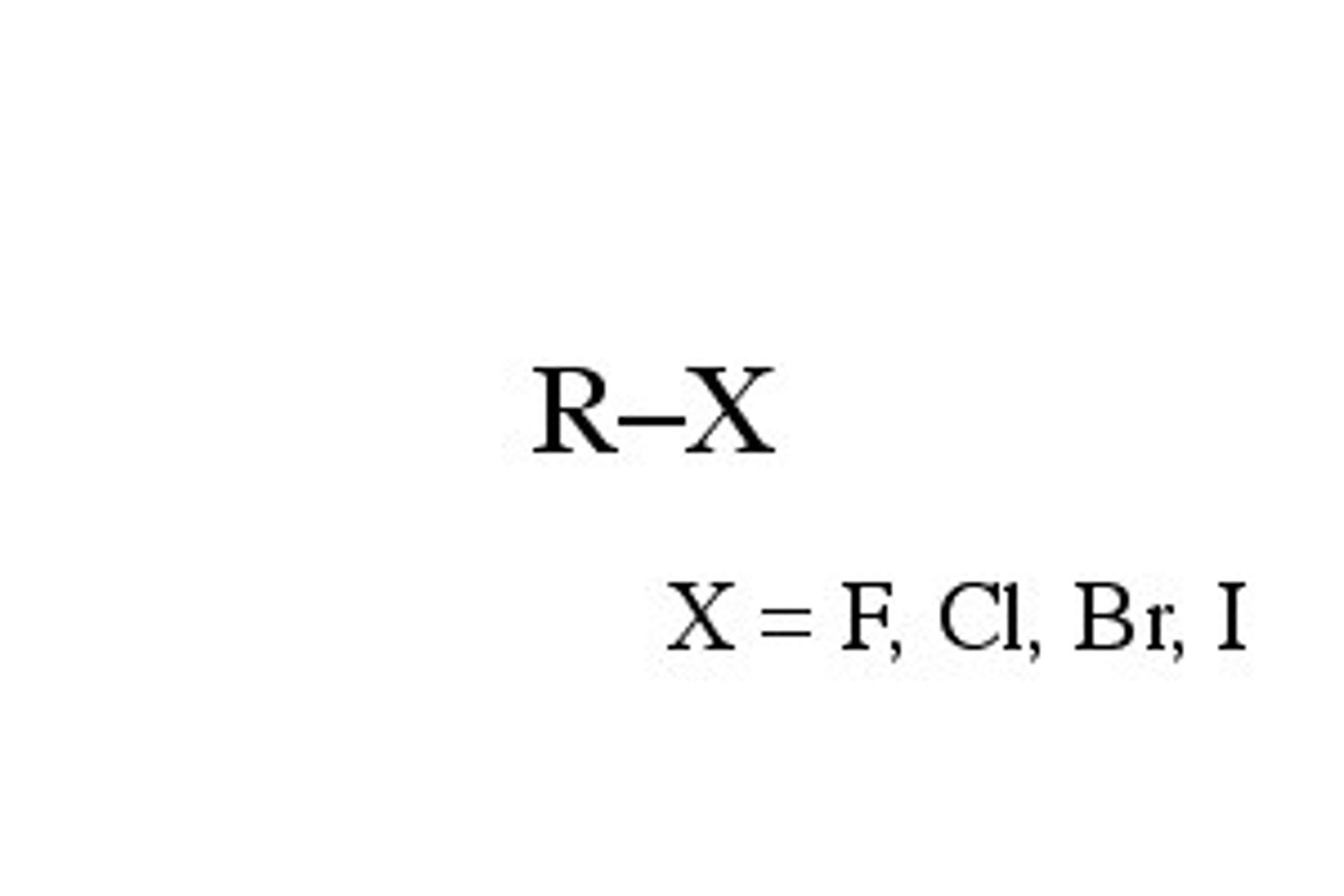
Which reactions can an alkyl halide undergo?
SN1, SN2, E1, E2
What is an SN1 reaction?
An SN1 reaction proceeds by way of a mechanism in which the leaving group dissociates in a kinetically slow step, producing a planar carbocation which is then rapidly attacked by a nucleophile.
Steric congestion tends to promote SN1-type mechanisms, so a tertiary alkyl halide, like t-butyl chloride, would favor pushing the leaving group off to form the carbocation.
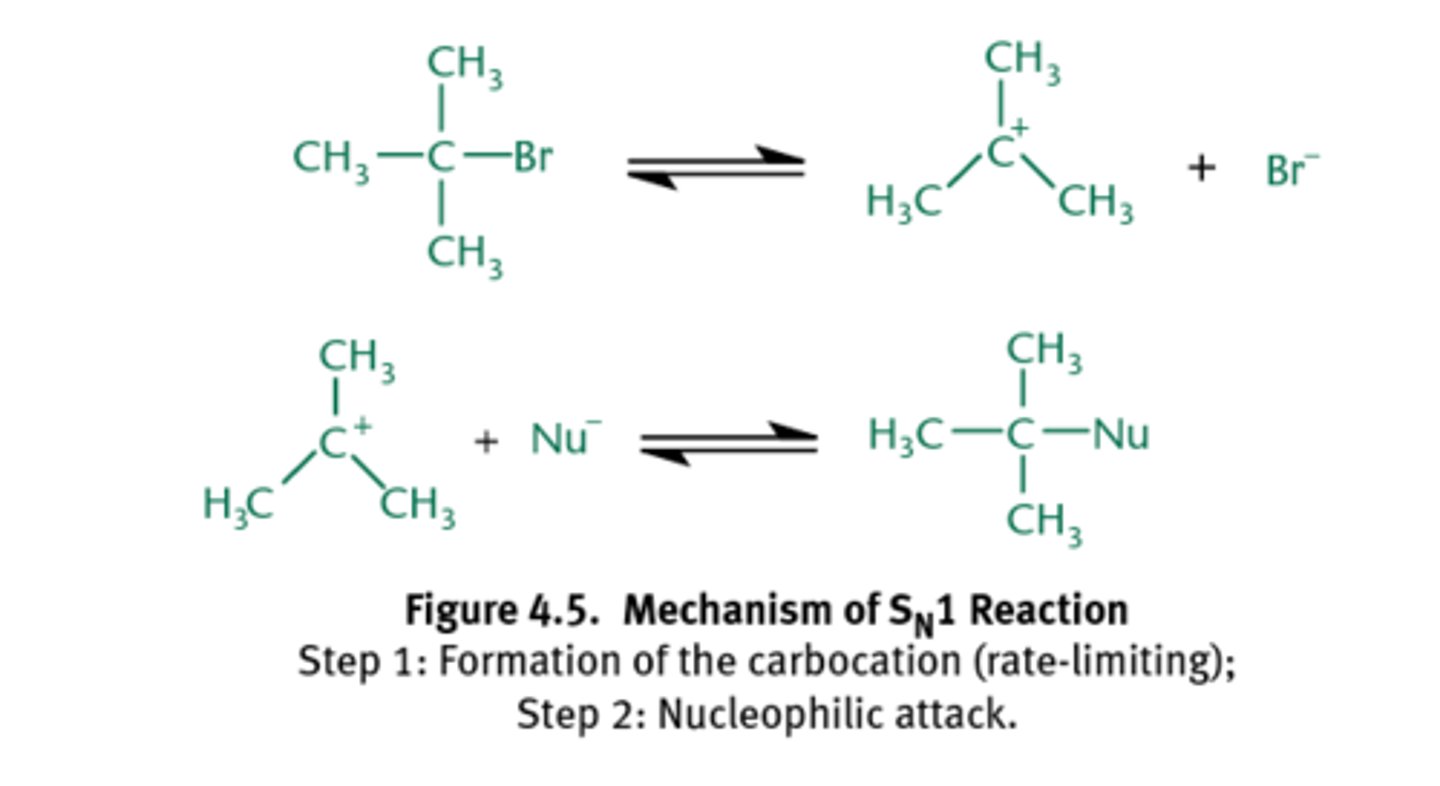
What is an SN2 reaction?
In this mechanism, one bond is broken and one bond is formed synchronously, ie. in one step.
SN2 is a kind of nucleophilic substitution reaction mechanism.
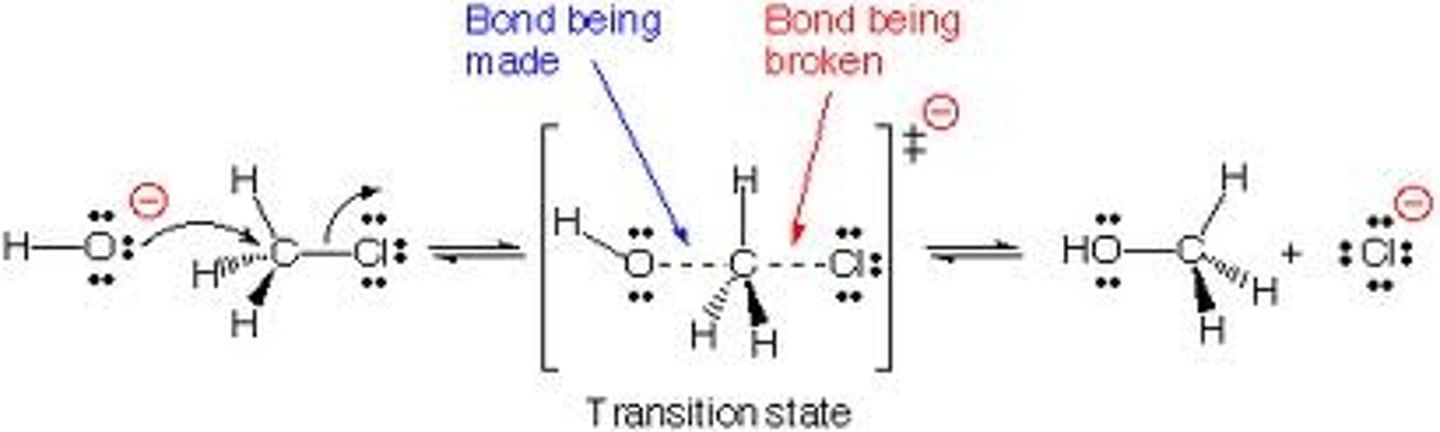
What is an E1 reaction?
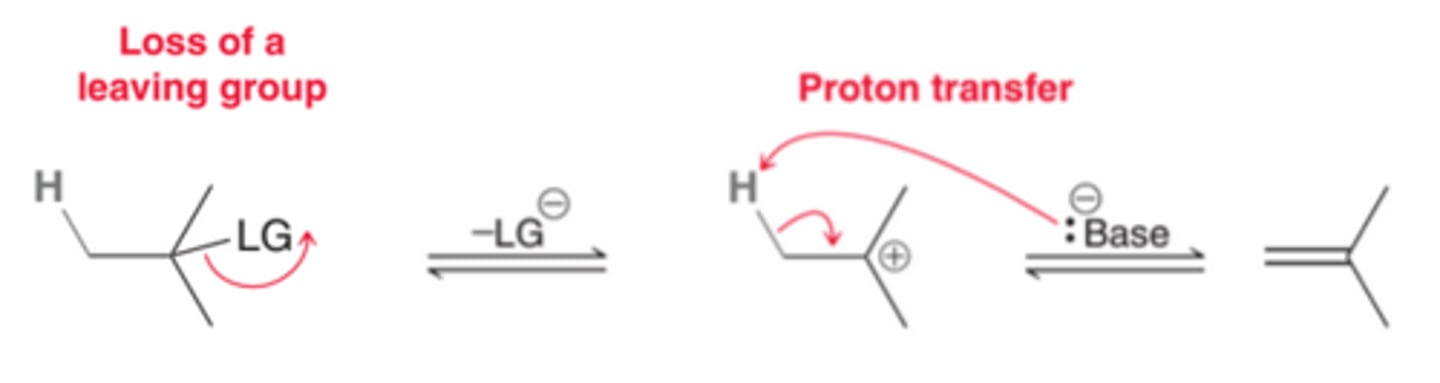
What is an E2 reaction?
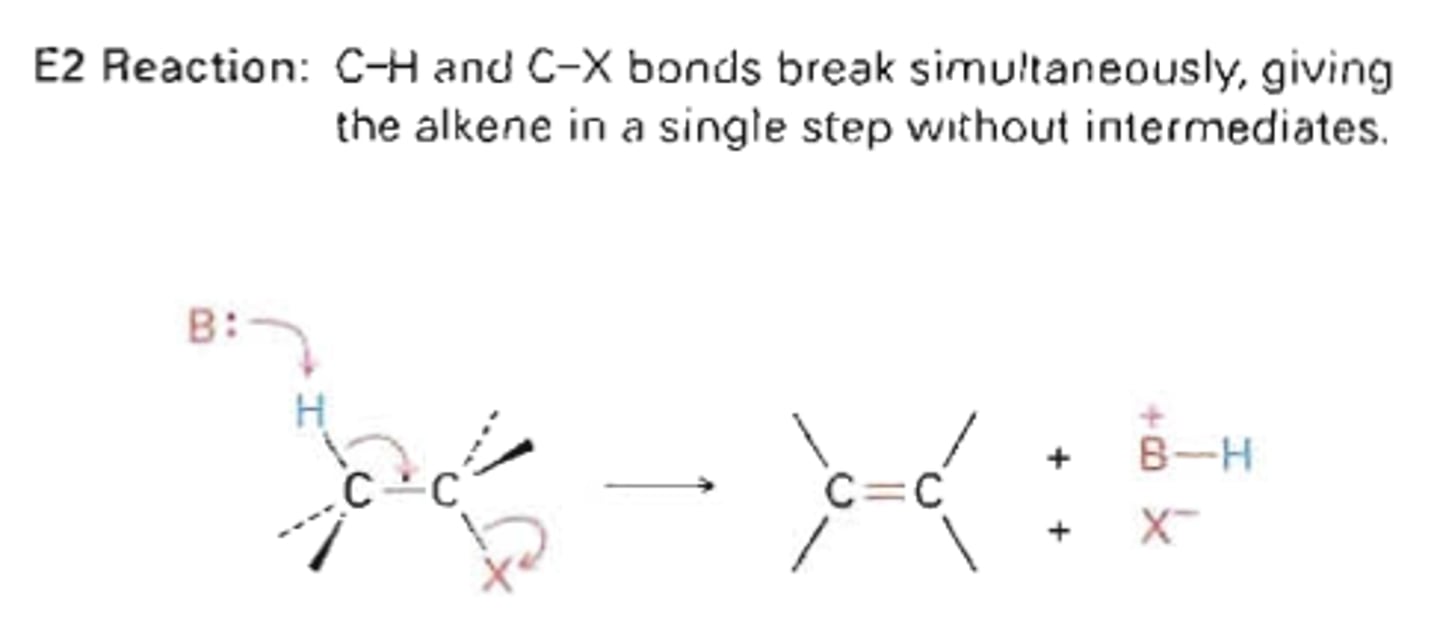
SN1 vs SN2
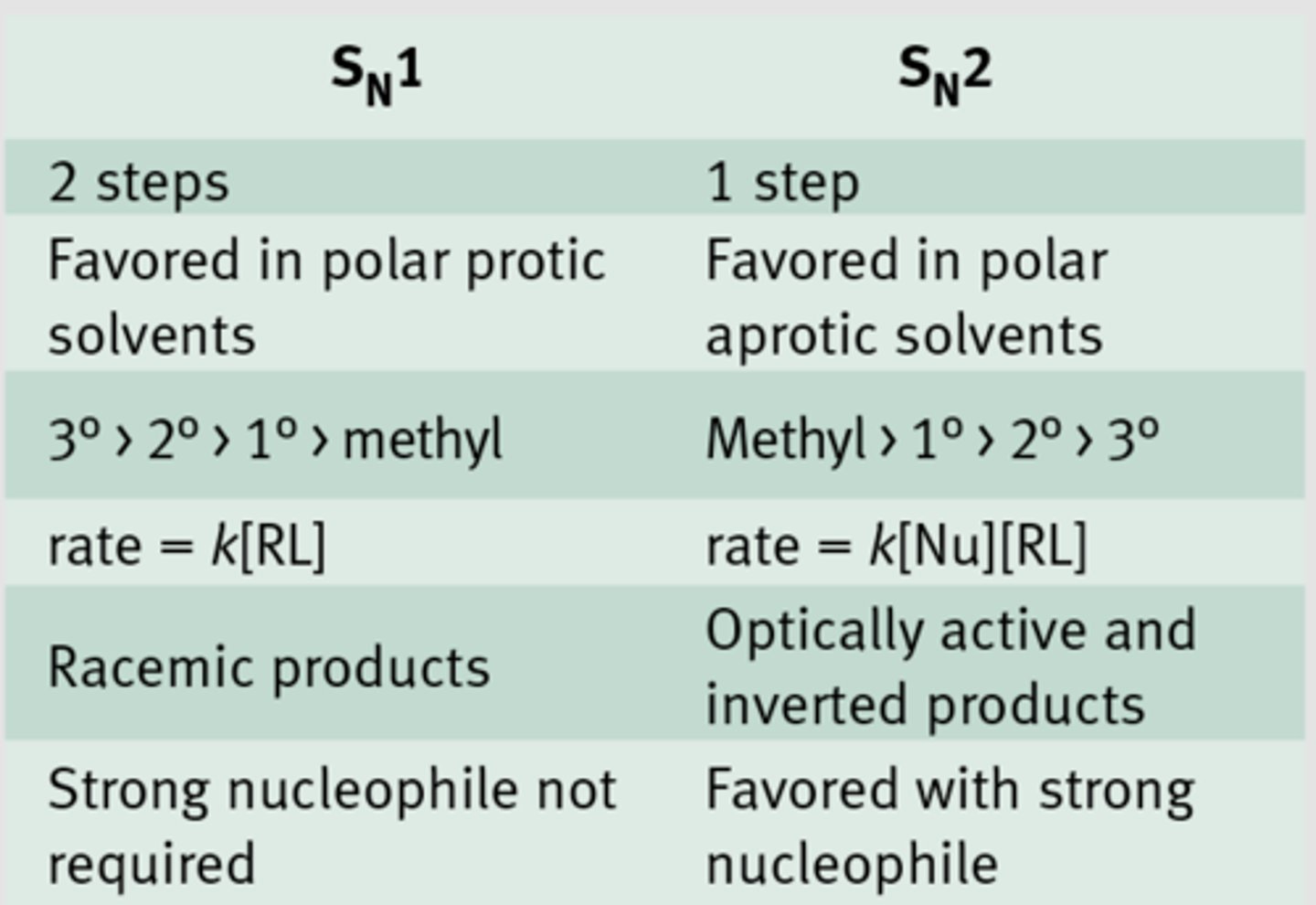
SN1 vs SN2
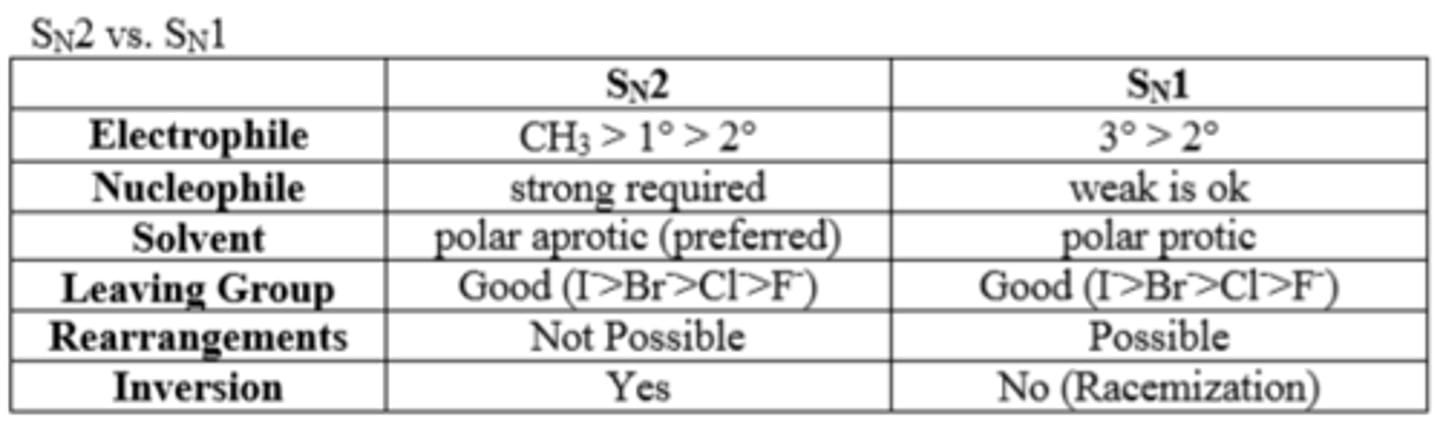
Mechanism diagram:
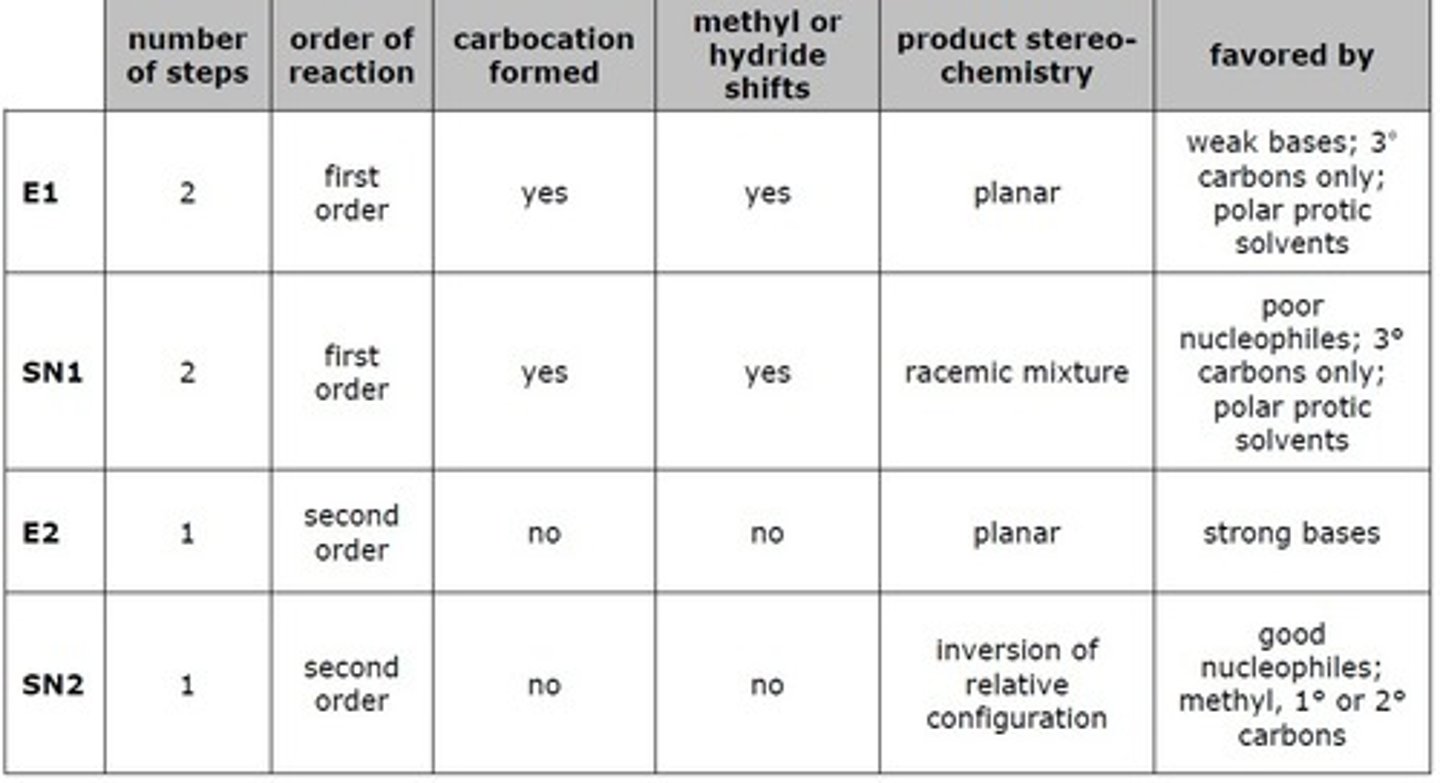
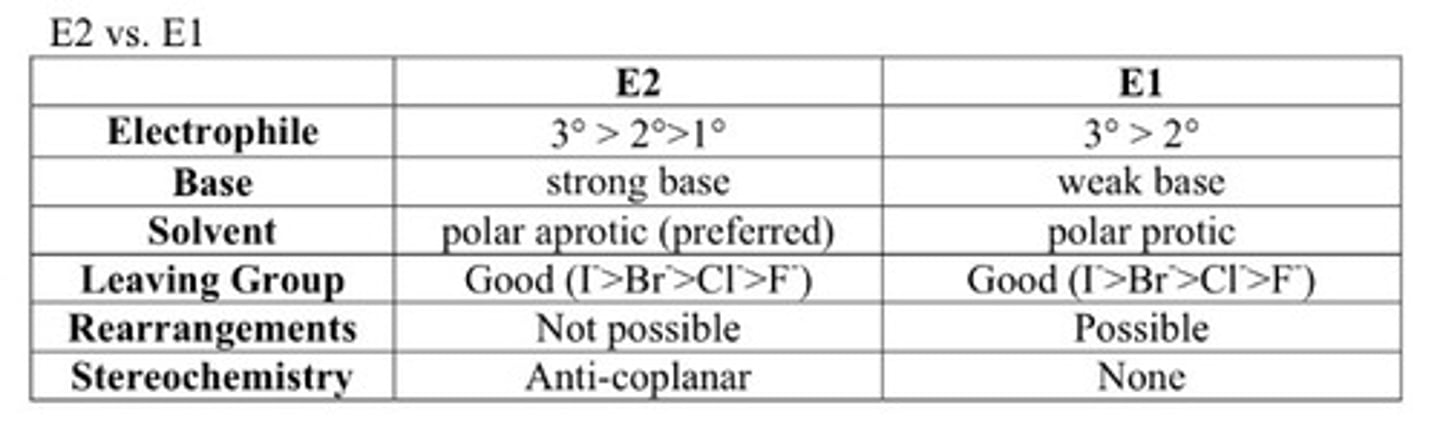
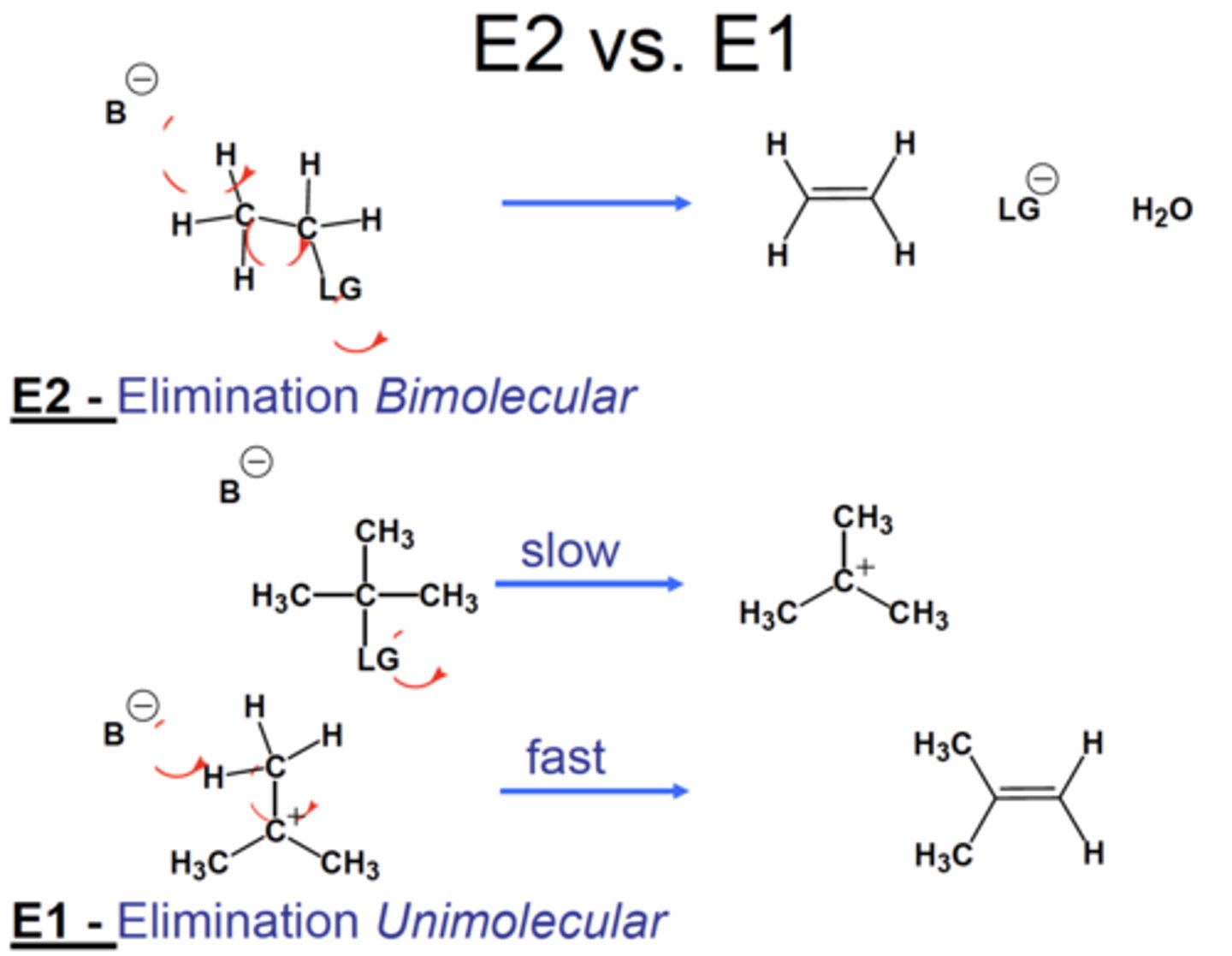
E1 vs E2
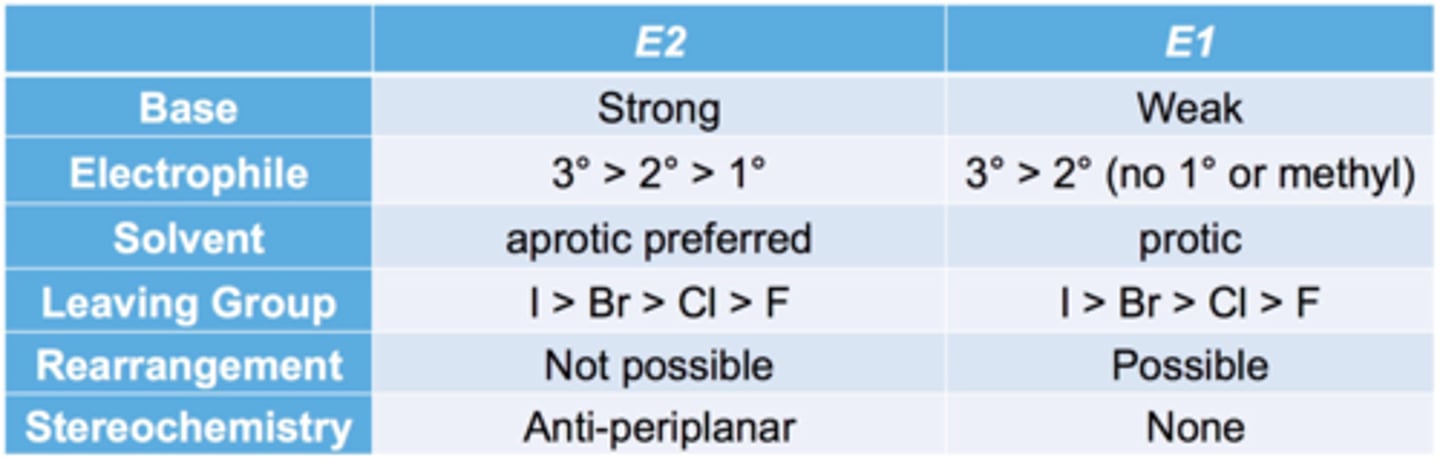
E1 vs E2
Mechanism comparisons:

SN1 vs E1
SN2 vs E2
SN2 vs E2 rates
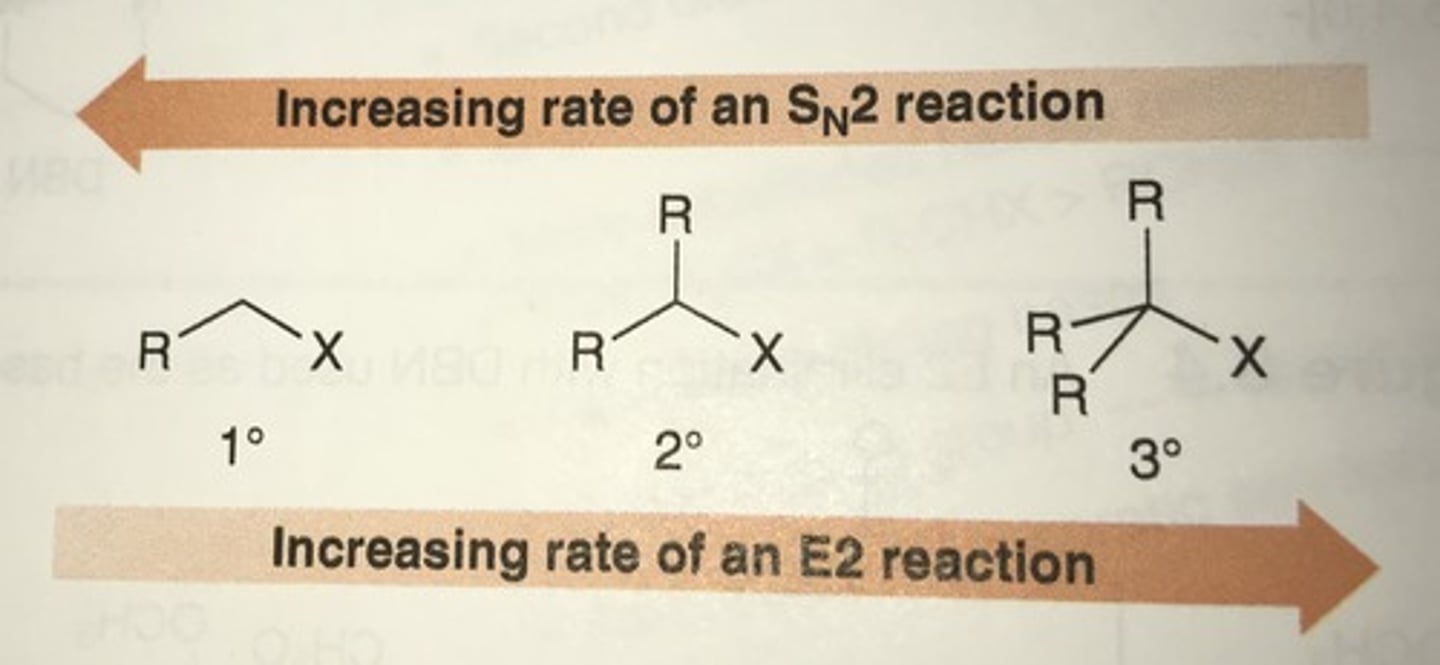
SN1 vs SN2: The nucleophile
SN2 requires a strong nucleophile, while nucleophilic strength doesn't affect SN1.
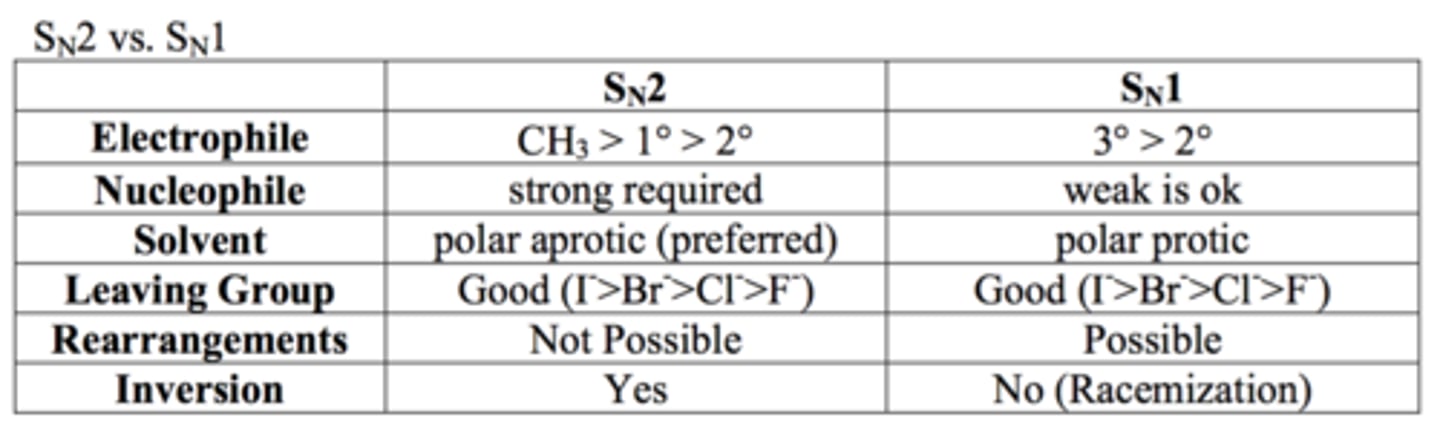
SN1 vs SN2: Solvents
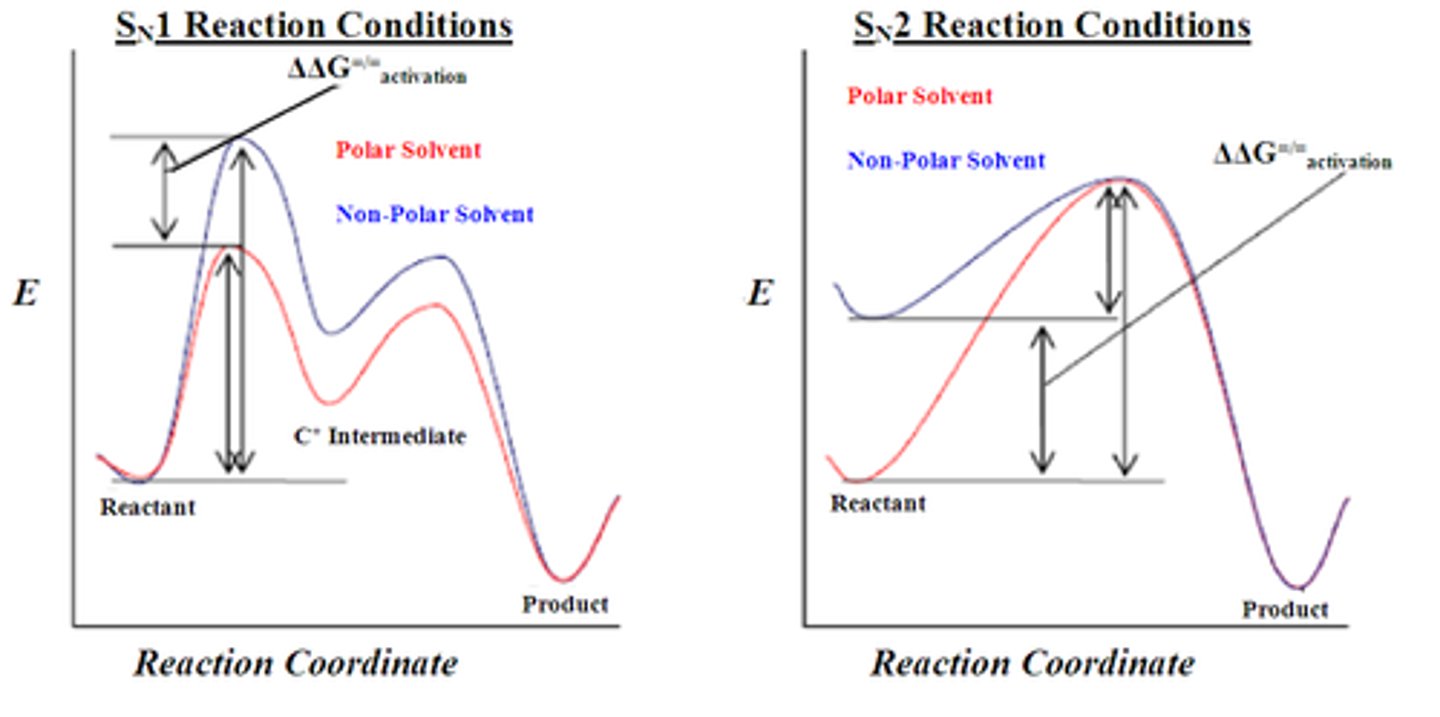
SN1 vs. SN2 reactions
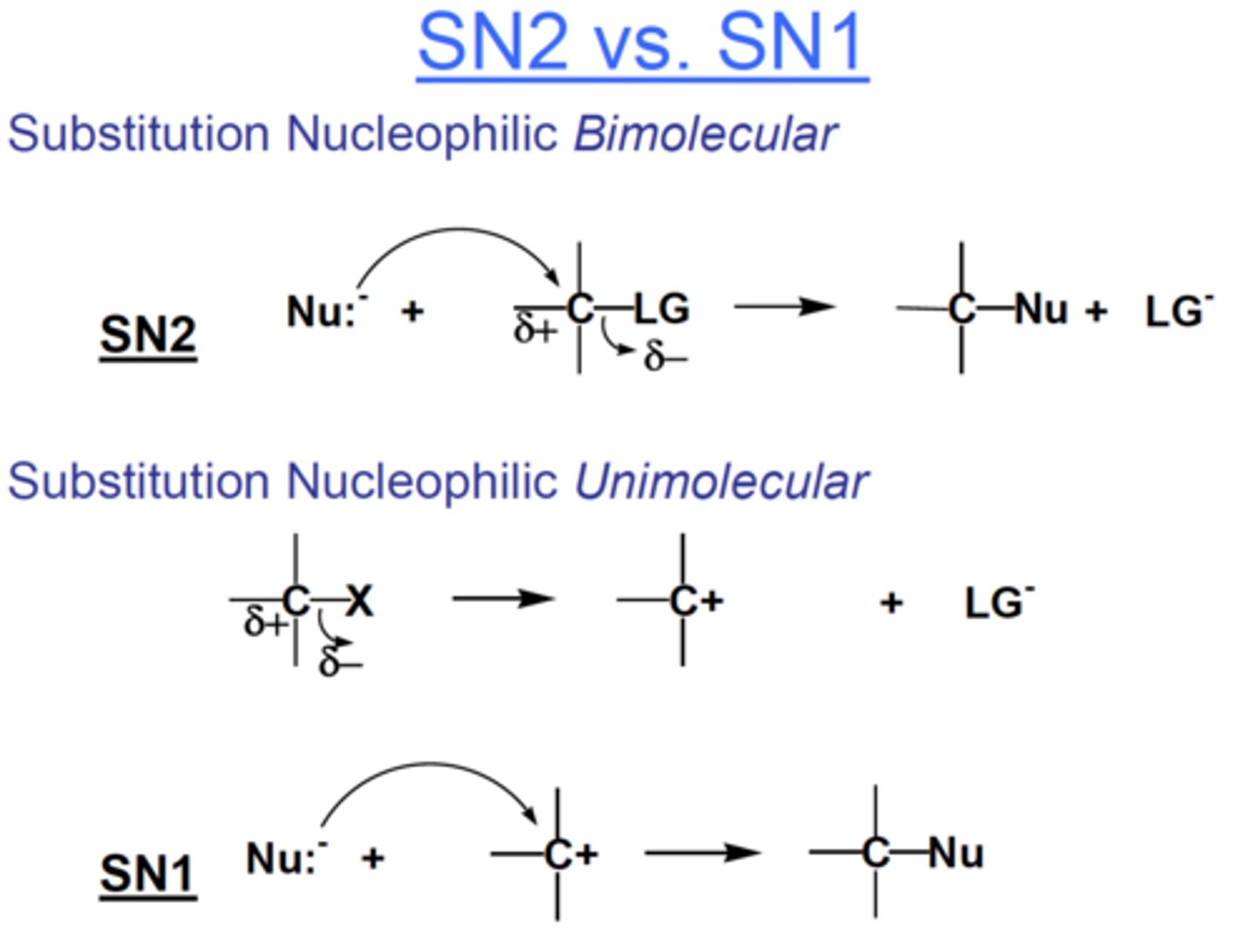
E2 vs. E1 reactions
What is the rate of the reaction dependent on for SN2 reactions?
Concentration of the alkyl halides AND the nucleophile
In SN2 reactions, the rate of the reaction with a given nucleophile ____ with INCREASING size of the alkyl halides
decreases
In SN2 reactions, the configuration of the substituted product is ____
compared to the starting material
inverted
What does the rate of the reaction depend on for SN1 reactions?
ONLY on the concentration of the alkyl halide
In SN1 reactions, the rate of the reaction is favored by..
- the bulkiness of the alkyl substituent
- the bulkier the better
In SN1 reactions, in the substitution of a chiral alkyl halide, a ___________ of product is obtained
racemic mixture
How do you determine whether or not you have a substitution or elimination reaction?
1. Look at reagent
2. Look at substrate
3. Determine mechanism
4. Consider stereochemistry, rearrangements and stability of potential products when applicable.
SN2/E2 reactions are favored by?
- HIGH concentration of nucleophile
- STRONG base
Favored by a HIGH concentration of nucleophile and a STRONG base
SN2/E2 reactions
SN1/E1 reactions are favored by?
POOR nucleophile
WEAK base
Favored by a POOR nucleophile and WEAK base
SN1/E1 reactions
SN2 vs. E2
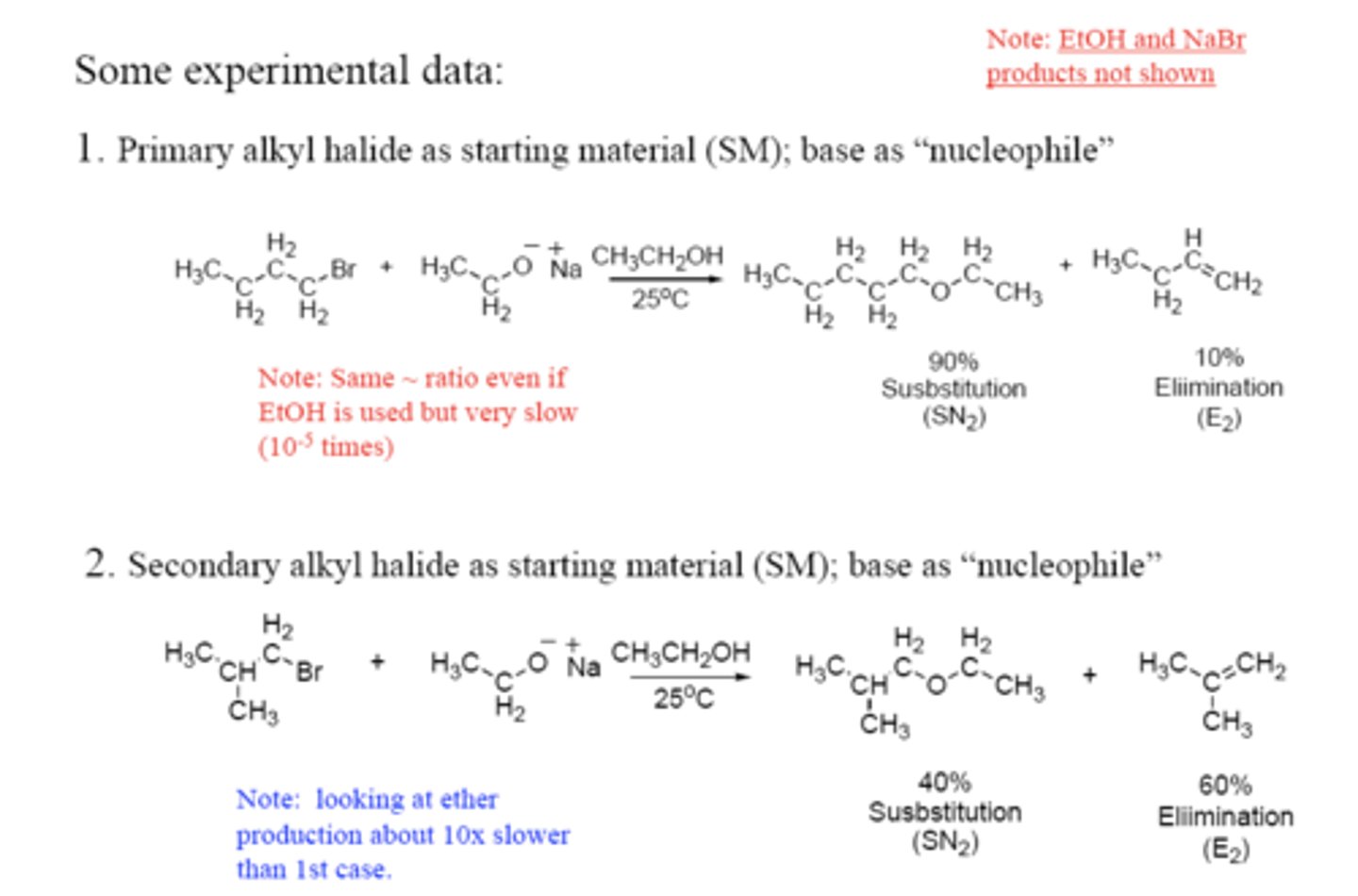
SN1 vs. E1
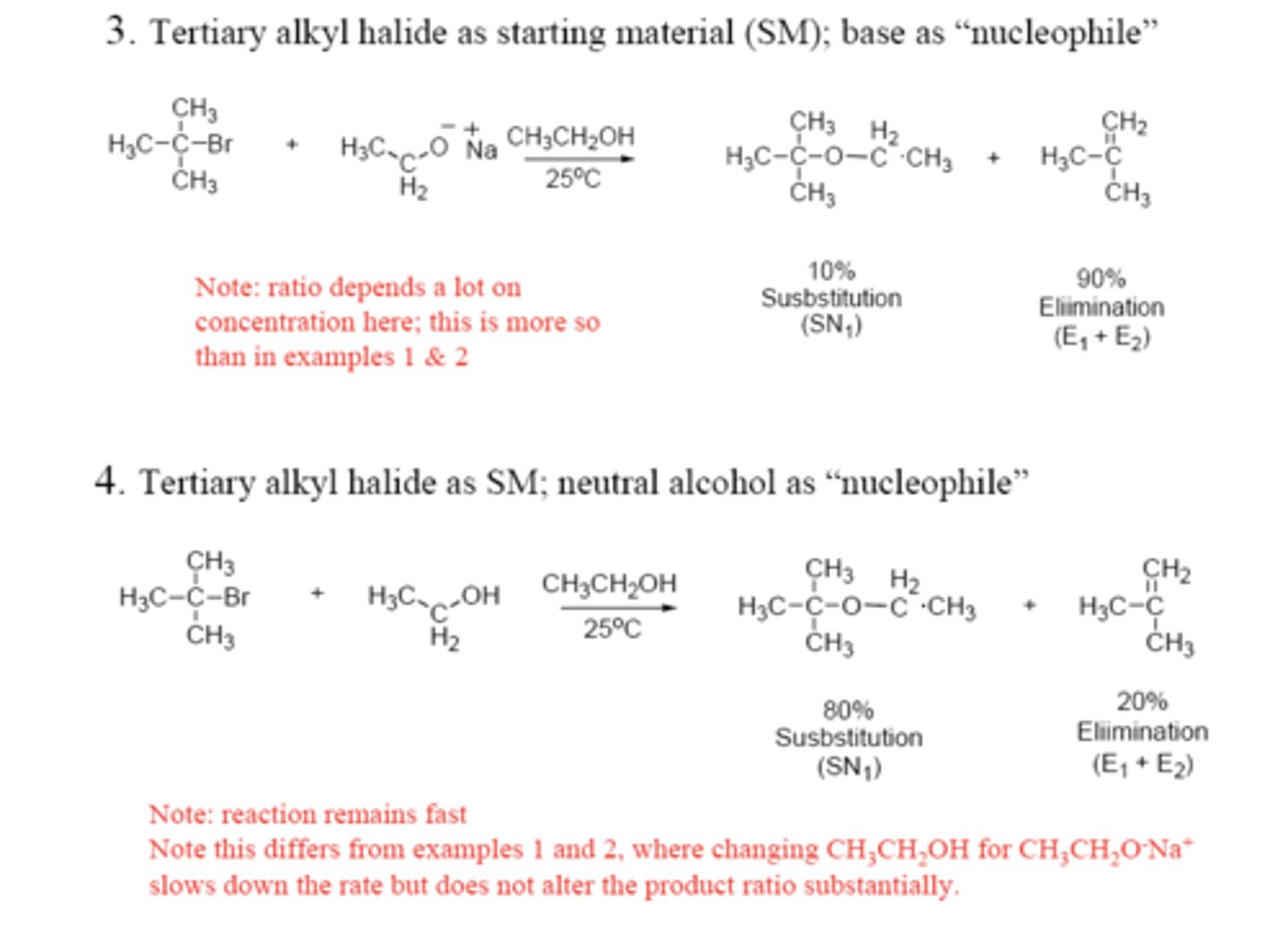
- Slows SN2
- Helps E2
Steric effects
Helps SN2 but helps E2 MORE
Basicity
- Kinetic idea; Polarizability
- Helps SN2
- Helps SN1 2nd step
Nucleophilicity
Charge of leaving group?
Leave NEUTRAL if possible
Charge of attacking group?
NEGATIVE if possible
Helps E2 and E1
High base concentration
Helps SN2 only
solvent
All reactions increase but E2 > SN2
Temperature
If good enough, SN1 or E1
Stability of possible carbocations
What type of reaction does an ethoxide ion favor?
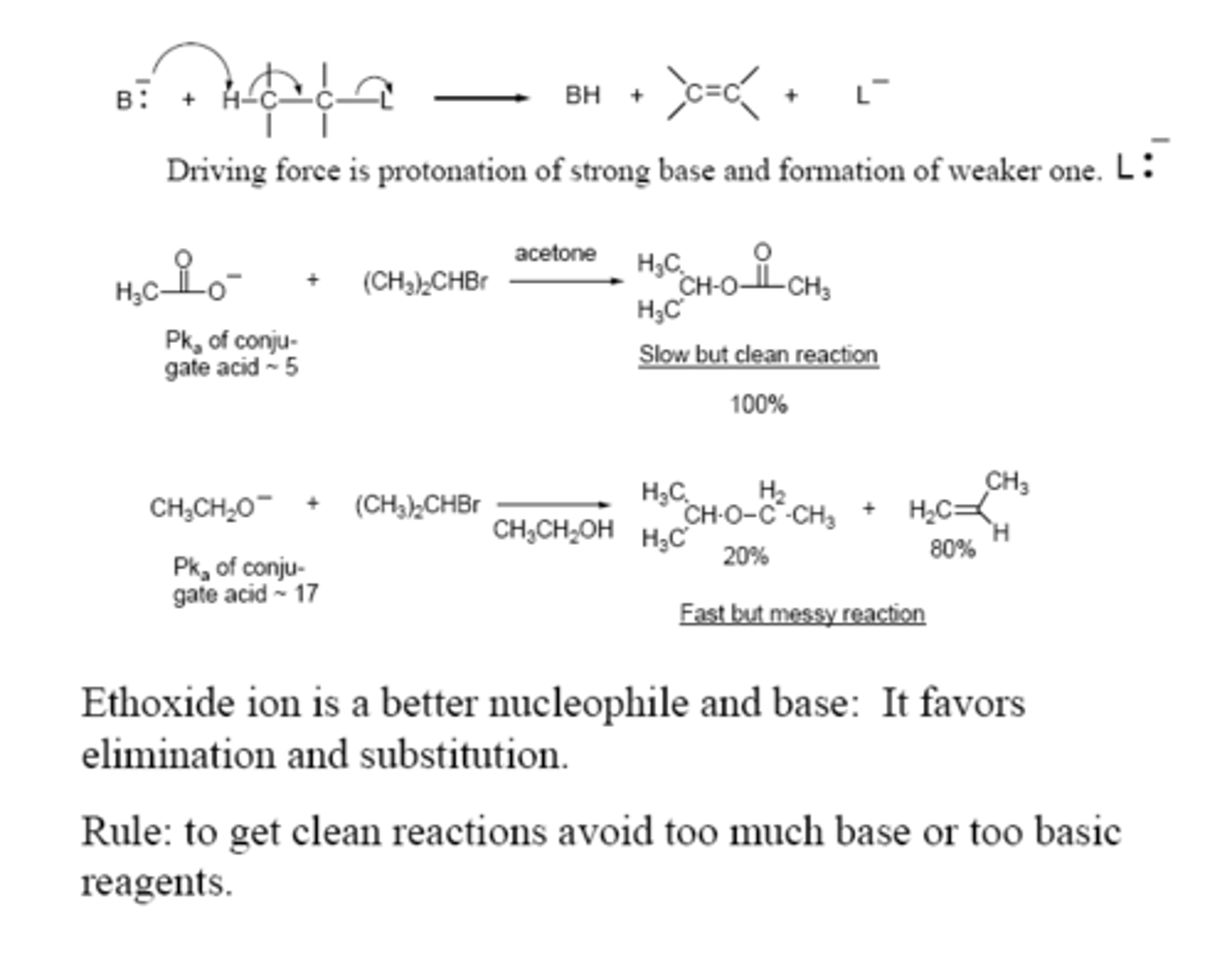
Steric hindrance at alpha and beta carbons produce what effect on SN2/E2 when tertiary or secondary?
- SN2 is slowed
- E2 becomes FASTER
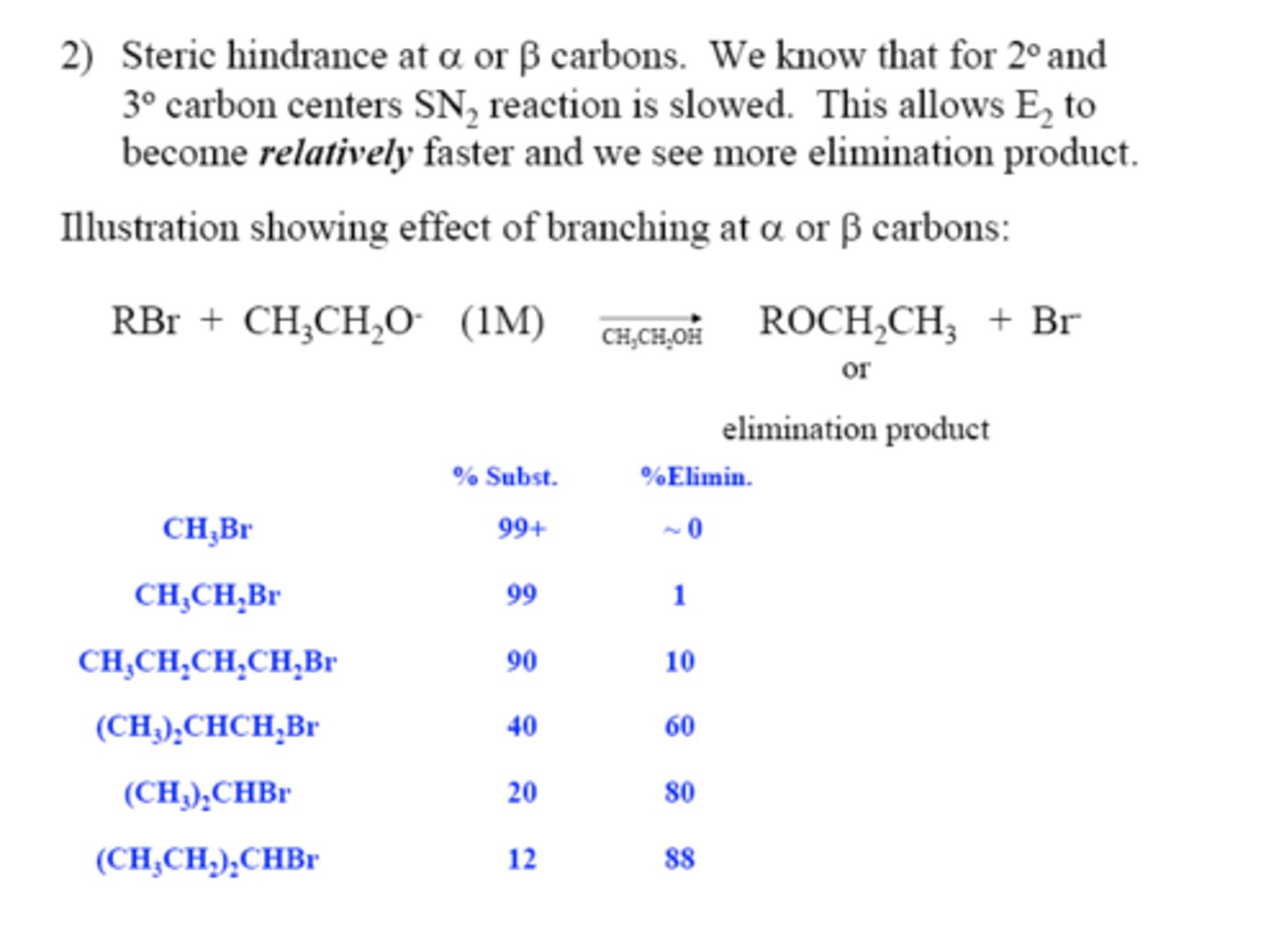
When is substitution favored?
Given that the major reaction of a secondary alkyl halide with an alkoxide ion is elimination by the E2 mechanism, we can expect the proportion of substitution to increase with?
1) decreased crowding at the carbon that bears the leaving group
2) decreased basicity of the nucleophile
What does primary mean?
Attached to one thing
What does secondary mean?
Attached to 2 things
What does tertiary mean?
Attached to 3 things
Strong base
Weak nucleophile
E2
*primary, secondary or tertiary does not matter
Strong base
Strong nucleophile
*Primary
SN2
Strong base
Strong nucleophile
*Secondary
E2
Strong base
Strong nucleophile
*Tertiary
E2
Weak base
Strong nucleophile
*Primary
SN2
Weak base
Strong nucleophile
*Secondary
SN2
Weak base
Strong nucleophile
*Tertiary
SN1
Weak base
Weak nucleophile
*Primary
No reaction
Weak base
Weak nucleophile
*Secondary
SN1/E1
*only when solvolysis occurs at secondary
Weak base
Weak nucleophile
*Tertiary
SN1 and E1
NaH, DBN, DBU
Strong base
Weak nucleophile
HO-, MeO-, EtO-
Strong base
Strong nucleophile
Halides: I-, Br-, Cl-
Sulfur groups: RS-, SH-, RSH, H2S
Weak base
STRONG nucleophile
H2O, MeOH, EtOH, Alcohols (R-OH)
Weak base
Weak nucleophile
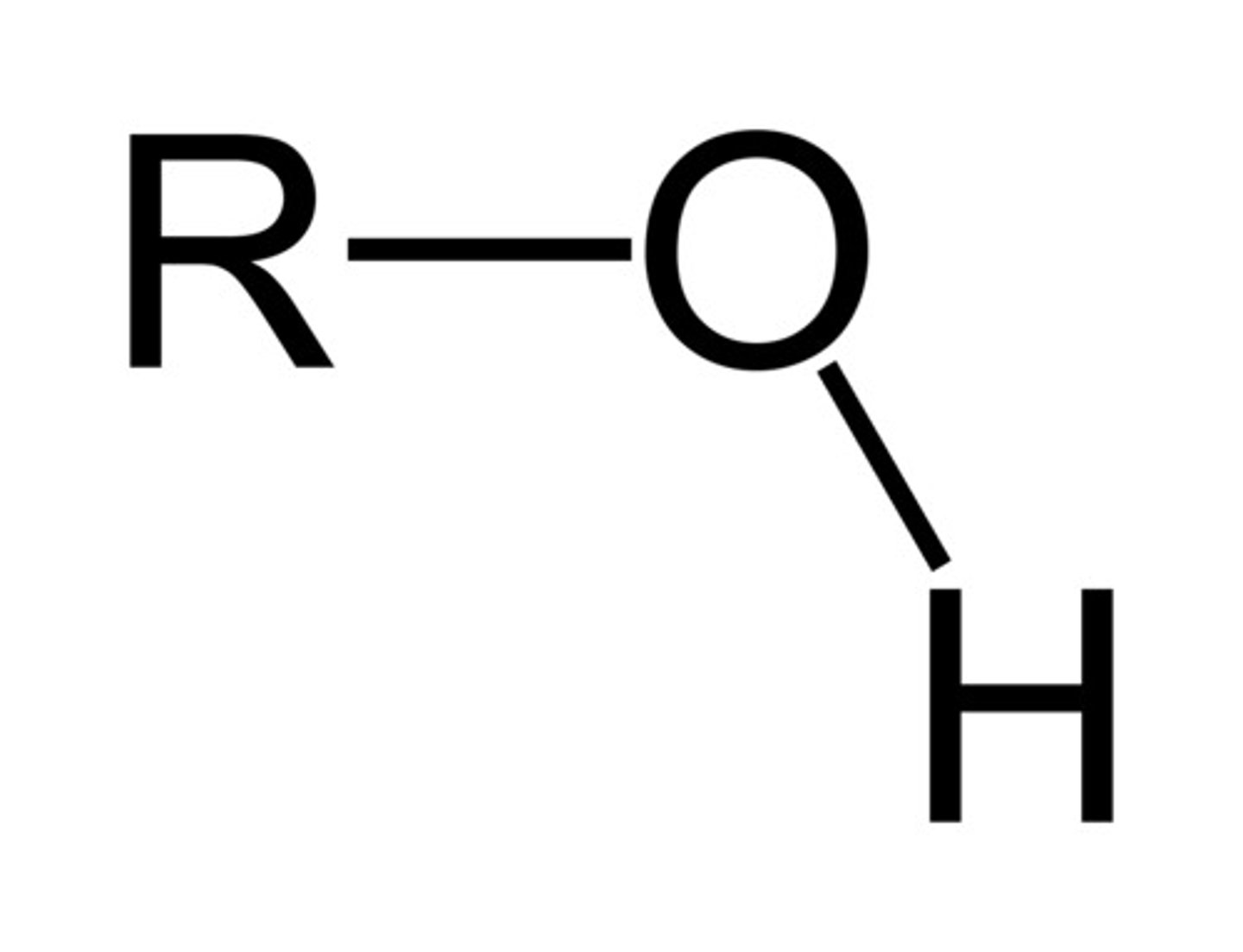
What is an alcohol?
What are the strong nucleophiles?
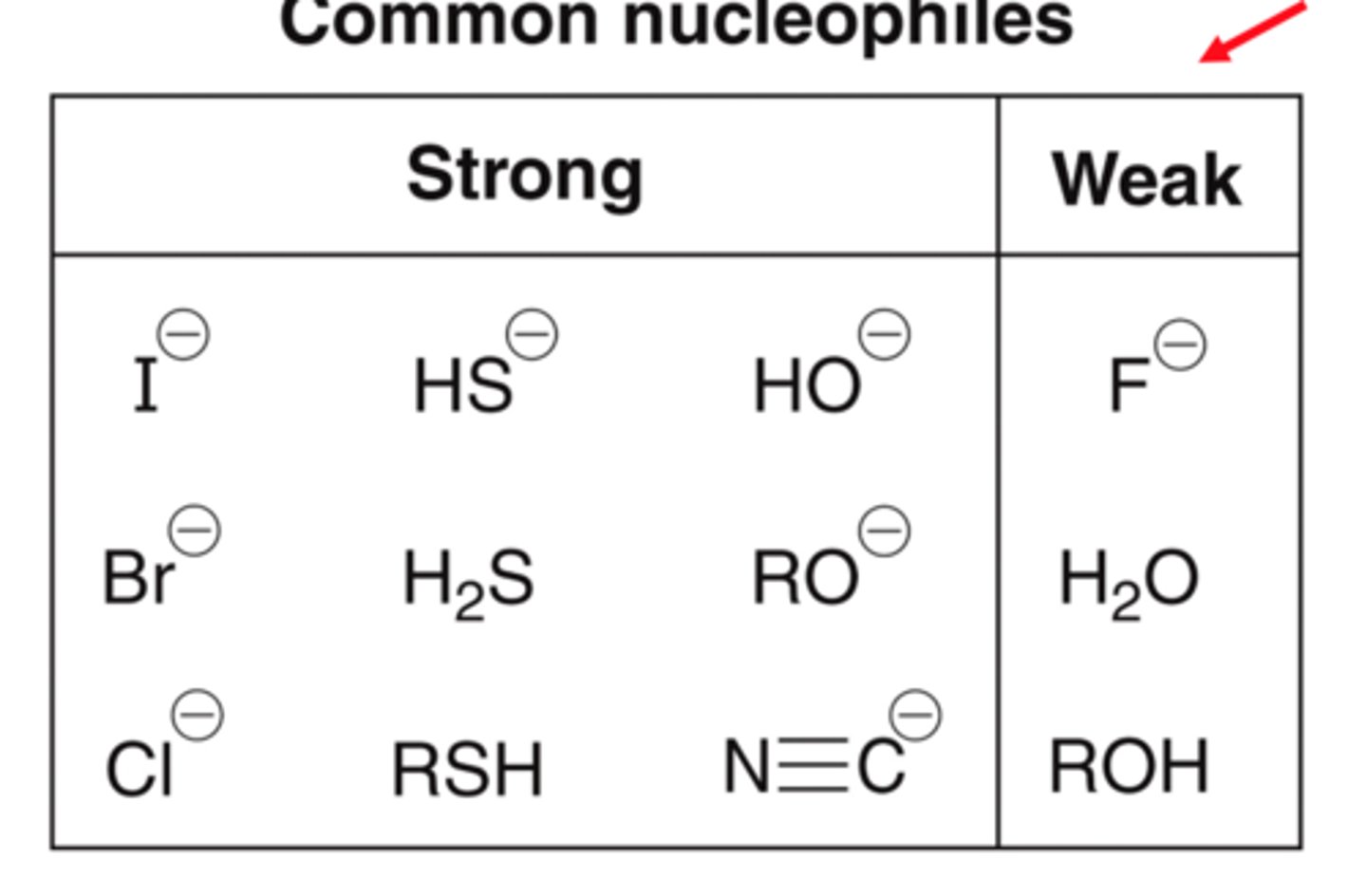
What are the weak nucleophiles?
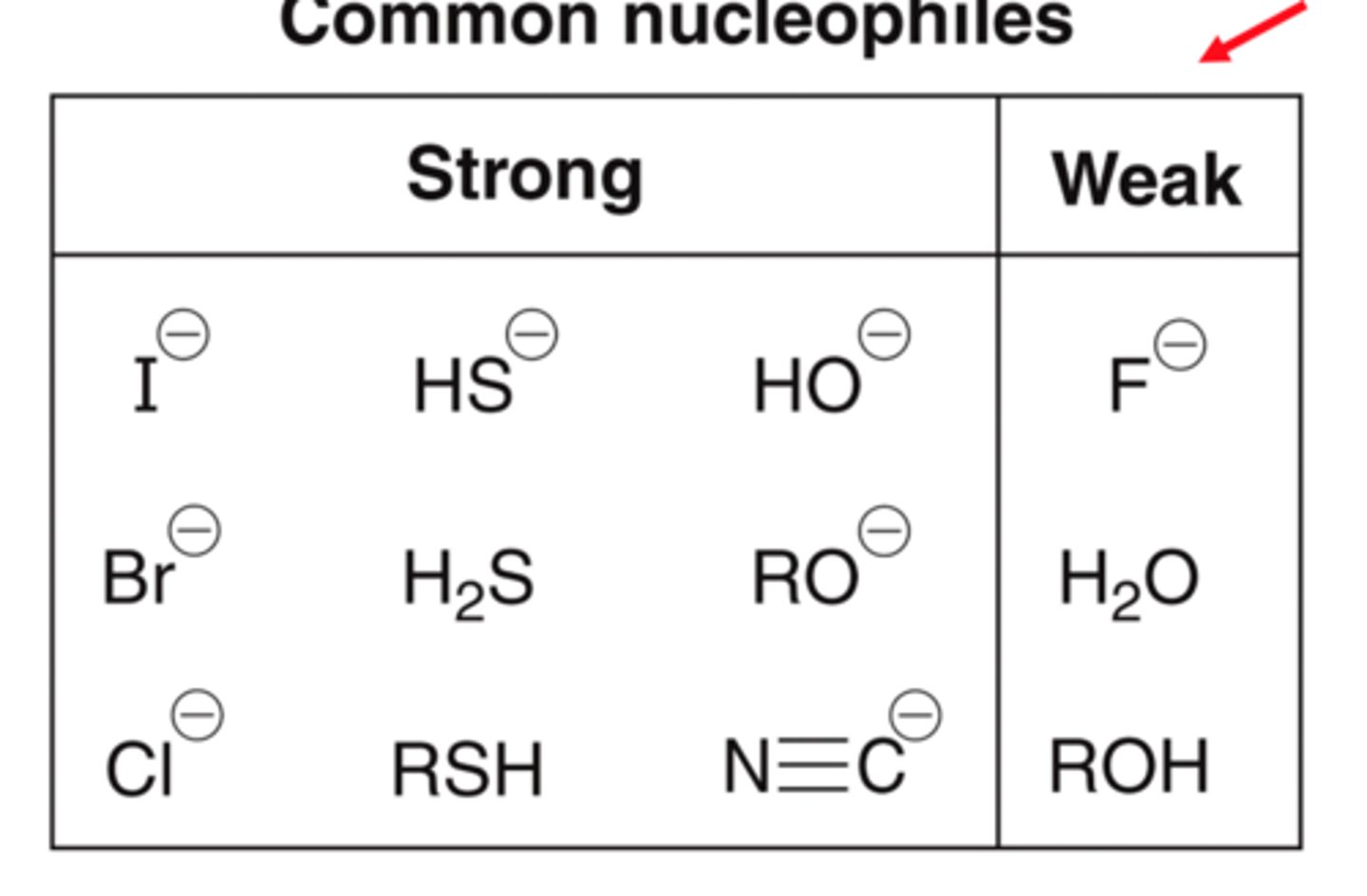
What are the strong bases?
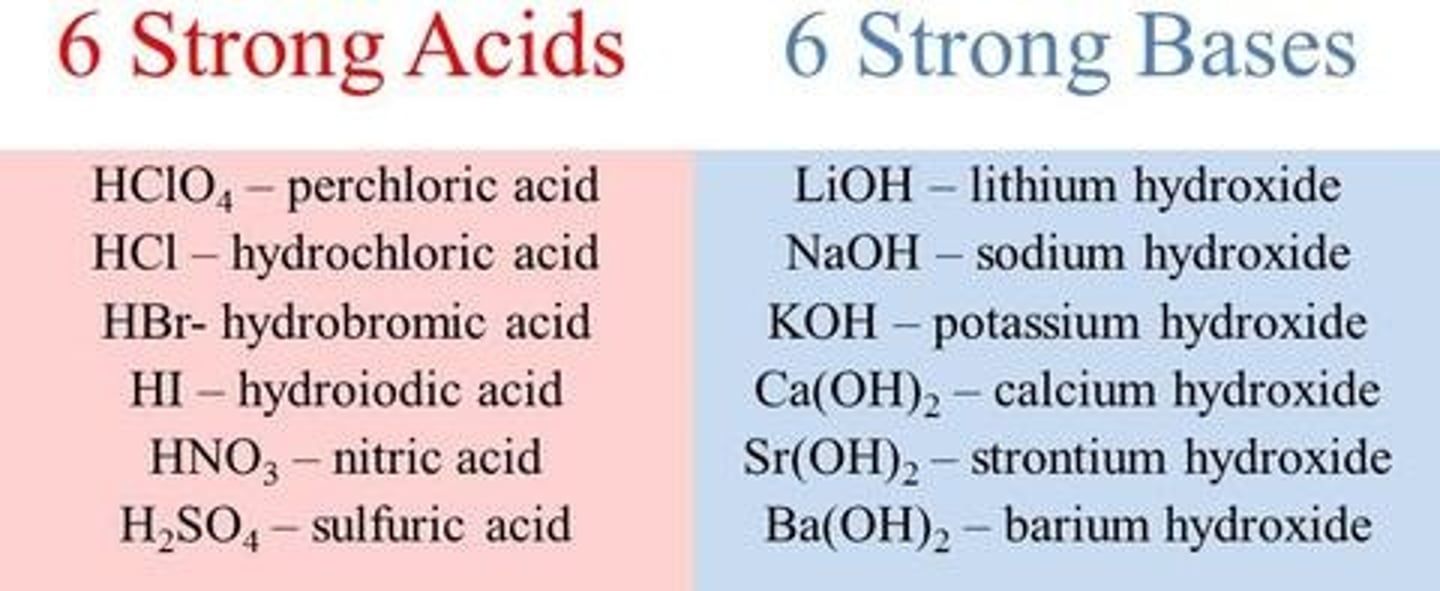
Determine the reagent:
NaH
SB, WN
Determine the reagent:
DBN
SB, WN
Determine the reagent:
DBU
SB, WN
Determine the reagent:
HO-
SB, SN
Determine the reagent:
MeO-
SB, SN
Determine the reagent:
EtO-
SB, SN
Determine the reagent:
I-
WB, SN
Determine the reagent:
Br-
WB, SN
Determine the reagent:
Cl-
WB, SN
Determine the reagent:
RS-
WB, SN
Determine the reagent:
HS-
WB, SN
Determine the reagent:
RSH
WB, SN
Determine the reagent:
H2S
WB, SN
Determine the reagent:
H2O
WB, WN